Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion Criteria
2.3. Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
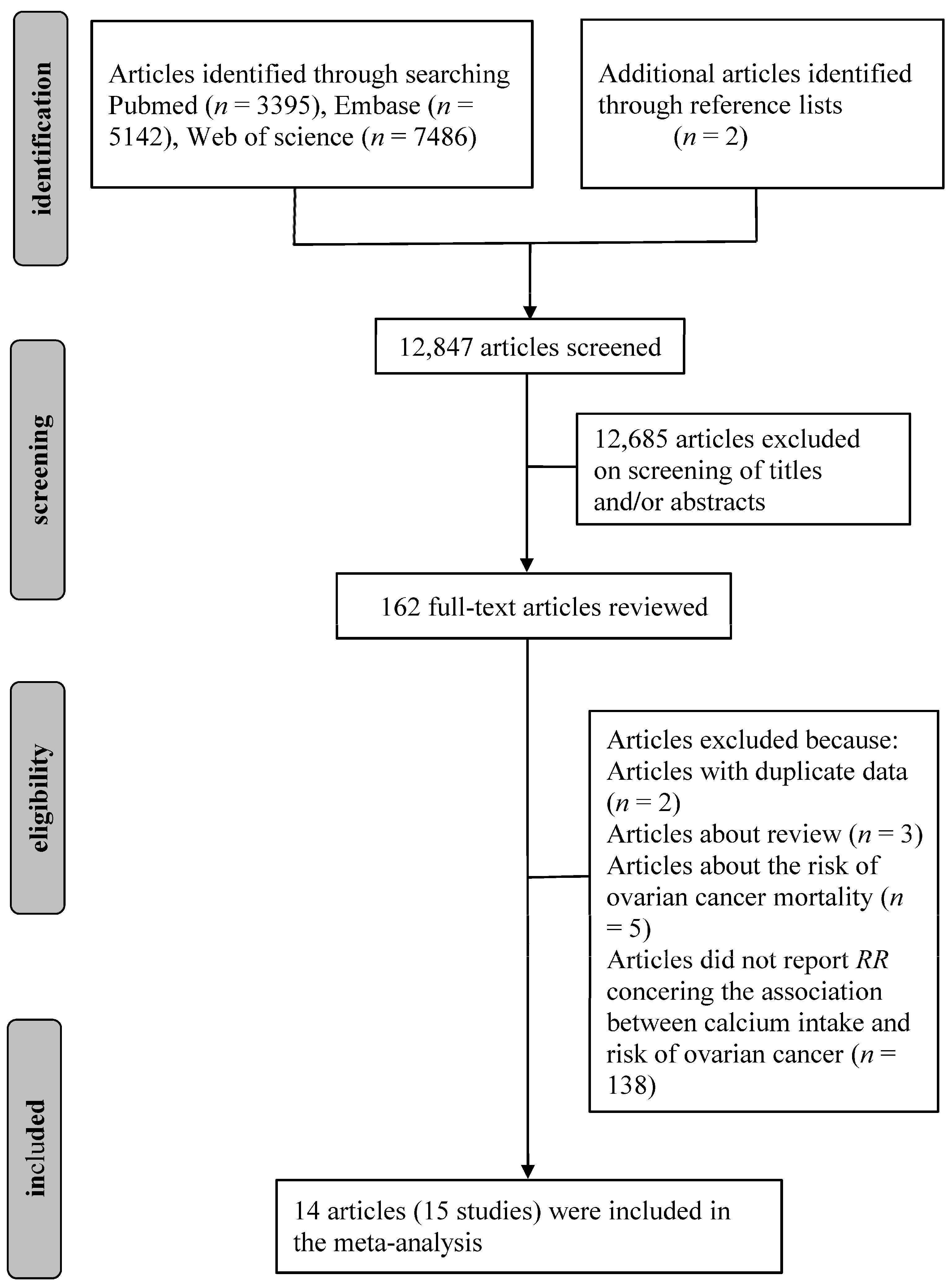
3. Results
3.1. Literature Search and Study Characteristics
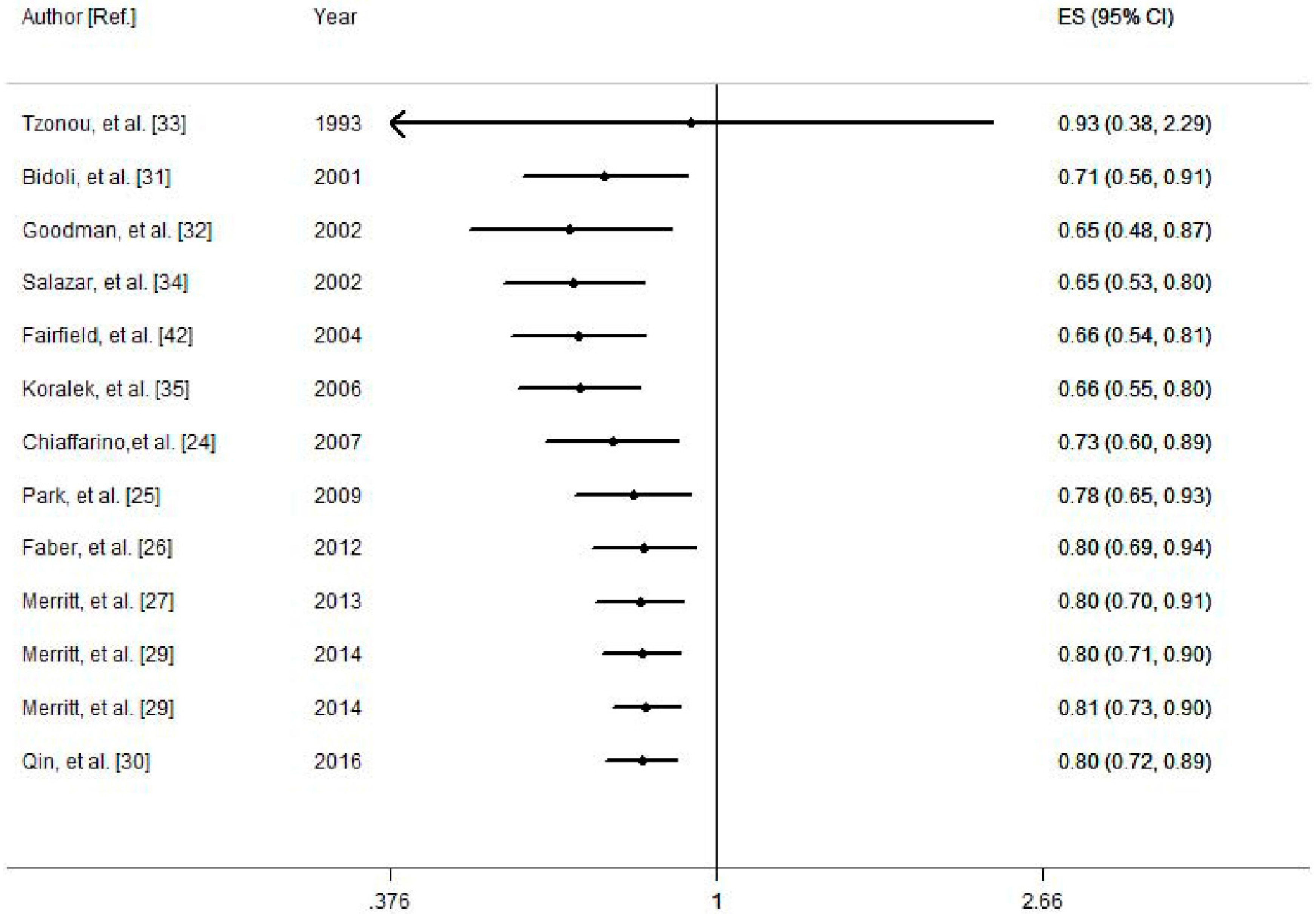
3.2. Quantitative Synthesis
3.2.1. Dietary Calcium and the Risk of Ovarian Cancer
3.2.2. Dietary Plus Supplemental Calcium Intake and the Risk of Ovarian Cancer
3.2.3. Dairy Calcium Intake and the Risk of Ovarian Cancer
3.3. Cumulative Meta-Analysis
3.4. Meta-Regression and Influence Analysis
3.5. Small-Study Effect Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| RR | relative risk |
| CI | confidence interval |
| REM | random effect model |
| EOC | epithelial ovarian cancer |
| FFQs | food frequency questionnaires |
| PTH | parathyroid hormone |
| IGF-1 | insulin-like growth factor-1 |
| US | United States |
References
- Davidson, B.; Trope, C.G. Ovarian cancer: Diagnostic, biological and prognostic aspects. Womens Health (Lond.) 2014, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, S.E.; Danforth, K.N. Ovarian cancer. In Cancer Epidemiology and Prevention, 3rd ed.; Schottenfeld, D., Fraumeni, J., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 1013–1026. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Holschneider, C.H.; Berek, J.S. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin. Surg. Oncol. 2000, 19, 3–10. [Google Scholar] [CrossRef]
- Webb, P.M.; Purdie, D.M.; Grover, S.; Jordan, S.; Dick, M.L.; Green, A.C. Symptoms and diagnosis of borderline, early and advanced epithelial ovarian cancer. Gynecol. Oncol. 2004, 92, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Goff, B.A.; Mandel, L.; Muntz, H.G.; Melancon, C.H. Ovarian carcinoma diagnosis. Cancer 2000, 89, 2068–2075. [Google Scholar] [CrossRef]
- Pu, D.; Jiang, S.W.; Wu, J. Association between MTHFR gene polymorphism and the risk of ovarian cancer: A meta-analysis of the literature. Curr. Pharm. Des. 2014, 20, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Navarro Rosenblatt, D.A.; Chan, D.S.; Abar, L.; Vingeliene, S.; Vieira, A.R.; Greenwood, D.C.; Norat, T. Anthropometric factors and ovarian cancer risk: A systematic review and nonlinear dose-response meta-analysis of prospective studies. Int. J. Cancer 2015, 136, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.M.; Merritt, M.A.; Jordan, S.J.; Yang, H.P.; Hankinson, S.E.; Park, Y.; Rosner, B.; Webb, P.M.; Cramer, D.W.; Wentzensen, N.; et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiol. Biomark. Prev. 2013, 22, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Crane, T.E.; Khulpateea, B.R.; Alberts, D.S.; Basen-Engquist, K.; Thomson, C.A. Dietary