Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli Preparation for Sensory Measurements
2.2.1. Stimuli for Fatty Acid Taste Measurement
2.2.2. Stimuli for Fatty Acid Olfactory Measurement
2.2.3. n-Butanol Threshold Test
2.2.4. Subjective Mouthfeel Measurement Test
2.3. Eating Behaviour and Dietary Intake Questionnaires
2.4. Testing Procedure
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Taste and Olfaction Detection Curves of Oleic Acid
3.3. Fatty Acid Taste Hypo- and Hypersensitivity
3.4. Relationship between Oleic Acid Taste Perception and Olfaction
3.5. Relationship between Oleic Acid Taste Hypo- and Hypersensitivity, Olfaction Detection Rate, n-Butanol Olfactory Threshold, and Eating Behaviour
3.6. Relationship between Oleic Acid Taste Hypo- and Hypersensitivity, Mouthfeel Rating, and Olfaction
3.7. Relationships between Oleic Acid Taste Perception, Oleic Acid Olfaction, Dietary Intake, Mouthfeel Rating, and Eating Behaviour
3.8. Oleic Acid Taste Perception and Olfaction Detection Rate and Body Composition
4. Discussion
4.1. Oleic Acid Taste and Olfactory Detection Rate
4.2. Fatty Acid Taste Hypo- and Hypersensitivity
4.3. Relationship between Oleic Acid Taste Perception and Olfaction
4.4. Oleic Acid Taste Perception and Disinhibited Eating Behaviour
4.5. Oleic Acid Taste Perception and Mouthfeel
4.6. Oleic Acid Taste Perception, Olfaction, and Body Composition
4.7. Oleic Acid Taste Perception, Olfaction, and Dietary Intake
4.8. Additional Strengths and Limitations of this Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trivedi, B.P. Gustatory system: The finer points of taste. Nature 2012, 486, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, B.P. Neuroscience: Hardwired for taste. Nature 2012, 486, S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chang, R.B.; Bushman, J.D.; Tu, Y.H.; Mulhall, E.M.; Wilson, C.E.; Copper, A.J.; Chick, W.S.; Hill-Eubanks, D.C.; Nelson, S.C.; et al. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc. Natl. Acad. Sci. USA 2016, 113, E229–E238. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Preference 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Chen, J.; Eaton, L. Multimodal mechanisms of food creaminess sensation. Food Funct. 2012, 3, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Craig, B.A.; Mattes, R.D. Oleogustus: The Unique Taste of Fat. Chem. Senses 2015, 40, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Costanzo, A. Is fat the sixth taste primary? Evidence and implications. Flavour 2015, 4, 5. [Google Scholar] [CrossRef]
- Chalé-Rush, A.; Burgess, J.R.; Mattes, R.D. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem. Senses 2007, 32, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S.J. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Newman, L.P.; Keast, R.S.J. Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin. Nutr. 2011, 30, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Mattes, R.D.; Tucker, R.M. Fat taste in humans: sources of within- and between-subject variability. Prog. Lipid Res. 2013, 52, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem. Senses 2009, 34, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; Lundström, J.N. Detecting fat content of food from a distance: Olfactory-based fat discrimination in humans. PLoS ONE 2014, 9, e85977. [Google Scholar] [CrossRef] [PubMed]
- Chalé-Rush, A.; Burgess, J.R.; Mattes, R.D. Multiple routes of chemosensitivity to free fatty acids in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1206–G1212. [Google Scholar] [CrossRef] [PubMed]
- Liou, B.K.; Grün, I.U. Effect of fat level on the perception of five flavor chemicals in ice cream with or without fat mimetics by using a descriptive test. J. Food Sci. 2007, 72, S595–S604. [Google Scholar] [CrossRef] [PubMed]
- Cvijanovic, N.; Feinle-Bisset, C.; Young, R.L.; Little, T.J. Oral and intestinal sweet and fat tasting: impact of receptor polymorphisms and dietary modulation for metabolic disease. Nutr. Rev. 2015, 73, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Archer, N.; Duesing, K.; Hannan, G.; Keast, R. Mechanism of fat taste perception: Association with diet and obesity. Prog. Lipid Res. 2016, 63, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Keast, R.S.J. Recent fat intake modulates fat taste sensitivity in lean and overweight subjects. Int. J. Obes. 2012, 36, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.N.; Hendrie, G.A.; Carty, D. Sensitivity, hedonics and preferences for basic tastes and fat amongst adults and children of differing weight status: A comprehensive review. Food Qual. Preference 2015, 41, 112–120. [Google Scholar] [CrossRef]
