Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Clinical Parameters and Blood Sample Collection
2.2. Steatosis Evaluation
2.3. Liver Fibrosis Assessment
3. Data Analysis
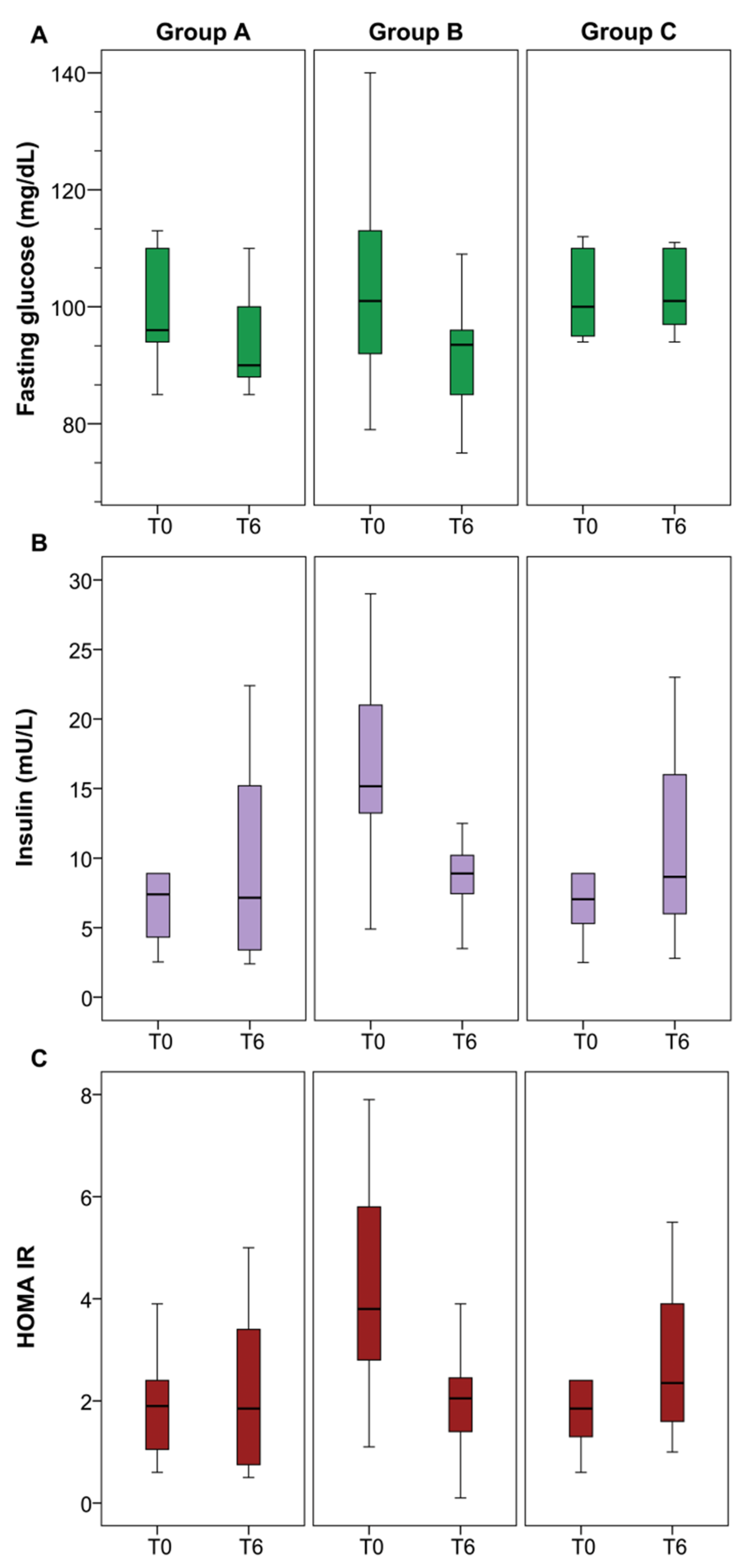
4. Results
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Masarone, M.; Federico, A.; Abenavoli, L.; Loguercio, C.; Persico, M. Non alcoholic fatty liver: Epidemiology and natural history. Rev. Recent Clin. Trials 2014, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Guaraldi, G.; Nascimbeni, F.; Romagnoli, D.; Zona, S.; Targher, G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease—Evidence from three different disease models: NAFLD, HCV and HIV. World J. Gastroenterol. 2016, 22, 9674–9693. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L. The role of liver biopsy to assess non-alcoholic fatty liver disease. Rev. Recent Clin. Trials 2014, 9, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Miglioli, L.; Masutti, F.; Castiglione, A.; Crocè, L.S.; Tiribelli, C.; Bellentani, S. Incidence and natural course of fatty liver in the general population: The Dionysos study. Hepatology 2007, 46, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Browning, J.D.; Ayers, C.R.; Das, S.R.; Khera, A.; Vega, G.L.; Grundy, S.M.; Scherer, P.E. Adiponectin as an independent predictor of the presence and degree of hepatic steatosis in the Dallas heart study. J. Clin. Endocrinol. Metab. 2012, 97, E982–E986. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Tanyolaç, S.; Iiritano, S.; Sciacqua, A.; Capula, C.; Arcidiacono, B.; Nocera, A.; Possidente, K.; Baudi, F.; Ventura, V.; et al. A polymorphism of HMGA1 is associated with increased risk of metabolic syndrome and related components. Sci. Rep. 2013, 3, 1491. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. Pathogenesis of steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Gambino, R.; Musso, G.; Cassader, M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: Mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1325–1365. [Google Scholar] [CrossRef] [PubMed]
- Beltowsky, J.; Wójcicka, G.; Górny, D.; Marciniak, A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. Physiol. Pharmacol. 2000, 51, 883–896. [Google Scholar]
- Accattato, F.; Greco, M.; Pullano, S.A.; Carè, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; De Simone, T.; D’Auria, M.V.; de Sio, I.; Federico, A.; Tuccillo, C.; Abbatecola, A.M.; Del Vecchio Blanco, C.; Italian AISF Clinical Group. Non-alcoholic fatty liver disease: A multicentre clinical study by the Italian association for the study of the liver. Dig. Liver Dis. 2004, 36, 398–405. [Google Scholar] [CrossRef]
- Garcia-Monzon, C.; Martin-Perez, E.; Iacono, O.L.; Fernández-Bermejo, M.; Majano, P.L.; Apolinario, A.; Larrañaga, E.; Moreno-Otero, R. Characterization of pathogenic and prognostic factors of non-alcoholic steatohepatitis associated with obesity. J. Hepatol. 2000, 33, 716–724. [Google Scholar] [CrossRef]