intake and ovarian cancer risk: A systematic review. Cancer Epidemiol. Biomark. Prev. 2014, 23, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Milk, milk products and lactose intake and ovarian cancer risk: A meta-analysis of epidemiological studies. Int. J. Cancer 2006, 118, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.T.; Guo, L.; Liu, S.K.; Wang, D.H.; Xi, J.; Huang, P.; Liu, D.T.; Gao, J.F.; Feng, J.; Zhang, L. Egg consumption is associated with increased risk of ovarian cancer: Evidence from a meta-analysis of observational studies. Clin. Nutr. 2015, 34, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kolahdooz, F.; van der Pols, J.C.; Bain, C.J.; Marks, G.C.; Hughes, M.C.; Whiteman, D.C.; Webb, P.M. Meat, fish, and ovarian cancer risk: Results from 2 Australian case-control studies, a systematic review, and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.K.; Ju, W.; Choi, H.J.; Kim, S.C. Soy intake and risk of endocrine-related gynaecological cancer: A meta-analysis. BJOG 2009, 116, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, Y.; Hu, Y.; Zheng, S. Intake of cruciferous vegetables is associated with reduced risk of ovarian cancer: A meta-analysis. Asia Pac. J. Clin. Nutr. 2015, 24, 101–109. [Google Scholar] [PubMed]
- Asemi, Z.; Saneei, P.; Sabihi, S.S.; Feizi, A.; Esmaillzadeh, A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary calcium intake and risk of stroke: A dose-response meta-analysis. Am. J. Clin. Nutr. 2013, 97, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Tian, C.; Zhang, X. Dietary calcium intake, vitamin D levels, and breast cancer risk: A dose-response analysis of observational studies. Breast Cancer Res. Treat. 2012, 136, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Surcel, H.M.; Calypse, A.; Grankvist, K.; Luostarinen, T.; Lukanova, A.; Pukkala, E.; Lehtinen, M. Independent and joint effects of serum 25-hydroxyvitamin D and calcium on ovarian cancer risk: A prospective nested case-control study. Eur. J. Cancer 2010, 46, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Wu, A.H.; Tung, K.H.; McDuffie, K.; Cramer, D.W.; Wilkens, L.R.; Terada, K.; Reichardt, J.K.; Ng, W.G. Association of galactose-1-phosphate uridyltransferase activity and N314D genotype with the risk of ovarian cancer. Am. J. Epidemiol. 2002, 156, 693–701. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Parathyroid hormone may be a cancer promoter—An explanation for the decrease in cancer risk associated with ultraviolet light, calcium, and vitamin D. Med. Hypotheses 2000, 54, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Lee, V.S.; Canchola, A.J.; Clarke, C.A.; Purdie, D.M.; Reynolds, P.; Anton-Culver, H.; Bernstein, L.; Deapen, D.; Peel, D.; et al. Diet and risk of ovarian cancer in the California Teachers Study cohort. Am. J. Epidemiol. 