- Swinburn, B.; Sacks, G.; Vandevijvere, S.; Kumanyika, S.; Lobstein, T.; Neal, B.; Barqueraet, S.; Friel, S.; Hawkes, C.; Kelly, B.; et al. INFORMAS (International Network for Food and Obesity/non-communicable diseases Research, Monitoring and Action Support): Overview and key principles. Obes. Rev. 2013, 14 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, N.R.; López-Díaz, J.A.; Wall-Medrano, A.; Jiménez-Castro, J.A.; Angulo, O. Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiol. Behav. 2014, 129, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. Chemosensory abilities in consumers of a western-style diet. Chem. Senses 2016, 41, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered olfactory acuity in the morbidly obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Jurowich, C.F.; Seyfried, F.; Miras, A.D.; Bueter, M.; Deckelmann, J.; Fassnacht, M.; Germer, C.T.; Thalheimer, A. Does bariatric surgery change olfactory perception? Results of the early postoperative course. Int. J. Colorectal Dis. 2014, 29, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, N.; Johnson, M.C.; Butler, J.L.; Bell, G.A.; Ferguson, K.L.; Fadool, A.R.; Fadool, J.C.; Gale, A.M.; Gale, D.S.; Fadool, D.A. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J. Neurosci. 2014, 34, 6970–6984. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D.; Whittle, A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choquette, A.C.; Bouchard, L.; Drapeau, V.; Lemieux, S.; Tremblay, A.; Bouchard, C.; Vohl, M.C.; Pérusse, L. Association between olfactory receptor genes, eating behavior traits and adiposity: Results from the Quebec Family Study. Physiol. Behav. 2012, 105, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.D.; Welbeck, K. High hunger state increases olfactory sensitivity to neutral but not food odors. Chem. Senses 2011, 36, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.C.H.; Sakimura, J.; May, D.; Breen, C.; Driggin, E.; Tepper, B.J.; Chung, W.K.; Keller, K.L. Fat discrimination: A phenotype with potential implications for studying fat intake behaviors and obesity. Physiol. Behav. 2012, 105, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist, I.A.M.; Poelman, A.A.M.; Cochet-Broch, M.; Delahunty, C.M. Impact of model fat emulsions on sensory perception using repeated spoon to spoon ingestion. Physiol. Behav. 2016, 160, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shen, Y.; Parker, J.K.; Kennedy, O.B.; Methven, L. Relative Effects of Sensory Modalities and Importance of Fatty Acid Sensitivity on Fat Perception in a Real Food Model. Chemosens. Percept. 2016, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, C.; Laureati, M.; Invitti, C.; Pasqualinotto, L.; Bergamaschi, V.; Pagliarini, E. Cross-modal interactions for custard desserts differ in obese and normal weight Italian women. Appetite 2016, 100, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, S.; Hummel, T.; Böhner, C.; Berktold, S.; Hundt, W.; Kriner, M.; Heinrich, P.; Sommer, H.; Hanusch, C.; Prechtl, A.; et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J. Clin. Oncol. 2009, 27, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; Shultz, S.P.; McNaughton, S.A.; Russell, A.P.; Firestone, R.T.; George, L.; Beck, K.L.; Conlon, C.A.; von Hust, P.R.; Breier, B.; et al. Predictors and risks of body fat profiles in young New Zealand European, Māori and Pacific women: Study protocol for the women’s EXPLORE study. Springerplus 2015, 4, 128. [Google Scholar] [CrossRef] [PubMed]
- Von Hurst, P.R.; Walsh, D.C.I.; Conlon, C.A.; Ingram, M.; Kruger, R.; Stonehouse, W. Validity and reliability of bioelectrical impedance analysis to estimate body fat percentage against air displacement plethysmography and dual-energy X-ray absorptiometry. Nutr. Diet. 2015, 73, 197–204. [Google Scholar] [CrossRef]
- Haryono, R.Y.; Sprajcer, M.A.; Keast, R.S.J. Measuring oral fatty acid thresholds, fat perception, fatty food liking, and papillae density in humans. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Keast, R.S.J.; Azzopardi, K.M.; Newman, L.P.; Haryono, R.Y. Impaired oral fatty acid chemoreception is associated with acute excess energy consumption. Appetite 2014, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Panek-Scarborough, L.M.; Dewey, A.M.; Temple, J.L. Sensation and perception of sucrose and fat stimuli predict the reinforcing value of food. Physiol. Behav. 2012, 105, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: An upgrade based on a group of more than 3000 subjects. Eur. Arch. Otorhinolaryngol. 2007, 264, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Denzer, M.Y.; Gailer, S.; Kern, D.W.; Schumm, L.P.; Thuerauf, N.; Kornhuber, J.; Buettner, A.; Buettner, A.; Beauchamp, J. Quantitative Validation of the n-Butanol Sniffin’ Sticks Threshold Pens. Chemosens. Percept. 2014, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoeds, M.H.; de Wijk, R.A.; Zoet, F.D.; Nixdorf, R.R.; van Aken, G.A. How emulsion composition and structure affect sensory perception of low-viscosity model emulsions. Food Hydrocoll. 2008, 22, 631–646. [Google Scholar] [CrossRef]
