Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
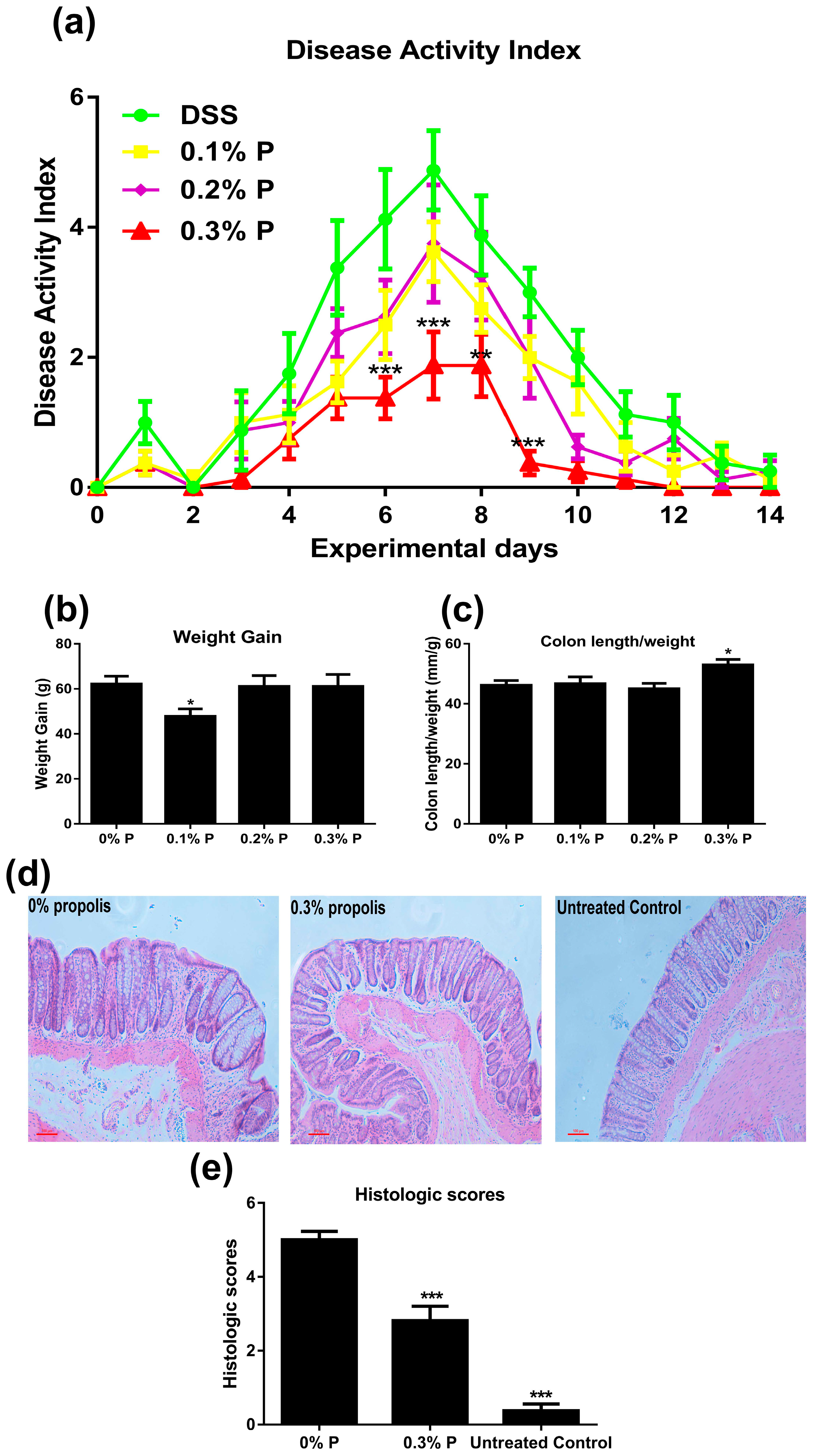
2.2. Disease Activity Index (DAI) Observations
2.3. Histological Analyses
2.4. Short Chain Fatty Acids (SCFA) Analysis
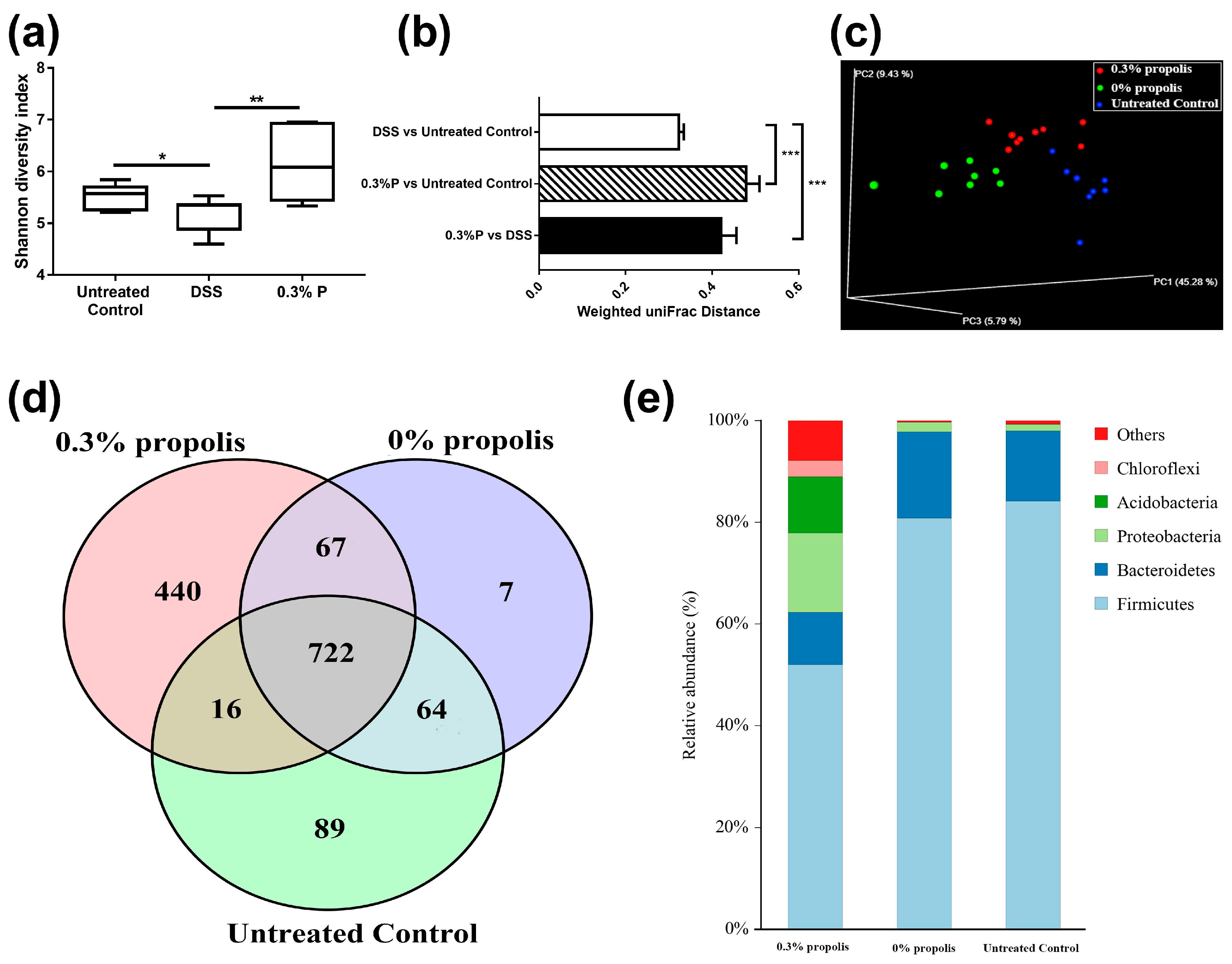
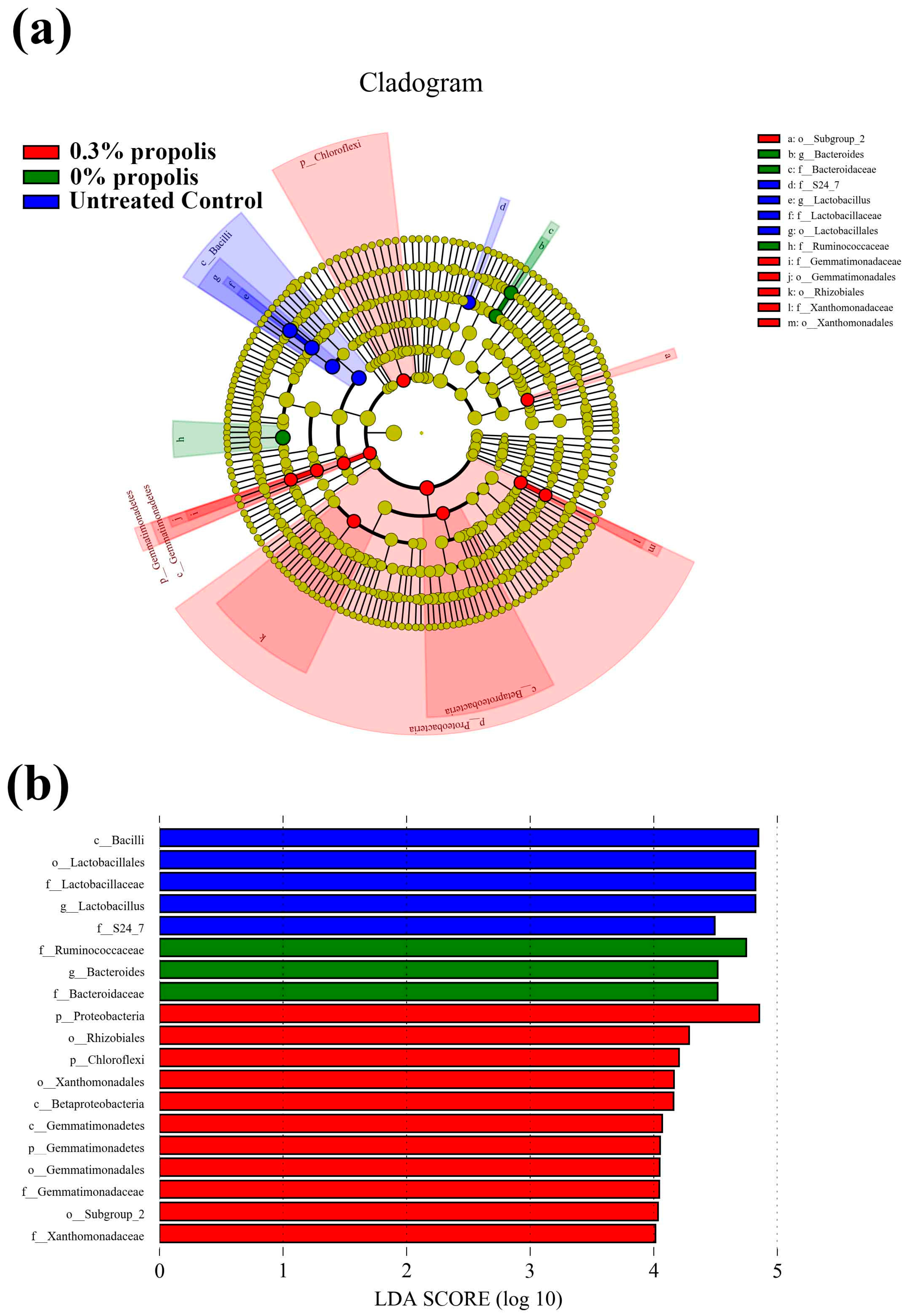
2.5. Measurement of the Gut Microbiota
2.6. Statistical Analyses
3. Results
3.1. Effects of Propolis on Colitis Symptoms
3.2. Effects on SCFA Production
3.3. Propolis Altered the Composition of the Intestinal Microbiota in Colitis Rats
3.4. Key Phylotypes of Gut Microbiota Altered by Propolis Supplement in Colitis Rats
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.E.; Sturino, J.M.; Carroll, R.J.; Rooney, L.W.; Azcarate-Peril, M.A.; Turner, N.D. Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Chang, E.B.; Devkota, S. Diet, microbes, and host genetics: The perfect storm in inflammatory bowel diseases. J. Gastroenterol. 2013, 48, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Richoux, R.; Jardin, J.; Nurdin, M.; Briard-Bion, V.; Parayre, S.; Ferreira, S.; Pot, B.; Bouguen, G.; Deutsch, S.M.; et al. Single-strain starter experimental cheese reveals antiinflammatory effect of propionibacterium freudenreichii in tnbs colitis model. J. Funct. Foods 2015, 18, 575–585. [Google Scholar] [CrossRef]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernándezquintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Yong, K.P. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular mechanisms underlying the in vitro anti-inflammatory effects of a flavonoid-rich ethanol extract from chinese propolis (poplar type). Evid. Based Complement. Altern. Med. 2013, 2013, 127672. [Google Scholar]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (populusxcanadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.K.; Torok, V.A.; Percy, N.J.; Abimosleh, S.M.; Howarth, G.S. Microbial fingerprinting detects unique bacterial communities in the faecal microbiota of rats with experimentally-induced colitis. J. Microbiol. 