2007, 165, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Parazzini, F.; Bosetti, C.; Franceschi, S.; Talamini, R.; Canzonieri, V.; Montella, M.; Ramazzotti, V.; Franceschi, S.; La Vecchia, C. Risk factors for ovarian cancer histotypes. Eur. J. Cancer 2007, 43, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Leitzmann, M.F.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2009, 169, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.T.; Jensen, A.; Sogaard, M.; Hogdall, E.; Hogdall, C.; Blaakaer, J.; Kjaer, S.K. Use of dairy products, lactose, and calcium and risk of ovarian cancer—Results from a Danish case-control study. Acta Oncol. 2012, 51, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.A.; Cramer, D.W.; Vitonis, A.F.; Titus, L.J.; Terry, K.L. Dairy foods and nutrients in relation to risk of ovarian cancer and major histological subtypes. Int. J. Cancer 2013, 132, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Hunter, D.J.; Spiegelman, D.; Anderson, K.E.; Arslan, A.; Beeson, W.L.; Buring, J.E.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Dairy products and ovarian cancer: A pooled analysis of 12 cohort studies. Cancer Epidemiol. Biomark. Prev. 2006, 15, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.A.; Poole, E.M.; Hankinson, S.E.; Willett, W.C.; Tworoger, S.S. Dairy food and nutrient intake in different life periods in relation to risk of ovarian cancer. Cancer Causes Control 2014, 25, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Moorman, P.G.; Alberg, A.J.; Barnholtz-Sloan, J.S.; Bondy, M.; Cote, M.L.; Funkhouser, E.; Peters, E.S.; Schwartz, A.G.; Terry, P.; et al. Dairy, calcium, vitamin D and ovarian cancer risk in African-American women. Br. J. Cancer 2016, 115, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Bidoli, E.; La Vecchia, C.; Talamini, R.; Negri, E.; Parpinel, M.; Conti, E.; Montella, M.; Carbone, M.A.; Franceschi, S. Micronutrients and ovarian cancer: A case-control study in Italy. Ann. Oncol. 2001, 12, 1589–1593. [Google Scholar] [CrossRef]
- Goodman, M.T.; Wu, A.H.; Tung, K.H.; McDuffie, K.; Kolonel, L.N.; Nomura, A.M.; Terada, K.; Wilkens, L.R.; Murphy, S.; Hankin, J.H. Association of dairy products, lactose, and calcium with the risk of ovarian cancer. Am. J. Epidemiol. 