- Ling, C.H.; de Craen, A.J.; Slagboom, P.E.; Gunn, D.A.; Stokkel, M.P.; Westendorp, R.G.J.; Maier, A.B. Accuracy of direct segmental multi-frequency bioimpedance analysis in the assessment of total body and segmental body composition in middle-aged adult population. Clin. Nutr. 2011, 30, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.E.; Feinle-Bisset, C.; Keast, R.S.J. Fatty acid detection during food consumption and digestion: Associations with ingestive behavior and obesity. Prog. Lipid Res. 2011, 50, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.M.; Mattes, R.D. Influences of repeated testing on nonesterified fatty acid taste. Chem. Senses 2013, 38, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A. High false positive rates in common sensory threshold tests. Atten. Percept. Psychophys. 2014, 77, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Effects of linoleic acid on sweet, sour, salty, and bitter taste thresholds and intensity ratings of adults. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1243–G1248. [Google Scholar] [CrossRef] [PubMed]
- Kallas, O.; Halpern, B.P. Retronasal Discrimination Between Vapor-Phase Long-Chain, Aliphatic Fatty Acids. Chemosens. Percept. 2011, 4, 16–24. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ares, G.; Barreiro, C.; Giménez, A. Comparison of attribute liking and JAR scales to evaluate the adequecy of sensory attributes of milk desserts. J. Sens. Stud. 2009, 24, 664–676. [Google Scholar] [CrossRef]
- Worch, T.; Lê, S.; Punter, P.; Pagès, J. Extension of the consistency of the data obtained with the Ideal Profile Method: Would the ideal products be more liked than the tested products? Food Qual. Preference 2012, 26, 74–80. [Google Scholar] [CrossRef]
- Popper, R. Use of just-about-right scales in consumer research. Nov. Tech. Sens. Charact. Consum. Profiling 2014, 137–155. [Google Scholar]
- Keller, K.L.; Liang, L.C.H.; Sakimura, J.; May, D.; van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring) 2012, 20, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Houston, Z.L. Development and Validation of A Semi-Quantitative Food Frequency Questionnaire to Assess Dietary Intake of Adult Women Living in New Zealand. Master’s Thesis, Massey University, Albany, New Zealand, 2014. [Google Scholar]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Westenhoefer, J. Dietary restraint and disinhibition: Is restraint a homogeneous construct? Appetite 1991, 16, 45–55. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Stunkard, A.J.; Pudel, V. Validation of the flexible and rigid control dimensions of dietary restraint. Int. J. Eat. Disord. 1999, 26, 53–64. [Google Scholar] [CrossRef]
- Bond, M.J.; McDowell, A.J.; Wilkinson, J.Y. The measurement of dietary restraint, disinhibition and hunger: An examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ). Int. J. Obes. 2001, 25, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.; Parnell, W.; Wilson, N. NZ Food: NZ People Key Results of the 1997 National Nutrition Survey NZ Food: NZ People Key Results of the 1997; Ministry of Health: Wellington, New Zealand, 1999. [Google Scholar]
- Quigley, R.; Watts, C. Food Comes First: Methodologies for the National Nutrition Survey of New Zealand; Public Health Group, Ministry of Health: Wellington, New Zealand, 1997. [Google Scholar]
- Ministry of Health. Eating and Activity Guidelines for New Zealand Adults; Ministry of Health: Wellington, New Zealand, 2015. [Google Scholar]
- Da Silva, N.F.; Sichieri, R.; Pereira, R.A.; da Silva, R.M.V.G.; Ferreira, M.G. Reproducibility, relative validity and calibration of a food frequency questionnaire for adults|Reprodutibilidade, validade relativa e calibração de um questionário de frequência alimentar elaborado para adultos. Cadernos Saúde Pública 2013, 29. [Google Scholar] [CrossRef]
- New Zealand Institute for Plant and Food Research. New Zealand FOODfiles (2010) Version 01; New Zealand Institute for Plant and Food Research and Ministry of Health: Palmerston North, New Zealand, 2011. [Google Scholar]
- Keast, R.S.J.; (Deakin University, Burwood, VIC, Australia). Personal communication, 2015.