- Seki, S.; Kitada, T.; Yamada, T.; Sakaguchi, H.; Nakatani, K.; Wakasa, K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver disease. J. Hepatol. 2002, 37, 56–62. [Google Scholar] [CrossRef]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with non-alcoholic steatohepatitis. Am. J. Gastroenterol. 2004, 99, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Merat, S.; Malekzadeh, R.; Sohrabi, M.R.; Sotoudeh, M.; Rakhshani, N.; Sohrabpour, A.A.; Naserimoghadam, S. Probucol in the treatment of non-alcoholic steatohepatitis: A double-blind randomized controlled study. J. Hepatol. 2003, 38, 414–418. [Google Scholar] [CrossRef]
- Harrison, S.A.; Torgerson, S.; Hayashi, P.; Ward, J.; Schenker, S. Vitamin E and vitamin C treatment improves fibrosis in patients with non-alcoholic steatohepatitis. Am. J. Gastroenterol. 2003, 98, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milic, N.; Peta, V.; Alfieri, F.; De Lorenzo, A.; Bellentani, S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J. Gastroenterol. 2014, 20, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Federico, A.; Dallio, M.; Scazzina, F. Mediterranean diet and nonalcoholic fatty liver disease: Molecular mechanisms of protection. Int. J. Food Sci. Nutr. 2017, 68, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Abellán Alemán, J.; Zafrilla Rentero, M.P.; Montoro-García, S.; Mulero, J.; Pérez Garrido, A.; Leal, M.; Guerrero, L.; Ramos, E.; Ruilope, L.M. Adherence to the “Mediterranean diet” in Spain and its relationship with cardiovascular risk (DIMERICA study). Nutrients 2016, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Park, C.Y.; Park, S.W. Role of thiazolidinediones, insulin sensitizers, in non-alcoholic fatty liver disease. J. Diabetes Investig. 2013, 4, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Foti, D.; Paonessa, F.; Chiefari, E.; Palaia, L.; Brunetti, G.; Gulletta, E.; Fusco, A.; Brunetti, A. The insulin receptor: A new anticancer target for peroxisome proliferator-activated receptor-gamma (PPARgamma) and thiazolidinedione-PPARgamma agonists. Endocr.-Relat. Cancer 2008, 15, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Peta, V.; Milic, N. Lifestyle changes associated with a new antioxidant formulation in non-alcoholic fatty liver disease: A case series. Ann. Hepatol. 2015, 14, 121–126. [Google Scholar] [PubMed]
- De Lorenzo, A.; Noce, A.; Bigioni, M.; Calabrese, V.; Della Rocca, D.G.; Di Daniele, N.; Tozzo, C.; Di Renzo, L. The effects of Italian Mediterranean organic diet (IMOD) on health status. Curr. Pharm. Des. 2010, 16, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Montalcini, T.; Accattato, F.; Costanzo, F.S.; Pujia, A.; Foti, D.; Brunetti, A.; Gulletta, E. Early effects of a hypocaloric, Mediterranean diet on laboratory parameters in obese individuals. Mediat. Inflamm. 2014, 2014, 750860. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; George, J.; Johnson, N.A. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Karne, R.J.; Chen, H.; Quon, M.J. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2004, 27, 1247–1248. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okudam, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Beaugrand, M. Transient elastography in non-alcoholic fatty liver disease. Ann. Hepatol. 2012, 11, 172–178. [Google Scholar] [PubMed]
- Berzigotti, A. Non-invasive assessment of non-alcoholic fatty liver disease: Ultrasound and transient elastography. Rev. Recent Clin. Trials 2014, 9, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sugimoto, K.; Inui, H.; Fukusato, T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3777–3785. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Milic, S.; Turk Wensveen, T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Wensveen, F.; Orlic, L. Nonalcoholic fatty liver disease—A multisystem disease? World J. Gastroenterol. 2016, 22, 9488–9505. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Peta, V. Role of adipokines and cytokines in non-alcoholic fatty liver disease. Rev. Recent Clin. Trials 2014, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Papamiltiadous, E.S.; Roberts, S.K.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Salim, A.; Tierney, A.C. A randomised controlled trial of a Mediterranean dietary intervention for adults with non-alcoholic fatty liver disease (MEDINA): Study protocol. BMC Gastroenterol. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Bihuniak, J.D.; Shook, J.; Kenny, A.; Kerstetter, J.; Huedo-Medina, T.B. The effect of the traditional Mediterranean-style diet on metabolic risk factors: A meta-analysis. Nutrients 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Rustgi, V.K. Current pharmacologic therapy for nonalcoholic fatty liver disease. Clin. Liver Dis. 2016, 20, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.F.; Sheen, L.Y.; Lin, H.J.; Chang, H.H. A review of western and traditional chinese medical approaches to managing nonalcoholic fatty liver disease. Evid.-Based Complement. Altern. Med. 2016, 2016, 6491420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Xie, L.Z.; Zhu, J.; Li, G.Q.; Grant, S.J.; Liu, J.P. Herbal medicines for fatty liver diseases. Cochrane Database Syst. Rev. 2013, CD009059. [Google Scholar] [CrossRef] [Green Version]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Trappoliere, M.; Tuccillo, C.; de Sio, I.; Di Leva, A.; Del Vecchio Blanco, C.; Loguercio, C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: Preliminary observations. Gut 2006, 55, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Izaola, O.; Gómez, S.; Tafur, C.; González, G.; Berroa, E.; Mora, N.; González, J.M.; de Luis, D.A. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3118–3124. [Google Scholar] [PubMed]