2002, 156, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Tzonou, A.; Hsieh, C.C.; Polychronopoulou, A.; Kaprinis, G.; Toupadaki, N.; Trichopoulou, A.; Karakatsani, A.; Trichopoulos, D. Diet and ovarian cancer: A case-control study in Greece. Int. J. Cancer 1993, 55, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martinez, E.; Lazcano-Ponce, E.C.; Gonzalez Lira-Lira, G.; Escudero-De los Rios, P.; Hernandez-Avila, M. Nutritional determinants of epithelial ovarian cancer risk: A case-control study in Mexico. Oncology 2002, 63, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Koralek, D.O.; Bertone-Johnson, E.R.; Leitzmann, M.F.; Sturgeon, S.R.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Relationship between calcium, lactose, vitamin D, and dairy products and ovarian cancer. Nutr. Cancer 2006, 56, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kushi, L.H.; Mink, P.J.; Folsom, A.R.; Anderson, K.E.; Zheng, W.; Lazovich, D.; Sellers, T.A. Prospective study of diet and ovarian cancer. Am. J. Epidemiol. 1999, 149, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Broadus, A.E.; Mangin, M.; Ikeda, K.; Insogna, K.L.; Weir, E.C.; Burtis, W.J.; Stewart, A.F. Humoral hypercalcemia of cancer. Identification of a novel parathyroid hormone-like peptide. N. Engl. J. Med. 1988, 319, 556–563. [Google Scholar] [PubMed]
- Coxam, V.; Davicco, M.J.; Durand, D.; Bauchart, D.; Lefaivre, J.; Barlet, J.P. The influence of parathyroid hormone-related protein on hepatic IGF-1 production. Acta Endocrinol. (Copenh.) 1992, 126, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Khandwala, H.M.; McCutcheon, I.E.; Flyvbjerg, A.; Friend, K.E. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr. Rev. 2000, 21, 215–244. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Saunders, B.; Fu, L.; Mao, W.; Nicosia, S.V. The insulin-like growth factor 1 receptor induces transformation and tumorigenicity of ovarian mesothelial cells and down-regulates their Fas-receptor expression. Cancer Res. 1999, 59, 3264–3270. [Google Scholar] [PubMed]
- Lund, P.K. Insulin-like growth factors: Gene structure and regulation. In Hormonal Control of Growth, 1st ed.; Kostyo, J.L., Goodman, H.M., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 537–571. [Google Scholar]
- Lukanova, A.; Lundin, E.; Toniolo, P.; Micheli, A.; Akhmedkhanov, A.; Rinaldi, S.; Muti, P.; Lenner, P.; Biessy, C.; Krogh, V.; et al. Circulating levels of insulin-like growth factor-I and risk of ovarian cancer. Int. J. Cancer 2002, 101, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Ramasamy, I. Recent advances in physiological calcium homeostasis. Clin. Chem. Lab. Med. 2006, 44, 237–273. [Google Scholar] [CrossRef] [PubMed]
- Munafo, M.R.; Flint, J. Meta-analysis of genetic association studies. Trends Genet. 2004, 20, 439–444. [Google Scholar] [CrossRef] [PubMed]