2012, 50, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Camuesco, D.; Rodríguezcabezas, M.E.; Garridomesa, N.; Cuetosola, M.; Bailón, E.; Comalada, M.; Arribas, B.; Merlos, M.; Balsa, D.; Zarzuelo, A.; et al. The intestinal anti-inflammatory effect of dersalazine sodium is related to a down-regulation in il-17 production in experimental models of rodent colitis. Br. J. Pharmacol. 2012, 165, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, L.; Jin, X.L.; Ma, Q.X.; Marcucci, M.C.; Netto, A.A.L.; Huang, S.; Ren, W.K.; Conlon, M.A. Polyphenol-rich propolis extracts from china and brazil exert anti-inflammatory effects by modulating ubiquitination of traf6 during the activation of nf-κb. J. Funct. Foods 2015, 19, 464–478. [Google Scholar] [CrossRef]
- Ying, H.; Leu, R.K.L.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; Mckinnon, R.; Young, G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar]
- Loher, F.; Schmall, K.; Freytag, P.; Landauer, N.; Hallwachs, R.; Bauer, C.; Siegmund, B.; Rieder, F.; Lehr, H.-A.; Dauer, M.; et al. The specific type-4 phosphodiesterase inhibitor mesopram alleviates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2003, 305, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Kerr, C.A.; Mcsweeney, C.S.; Dunne, R.A.; Shaw, J.M.; Kang, S.; Bird, A.R.; Morell, M.K.; Lockett, T.J.; Molloy, P.L.; et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a western diet. J. Nutr. 2012, 142, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.X.; Fang, D.Q.; Shi, D.; Chen, D.Y.; Yan, R.; Zhu, Y.X.; Chen, Y.F.; Shao, L.; Guo, F.F.; Wu, W.R.; et al. Alterations and correlations of the gut microbiome, metabolism and immunity in patients with primary biliary cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.A.; López-Miranda, V.; Lombó, F.; Abalo, R. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol. Rep. 2016, 68, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohn’s Colitis 2012, 142, 271–279. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. Population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.A.; Topping, D.L. Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating ampk and erk signaling. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.K.; Kwon, H.S.; Kim, Y.H.; Shin, H.K.; Kim, J.K. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem. Biophys. Res. Commun. 2009, 381, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Ji, G.E.; Sung, M.K. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Digest. Dis. Sci. 2012, 57, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; González, R.; Suárez, M.D.; Zarzuelo, A.; Sánchez, M.D.; Zarzuelo, A.; Martínez-Augustin, O. Intestinal anti-inflammatory activity of apigenin k in two rat colitis models induced by trinitrobenzenesulfonic acid and dextran sulphate sodium. Br. J. Nutr. 2015, 113, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Liu, Z.A. Increased cyp4b1 mrna is associated with the inhibition of dextran sulfate sodium-induced colitis by caffeic acid in mice. Exp. Biol. Med. 2009, 234, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.C.; Hernandes, L.; Bersaniamado, C.A.; Franco, S.L.; Silva, J.F.; Natali, M.R. Use of propolis hydroalcoholic extract to treat colitis experimentally induced in rats by 2,4,6-trinitrobenzenesulfonic acid. Evid. Based Complement. Altern. Med. 2013, 2013, 853976. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Hara, T.; Ebato, T.; Fukui, T.; Masuzawa, T. Brazilian propolis ameliorates trinitrobenzene sulfonic acid-induced colitis in mice by inhibiting th1 differentiation. Int. Immunopharmacol. 