- Loguercio, C.; Andreone, P.; Brisc, C.; Brisc, M.C.; Bugianesi, E.; Chiaramonte, M.; Cursaro, C.; Danila, M.; de Sio, I.; Floreani, A.; et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Greco, M.; Nazionale, I.; Peta, V.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.; Pin, W.K.; Wong, M.H.; Kwok, C.Y.; Chan, R.Y.; Yu, P.H.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Şener, B.; Musharraf, S.G. Antioxidant and hepatoprotective activity appraisal of four selected Fumaria species and their total phenol and flavonoid quantities. Exp. Toxicol. Pathol. 2012, 64, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Pacana, T.; Cazanave, S.; Verdianelli, A.; Patel, V.; Min, H.K.; Mirshahi, F.; Quinlivan, E.; Sanyal, A.J. Dysregulated hepatic methionine metabolism drives homocysteine elevation in diet-induced nonalcoholic fatty liver disease. PLoS ONE 2015, 10, e0136822. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Bjornson, E.; Zhang, C.; Klevstig, M.; Söderlund, S.; Ståhlman, M.; Adiels, M.; Hakkarainen, A.; Lundbom, N.; Kilicarslan, M.; et al. Personal model-assisted identification of NAD+ and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 2017, 13, 916. [Google Scholar] [CrossRef] [PubMed]
| Group A (n = 20) | Group B (n = 20) | Group C (n = 10) | |
|---|---|---|---|
| Age (years) | 52 (40–60) | 46 (40–57) | 33 (28–43) |
| Weight (kg) | 83 (80–88) | 90 (81–92) | 84 (75–95) |
| BMI (kg/m2) | 31 (29–33) | 29 (28–32) | 29 (27–31) |
| Waist circumference (cm) | 108 (99–114) | 104 (100–105) | 102 (97–110) |
| Hip circumference (cm) | 105 (102–116) | 105 (102–110) | 105 (99–112) |
| Systolic blood pressure (mmHg) | 125 (120–140) | 130 (120–140) | 120 (110–130) |
| Diastolic blood pressure (mmHg) | 80 (70–90) | 80 (70–90) | 80 (60–82) |
| AST (U/L) | 22 (20–25) | 22 (20–25) | 25 (23–35) |
| ALT (U/L) | 22 (15–30) | 25 (21–40) | 35 (24–58) |
| γGT (U/L) | 20 (16–28) | 21 (14–31) | 21 (16–37) |
| Total bilirubin (mg/dL) | 0.45 (0.38–0.80) | 0.40 (0.30–0.60) | 0.80 (0.77–0.90) |
| Fasting glucose (mg/dL) | 96 (94–110) | 101 (90–113) | 100 (95–110) |
| Insulin (mU/L) | 8 (4–16) | 15 (13–21) | 7 (5–11) |
| Triglycerides (mg/dL) | 140 (129–157) | 101 (84–106) | 138 (115–173) |
| Total cholesterol (mg/dL) | 189 (178–206) | 198 (171–213) | 176 (147–191) |
| LDL-C (mg/dL) | 124 (105–134) | 122 (97–133) | 115 (99–125) |
| Creatinine (mg/dL) | 0.8 (0.7–0.8) | 0.8 (0.7–0.9) | 0.80 (0.70–0.90) |
| Urea (mg/dL) | 38 (31–43) | 38 (28–40) | 32 (26–40) |
| HOMA-IR | 2 (1–2) | 4 (3–6) | 2 (1–3) |
| TyG index | 4.7 (4.7–4.8) | 4.6 (4.5–4.8) | 4.7 (4.7–4.8) |
| FL index | 71 (56–85) | 58 (42–69) | 67 (63–80) |
| TE | 8.1 (6.7–9.2) | 6.9 (6.7–7.2) | 7.2 (5.3–10.1) |
| US score | 2 (2–3) | 2 (2–2) | 1 (0.75–2) |
| Group A | |||
|---|---|---|---|
| T0 | T6 | p | |
| Weight (kg) | 83 (80–88) | 78 (75–80) | 0.0001 |
| BMI (kg/m2) | 31 (29–33) | 29 (27–31) | 0.0001 |
| Waist circumference (cm) | 108 (99–114) | 102 (98–111) | 0.0001 |