| Author [Ref.] | Year | Country | Age Range/Mean Age (Case/Control) | Follow Years (Median) | Study Design | Dietary Assessment | Sample Size (Case) | Range of Calcium (Highest/Lowest) (mg/Day) | Exposure | Outcome | RR (95% CI) | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goodman, M.T. [32] | 2002 | US | 54.8 | NA | CC | Validated FFQ | 1165 (558) | Highest: >1107.9 Lowest: <528.1 | Dietary calcium | EOC | 0.46 (0.27, 0.76) | Age, ethnicity, study center, education, energy intake, parity, oral contraceptive use, tubal ligation |
| Highest: >631.4 Lowest: <182.9 | Dairy calcium | EOC | 0.55 (0.36, 0.84) | |||||||||
| Merritt, M.A. [29] | 2014 | US | 25–55 | 28 | Cohort | Validated FFQ | 76243 (609) | Highest: >1018 Lowest: <433 | Dairy calcium | EOC | 0.80 (0.59, 1.09) | Total caloric intake, menopausal status, number of pregnancies and parity, oral contraceptive use, tubal ligation and family history of ovarian cancer |
| Merritt, M.A. [29] | 2014 | US | 25–55 | 28 | Cohort | Validated FFQ | 88356 (155) | Highest: >675.4 Lowest: <277.7 | Dairy calcium | EOC | 0.86 (0.68, 1.10) | |
| Merritt, M.A. [27] | 2013 | US | 52.5/52.4 | NA | CC | Validated FFQ | 3898 (1909) | Highest: >859.3 Lowest: <543.7 | Dietary calcium | EOC | 0.74 (0.62, 0.89) | Age, number of pregnancies, oral contraceptive use, tubal ligation, family history of ovarian cancer in a first degree relative, study center, study phase and total calories |
| Highest: >1318.8 Lowest: <654.9 | Total calcium | EOC | 0.62 (0.49, 0.79) | Age, number of pregnancies, oral contraceptive use, tubal ligation, family history of ovarian cancer in a first degree relative, study center, study phase, total calories, total vitamin D and lactose | ||||||||
| Qin, B. [30] | 2016 | US | 57.3/54.9 | NA | CC | Validated FFQ | 1146 (490) | Highest: >819.6 Lowest: <362.4 | Dietary calcium | EOC | 0.52 (0.28, 0.98) | Age, region, and total energy intake, education, parity, oral contraceptive use, menopausal status, tubal ligation, family history of breast/ovarian cancer, daylight hours spent outdoors in summer months, pigmentation, recreational physical activity, BMI, other sugar intake excluding lactose, plus quartiles of total vitamin D, and lactose, supplemental intake of calcium |
| Highest: >1233.7 Lowest: <478.6 | Total calcium | EOC | 0.51 (0.30, 0.86) | Age, region, and total energy intake, education, parity, oral contraceptive use, menopausal status, tubal ligation, family history of breast/ovarian cancer, daylight hours spent outdoors in summer months, pigmentation, recreational physical activity, BMI, other sugar intake excluding lactose, plus quartiles of total vitamin D, and lactose | ||||||||