2013, 16, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Gundamaraju, R.; Shinde, T.; Eri, R. Therapeutic interventions for gut dysbiosis and related disorders in the elderly: Antibiotics, probiotics or faecal microbiota transplantation? Benef. Microbes 2017, 8, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Bibbo, S.; Gasbarrini, A. Gut microbiota modulation: Probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 2014, 9, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Aitken, J.D.; Vijay-Kumar, M.; Gewirtz, A.T. Toll-like receptor–gut microbiota interactions: Perturb at your own risk! Annu. Rev. Physiol. 2012, 74, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Yeom, Y.; Kim, B.-S.; Kim, S.-J.; Kim, Y. Sasa quelpaertensis leaf extract regulates microbial dysbiosis by modulating the composition and diversity of the microbiota in dextran sulfate sodium-induced colitis mice. BMC Complement. Altern. Med. 2016, 16, 481. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.; Schwab, C.; Milinovich, G.; Reichert, J.; Mahfoudh, K.B.; Decker, T.; Engel, M.; Hai, B.; Hainzl, E.; Heider, S.; et al. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012, 6, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T. Alterations in intestinal microflora, faecal bile acids and short chain fatty acids in dextran sulphate sodium-induced experimental acute colitis in rats. Eur. J. Gastroenterol. Hepatol. 2001, 13, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Takemura, N.; Ogasawara, T.; Sasajima, N.; Watanabe, J.; Ito, H.; Morita, T.; Sonoyama, K. Effects of fructo-oligosaccharide on dss-induced colitis differ in mice fed nonpurified and purified diets. J. Nutr. 2010, 140, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.; Debras, E.; Nieto, A.; Concha, A.; Gálvez, J.; López-Huertas, E.; Boza, J.; Obled, C.; Xaus, J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr. 2006, 25, 477–488. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, S.C.; Zeoula, L.M.; Franco, S.L.; Peres, L.P.; Arcuri, P.B.; Forano, E. Antimicrobial activity of brazilian propolis extracts against rumen bacteria in vitro. World. J. Microbiol. Biotechnol. 2013, 29, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, U.; Cheng, H.W.; Applegate, T. Functions of propolis as a natural feed additive in poultry. World’s Poult. Sci. J. 2016, 72, 37–48. [Google Scholar] [CrossRef]
- Roquetto, A.R.; Monteiro, N.E.S.; Moura, C.S.; Toreti, V.C.; de Pace, F.; dos Santos, A.; Park, Y.K.; Amaya-Farfan, J. Green propolis modulates gut microbiota, reduces endotoxemia and expression of tlr4 pathway in mice fed a high-fat diet. Food Res. Int. 2015, 76, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. Ibd—What role do proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-L.; Zhu, Y.-Y.; Ma, Y.-L.; Xiang, Q.-S.; Shen, R.-L.; Liu, Y.-Q. Oat products modulate the gut microbiota and produce anti-obesity effects in obese rats. J. Funct. Foods 2016, 25, 408–420. [Google Scholar] [CrossRef]
- Bloom, S.M.; Bijanki, V.N.; Nava, G.M.; Sun, L.; Malvin, N.P.; Donermeyer, D.L.; Dunne, W.M.; Allen, P.M.; Stappenbeck, T.S. Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 2011, 9, 390–403. [Google Scholar] [CrossRef] [PubMed]
| Components | g/kg Diet |
|---|---|
| Casein | 250 |
| Cornstarch | 350 |
| Sucrose | 100 |
| Fat Blend (Canola and Palm oils) | 200 |
| Wheat bran | 50 |
| l-Cystine | 3 |
| Choline bitartrate | 2.5 |
| Vitamins (AIN 93) | 10 |
| Minerals | 35 |
| Tert-butyl hydroquinol | 0.014 |
| Indices | 0% Propolis | 0.1% Propolis | 0.2% Propolis | 0.3% Propolis |
|---|---|---|---|---|
| Total fat pad (mg/g) | 20.2 ± 1.3 | 18.4 ± 1.0 | 23.0 ± 2.4 | 20.4 ± 1.1 |
| Liver (mg/g) | 40.8 ± 1.3 | 38.5 ± 0.8 | 43.1 ± 2.1 | 40.8 ± 1.1 |
| Spleen(mg/g) | 2.7 ± 0.1 | 2.6 ± 0.1 | 2.9 ± 0.2 | 2.5 ± 0.1 |
| Kidney (mg/g) | 7.6 ± 0.2 | 7.4 ± 0.2 | 7.8 ± 0.4 | 7.4 ± 0.2 |