| Hip circumference (cm) | 105 (102–116) | 102 (99–110) | 0.0001 |
| Systolic blood pressure (mmHg) | 125 (120–140) | 125 (120–130) | 0.121 |
| Diastolic blood pressure (mmHg) | 80 (70–90) | 80 (70–80) | 0.755 |
| AST (U/L) | 22 (20–25) | 23 (21–25) | 0.101 |
| ALT (U/L) | 22 (15–30) | 25 (18–31) | 0.497 |
| γGT (U/L) | 20 (16–28) | 25 (21–31) | 0.024 |
| Total bilirubin (mg/dL) | 0.45 (0.38–0.80) | 0.45 (0.30–0.80) | 0.436 |
| Fasting glucose (mg/dL) | 96 (94–110) | 90 (88–102) | 0.258 |
| Insulin (mU/L) | 8 (4–16) | 7 (3–15) | 0.777 |
| Triglycerides (mg/dL) | 140 (129–157) | 85 (75–135) | 0.0001 |
| Total cholesterol (mg/dL) | 189 (178–206) | 156(143–185) | 0.0001 |
| LDL-C (mg/dL) | 124 (105–134) | 102 (92–115) | 0.005 |
| Creatinine (mg/dL) | 0.8 (0.7–0.8) | 0.8 (0.7–0.9) | 0.218 |
| Urea (mg/dL) | 38 (31–43) | 30 (27–34) | 0.007 |
| HOMA-IR | 1.9 (0.9–2.4) | 1.8 (0.6–3.4) | 0.985 |
| TyG index | 4.7 (4.7–4.8) | 4.5 (4.4–4.8) | 0.100 |
| FL index | 71 (56–85) | 45 (39–69) | 0.002 |
| TE | 8.1 (6.7–9.2) | 6.0 (5.1–7.0) | 0.0001 |
| US score | 2 (2–3) | 2 (1–2) | 0.0001 |
| Group B | |||
|---|---|---|---|
| T0 | T6 | p | |
| Weight (kg) | 90 (81–92) | 81 (74–86) | 0.002 |
| BMI (kg/m2) | 29 (28–32) | 27 (25–28) | 0.0001 |
| Waist circumference (cm) | 104 (100–105) | 98 (96–100) | 0.0001 |
| Hip circumference (cm) | 105 (102–110) | 101 (99–102) | 0.001 |
| Diastolic blood pressure (mmHg) | 80 (70–90) | 80 (70–80) | 0.285 |
| Systolic blood pressure (mmHg) | 130 (120–140) | 120 (120–130) | 0.012 |
| AST (U/L) | 22 (20–25) | 21 (18–32) | 0.955 |
| ALT (U/L) | 25 (21–40) | 25 (17–25) | 0.007 |
| γGT (U/L) | 21 (14–31) | 24 (16–30) | 0.175 |
| Total bilirubin (mg/dL) | 0.40 (0.30–0.60) | 0.50 (0.40–0.60) | 0.084 |
| Fasting glucose (mg/dL) | 101 (90–113) | 93 (85–96) | 0.007 |
| Insulin (mU/L) | 15 (13–21) | 9 (7–10) | 0.0001 |
| Triglycerides (mg/dL) | 106 (100–139) | 75 (61–92) | 0.011 |
| Total cholesterol (mg/dL) | 198 (171–213) | 152 (140–180) | 0.0001 |
| LDL-C (mg/dL) | 122 (97–133) | 98 (78–120) | 0.016 |
| Creatinine (mg/dL) | 0.8 (0.7–0.9) | 95 (78–120) | 0.409 |
| Urea (mg/dL) | 38 (28–40) | 39 (28–44) | 0.497 |
| HOMA-IR | 4 (3–6) | 2 (1–2) | 0.001 |
| TyG index | 4.6 (4.5–4.8) | 4.5 (4.5–4.7) | 0.005 |
| FL index | 58 (42–69) | 38 (29–45) | 0.003 |
| TE | 6.9 (6.7–7.2) | 5.0 (4.7–5.2) | 0.0001 |
| US score | 2 (2–2) | 0 (0–1) | 0.0001 |
| Group C | |||
|---|---|---|---|
| T0 | T6 | p | |
| Weight (kg) | 84 (75–95) | 85 (75–95) | 0.214 |
| BMI (kg/m2) | 29 (27–31) | 29 (27–30) | 0.223 |
| Waist circumference (cm) | 102 (97–110) | 102 (99–111) | 0.334 |
| Hip circumference (cm) | 105 (99–112) | 106 (99–113) | 0.389 |
| Systolic Blood Pressure (mmHg) | 120 (110–130) | 120 (120–132) | 0.066 |
| Diastolic Blood Pressure (mmHg) | 80 (60–82) | 80 (70–82) | 0.102 |
| AST (U/L) | 25 (23–35) | 29 (24–56) | 0.023 |
| ALT (U/L) | 35 (24–58) | 40 (32–52) | 0.878 |
| γGT (U/L) | 21 (16–37) | 29 (21–37) | 0.036 |