| Tzonou, A. [33] | 1993 | Greece | <75 | NA | CC | FFQ | 389 (189) | Highest: >1500 Lowest: <500 | Dietary calcium | EOC | 0.93 (0.38, 2.29) | Total calories |
| Chang, E.T. [23] | 2007 | US | 50 | 8.1 | Cohort | Validated FFQ | 97275 (280) | Highest: >1127 Lowest: <461 | Total calcium | EOC | 0.90 (0.57, 1.43) | Race, total energy intake, parity, oral contraceptive use, strenuous exercise, wine consumption, and menopausal status/hormone therapy use, use of dietary supplements, excluded short-term supplement users |
| Bidoli, E. [31] | 2001 | Italy | 56/57 | NA | CC | Validated FFQ | 3442 (1031) | NA | Dietary calcium | EOC | 0.70 (0.60, 1.00) | Age, study center, year of interview, education, BMI, parity, oral contraceptive use, occupational physical activity, and energy intake |
| Salazar, M.E. [34] | 2002 | Mexico | 53/54 | NA | CC | Validated FFQ | 713 (84) | Highest: ≥1205 Lowest: <800 | Dietary calcium | EOC | 0.59 (0.32, 1.10) | Age, total energy intake, number of live births, recent changes in weight, physical activity and diabetes |
| Kushi, L.H. [41] | 1999 | US | 55-69 | 10 | Cohort | FFQ | 29083 (139) | Highest: >1372 Lowest: <731 | Total calcium | EOC | 1.66 (0.96, 2.88) | Age, total energy intake, number of live births, age at menopause, family history of ovarian cancer in a first-degree relative, hysterectomy/unilateral oophorectomy status, waist-to-hip ratio, level of physical activity, cigarette smoking, and educational level |
| Fairfield, K.M. [42] | 2004 | US | 30-55 | 16 | Cohort | Validated FFQ | 80326 (301) | NA | Dietary calcium | OC | 0.85 (0.36, 2.00) | Age, BMI, caffeine intake, duration of oral contraceptive use, parity, tubal ligation and smoking, energy |
| NA | Total calcium | OC | 1.47 (0.88, 2.47) | |||||||||
| Koralek, D.O. [35] | 2006 | US | 61 | NA | Cohort | Validated FFQ | 31925 (146) | NA | Dietary calcium | OC | 0.67 (0.43, 1.04) | Age, menopause type, parity, oral contraceptive use, and postmenopausal hormone use at baseline, energy |
| NA | Total calcium | OC | 0.65 (0.36, 1.16) | Total vitamin D, lactose, age, menopause type, parity, age at menarche, oral contraceptive use, and postmenopausal hormone use at baseline, energy | ||||||||
| Chiaffarino, F. [24] | 2007 | Italy | 56/57 | NA | CC | FFQ | 2904 (493) | NA | Dietary calcium | EOC | 0.90 (0.89, 1.10) | Age, study center, year of interview, education, parity, oral contraceptive use, family history of ovarian and/or breast cancer in first degree relatives and energy intake |
| Faber, M.T. [26] | 2012 | Denmark | 58.9/57.1 | NA | CC | FFQ | 2208 (554) | Highest: ≥1200 Lowest: <400 | Dairy calcium | EOC | 1.00 (0.68, 1.48) | Age, pregnancy, number of pregnancies, oral contraceptive use, duration of oral contraceptive use, hormone replacement therapy use, and family history of breast and/or ovarian cancer, lactose intake |