| Variables | Treatment | |||
|---|---|---|---|---|
| Cecum weights, g | 0% propolis | 0.1% propolis | 0.2% propolis | 0.3% propolis |
| Tissue | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 |
| Digesta | 1.8 ± 0.2 | 1.7 ± 0.1 | 1.8 ± 0.2 | 1.8 ± 0.1 |
| Cecum pH | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.9 ± 0.1 | 7.7 ± 0.1 |
| Pool, μmol | 0% propolis | 0.1% propolis | 0.2% propolis | 0.3% propolis |
| Acetate | 79.6 ± 6.9 a,b | 89.5 ± 5.3 a | 66.8 ± 4.1 b | 74.9 ± 3.5 a,b |
| Propionate | 20.3 ± 1.4 | 19.5 ± 0.8 | 17.2 ± 0.8 | 19.2 ± 0.9 |
| Butyrate | 13.0 ± 1.1 | 12.6 ± 1.3 | 12.1 ± 0.9 | 12.7 ± 0.8 |
| Total SCFA | 116.1 ± 8.9 a,b | 124.7 ± 4.2 a | 99.3 ± 5.1 b | 110.1 ± 4.7 a,b |
| Percentage of total, % | 0% propolis | 0.1% propolis | 0.2% propolis | 0.3% propolis |
| Acetate | 68.2 ± 1.1 | 71.4 ± 2.1 | 67.0 ± 1.5 | 68.1 ± 0.8 |
| Propionate | 17.7 ± 0.7 | 15.8 ± 0.9 | 17.5 ± 0.8 | 17.5 ± 0.5 |
| Butyrate | 11.3 ± 0.7 | 10.3 ± 1.2 | 12.2 ± 0.8 | 11.5 ± 0.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Jin, X.; You, M.; Tian, W.; Leu, R.K.L.; Topping, D.L.; Conlon, M.A.; Wu, L.; Hu, F. Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients 2017, 9, 875. https://doi.org/10.3390/nu9080875
Wang K, Jin X, You M, Tian W, Leu RKL, Topping DL, Conlon MA, Wu L, Hu F. Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients. 2017; 9(8):875. https://doi.org/10.3390/nu9080875
Chicago/Turabian StyleWang, Kai, Xiaolu Jin, Mengmeng You, Wenli Tian, Richard K. Le Leu, David L. Topping, Michael A. Conlon, Liming Wu, and Fuliang Hu. 2017. "Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet" Nutrients 9, no. 8: 875. https://doi.org/10.3390/nu9080875
APA StyleWang, K., Jin, X., You, M., Tian, W., Leu, R. K. L., Topping, D. L., Conlon, M. A., Wu, L., & Hu, F. (2017). Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients, 9(8), 875. https://doi.org/10.3390/nu9080875