| Total Bilirubin (mg/dL) | 0.80 (0.77–0.90) | 0.80 (0.75–0.90) | 0.257 |
| Fasting Glucose (mg/dL) | 100 (95–110) | 101 (96–110) | 0.395 |
| Insulin (mU/L) | 7 (5–11) | 9 (6–16) | 0.041 |
| Triglycerides (mg/dL) | 138 (115–173) | 143 (138–173) | 0.241 |
| Total Cholesterol (mg/dL) | 176 (147–191) | 152 (140–180) | 0.025 |
| LDL-C (mg/dL) | 115 (99–125) | 78 (54–95) | 0.028 |
| Creatinine (mg/dL) | 0.80 (0.70–0.90) | 0.8 (0.77–0.90) | 0.083 |
| Urea (mg/dL) | 32 (26–40) | 31 (28–36) | 0.776 |
| HOMA-IR | 1.8 (1.2–2.8) | 2.3 (1.6–3.9) | 0.024 |
| TyG index | 4.7 (4.7–4.8) | 4.8 (4.8–4.9) | 0.132 |
| FL index | 67 (63–80) | 69 (68–83) | 0.066 |
| Fibroscan | 7.2 (5.3–10.1) | 8.5 (6.3–9.7) | 0.683 |
| US score | 1 (0.75–2) | 1 (1–2) | 0.705 |
| Group A | Group B | Group C | p | |
|---|---|---|---|---|
| Weight (kg) | 6% (−) | 7% (−) | 0.5% (−) | A vs. C 0.0001 |
| B vs. C 0.030 | ||||
| A vs. B 0.665 | ||||
| BMI (kg/m2) | 7.5% (−) | 9% (−) | 0.45% (−) | A vs. C 0.0001 |
| B vs. C 0.0001 | ||||
| A vs. B 0.935 | ||||
| Waist circumference (cm) | 2.8% (−) | 6% (−) | 0.3% (−) | A vs. C 0.0001 |
| B vs. C 0.0001 | ||||
| A vs. B 0.030 | ||||
| Hip circumference (cm) | 3.3% (−) | 4% (−) | 0.7% (−) | A vs. C 0.001 |
| B vs. C 0.001 | ||||
| A vs. B 0.206 | ||||
| Fasting glucose (mg/dL) | 3.5% (−) | 11% (−) | 0.5% (−) | A vs. C 0.724 |
| B vs. C 0.006 | ||||
| A vs. B 0.016 | ||||
| Insulin (mU/L) | 10% (+) | 38% (−) | 25% (+) | A vs. C 0.045 |
| B vs. C 0.0001 | ||||
| A vs. B 0.0001 | ||||
| Triglycerides (mg/dL) | 32.16% (−) | 21% (−) | 2.8% (+) | A vs. C 0.001 |
| B vs. C 0.002 | ||||
| A vs. B 0.935 | ||||
| Total cholesterol (mg/dL) | 14.8% (−) | 17% (−) | 9.3% (+) | A vs. C 0.0001 |
| B vs. C 0.0001 | ||||
| A vs. B 0.626 | ||||
| LDL-C (mg/dL) | 15% (−) | 9% (−) | 29% (−) | A vs. C 0.217 |
| B vs. C 0.234 | ||||
| A vs. B 0.705 | ||||
| HOMA-IR | 6.2% (+) | 43% (−) | 46% (+) | A vs. C 0.021 |
| B vs. C 0.001 | ||||
| A vs. B 0.0001 | ||||
| TyG index | 3.3% (−) | 1.2% (−) | 1% (+) | A vs. C 0.020 |
| B vs. C 0.010 | ||||
| A vs. B 0.131 | ||||
| FL index | 19% (−) | 27% (−) | 4.7% (+) | A vs. C 0.017 |
| B vs. C 0.0001 | ||||
| A vs. B 0.626 | ||||
| TE | 21% (−) | 27% (−) | 8.7% (+) | A vs. C 0.001 |
| B vs. C 0.0001 | ||||
| A vs. B 0.053 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenavoli, L.; Greco, M.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients 2017, 9, 870. https://doi.org/10.3390/nu9080870
Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E, Luzza F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients. 2017; 9(8):870. https://doi.org/10.3390/nu9080870
Chicago/Turabian StyleAbenavoli, Ludovico, Marta Greco, Natasa Milic, Francesca Accattato, Daniela Foti, Elio Gulletta, and Francesco Luzza. 2017. "Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study" Nutrients 9, no. 8: 870. https://doi.org/10.3390/nu9080870