| Park, Y. [25] | 2009 | US | 50–71 | 7 | Cohort | Validated FFQ | 74342 (515) | Highest: >1101 Lowest: <409 | Dietary calcium | OC | 1.02 (0.75, 1.37) | Energy, race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity, menopausal hormone therapy use, alcohol consumption, and intakes of red meat and total energy smoking, parity, oral contraceptive use, and duration of hormone replacement use, supplement calcium, and additional variables race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity |
| Highest: >1881 Lowest: <494 | Total calcium | OC | 1.14 (0.85, 1.52) | Race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity, menopausal hormone therapy use, alcohol consumption, and intakes of red meat and total energy smoking, parity, oral contraceptive use, and duration of hormone replacement use, and additional variables race/ethnicity, education, marital status, BMI, family history of cancer, vigorous physical activity |
| Exposure | Outcome | Subgroup | No. of Studies | Pooled RR (95% CI) | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|---|
| Dietary calcium | OC | All studies | 13 | 0.80 (0.72, 0.89) | 32.8 | 0.12 |
| Cohort | 5 | 0.86 (0.74, 0.99) | 0 | 0.614 | ||
| Case-control | 8 | 0.75 (0.64, 0.89) | 53.3 | 0.036 | ||
| North America | 9 | 0.76 (0.66, 0.87) | 26.4 | 0.209 | ||
| Europe | 4 | 0.86 (0.75, 0.99) | 18.9 | 0.296 | ||
| Validated FFQ | 10 | 0.75 (0.67, 0.85) | 20.5 | 0.254 | ||
| FFQ | 3 | 0.91 (0.82, 1.00) | 0 | 0.875 | ||
| Adjustment for parity | ||||||
| Yes | 9 | 0.79 (0.69, 0.91) | 42.5 | 0.084 | ||
| No | 4 | 0.77 (0.66, 0.90) | 0 | 0.424 | ||
| Adjustment for tubal ligation | ||||||
| Yes | 6 | 0.74 (0.64, 0.86) | 20.1 | 0.282 | ||
| No | 7 | 0.85 (0.75, 0.97) | 21.7 | 0.264 | ||
| Dietary calcium | EOC | All studies | 10 | 0.78 (0.69, 0.88) | 40.5 | 0.087 |
| Total calcium | OC | All studies | 7 | 0.90 (0.65, 1.24) | 76.1 | 0.000 |
| Dairy calcium | OC | All studies | 4 | 0.80 (0.66, 0.98) | 34.5 | 0.205 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Li, Z.; Ji, X.; Zhang, D. Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Nutrients 2017, 9, 679. https://doi.org/10.3390/nu9070679
Song X, Li Z, Ji X, Zhang D. Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis. Nutrients. 2017; 9(7):679. https://doi.org/10.3390/nu9070679
Chicago/Turabian StyleSong, Xingxing, Zongyao Li, Xinqiang Ji, and Dongfeng Zhang. 2017. "Calcium Intake and the Risk of Ovarian Cancer: A Meta-Analysis" Nutrients 9, no. 7: 679. https://doi.org/10.3390/nu9070679