- Tucker, R.M.; Edlinger, C.; Craig, B.A.; Mattes, R.D. Associations between BMI and fat taste sensitivity in humans. Chem. Senses 2014, 39, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T. A simple alternative analysis for threshold data determined by ascending forced-choice methods of limits. J. Sens. Stud. 2010, 25, 332–346. [Google Scholar] [CrossRef]
- ASTM Standard Practice for Determination of Odor and Taste Thresholds By a Forced Choice Ascending Concentration Series Method of Limits. Available online: http://www.astm.org/Standards/E679.htm (accessed on 4 Feburary 2015).
- Giguère, J.F.; de Moura Piovesana, P.; Proulx-Belhumeur, A.; Doré, M.; de Lemos Sampaio, K.; Gallani, M.-C. Reliability of a Simple Method for Determining Salt Taste Detection and Recognition Thresholds. Chem. Senses 2016, 41, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.I.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is sweet taste perception associated with sweet food liking and intake? Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Kennedy, O.B.; Methven, L. The effect of genotypical and phenotypical variation in taste sensitivity on liking of ice cream and dietary fat intake. Food Qual. Preference 2017, 55. [Google Scholar] [CrossRef]
- Hays, N.P.; Roberts, S.B. Aspects of eating behaviors disinhibition and restraint are related to weight gain and BMI in women. Obesity 2008, 16. [Google Scholar] [CrossRef] [PubMed]
- Baillie, A.G.S.; Coburn, C.T.; Abumrad, N.A. Reversible Binding of Long-chain Fatty Acids to Purified FAT, the Adipose CD36 Homolog. J. Membr. Biol. 1996, 153, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Galindo, M.M.; Voigt, N.; Stein, J.; Van lengerich, J.; Raguse, J.; Hofmann, T.; Meyerhof, W.; Behrens, M. G protein-coupled receptors in human fat taste perception. Chem. Senses 2012, 37, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, T.A.; Khan, N.A. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog. Lipid Res. 2014, 53, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Azize, S.; Selvakumar, S.; Sadou, H.; Besnard, P.; Khan, N.A. Ca2+ signaling in taste bud cells and spontaneous preference for fat: Unresolved roles of CD36 and GPR120. Biochimie 2014, 96, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.P.; Keast, R.S.J. The Test–Retest Reliability of Fatty Acid Taste Thresholds. Chemosens. Percept. 2013, 6, 70–77. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Canon, F. The role of saliva in aroma release and perception. Food Chem. 2017, 226. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.M.; Ludwig, R.G.; Nagai, M.H.; de Almeida, T.J.; Watanabe, H.M.; Hirata, M.Y.; Rosenstock, T.R.; Papes, F.; Malnic, B.; Glezer, L. CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium. Sci. Rep. 2016, 6, 25507. [Google Scholar] [CrossRef] [PubMed]
- Tomassini Barbarossa, I.; Ozdener, M.H.; Melis, M.; Love-Gregory, L.; Mitreva, M.; Abumrad, N.A.; Pepina, M.Y. Variant in a common odorant-binding protein gene is associated with bitter sensitivity in people. Behav. Brain Res. 2017, 329, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Fadel, V.; Hummel, T.; Sommer, J.U. Subjective olfactory desensitization and recovery in humans. Chem. Senses 2014, 39, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Spencer, W.A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966, 73, 16–43. [Google Scholar] [CrossRef] [PubMed]
- Bryant, E.J.; King, N.A.; Blundell, J.E. Disinhibition: Its effects on appetite and weight regulation. Obes. Rev. 2008, 9, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; De Bray, J.G.; Beck, K.L.; Conlon, C.A.; Stonehouse, W. Exploring the relationship between body composition and eating behavior using the three factor eating questionnaire (TFEQ) in young New Zealand women. Nutrients 2016, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Lesdéma, A.; Fromentin, G.; Daudin, J.-J.; Arlotti, A.; Vinoy, S.; Tome, D.; Marsset-Baglieri, A. Characterization of the Three-Factor Eating Questionnaire scores of a young French cohort. Appetite 2012, 59, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Hong, G.; Matsuyama, Y.; Wang, W.; Izumi, S.; Izumi, M.; Toda, T.; Kudo, T.A. Association of oral fat sensitivity with body mass index, taste preference, and eating habits in healthy Japanese young adults. Tohoku J. Exp. Med. 2016, 238, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Haryono, R.; Keast, R. Functionality of fatty acid chemoreception: A potential factor in the development of obesity? Nutrients 2013, 5, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Sensory processing in the brain related to the control of food intake. Proc. Nutr. Soc. 2007, 66, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Jervis, S.M.; Gerard, P.; Drake, S.; Lopetcharat, K.; Drake, M.A. The Perception of Creaminess in Sour Cream. J. Sens. Stud. 2014, 29, 248–257. [Google Scholar] [CrossRef]
- Shin, H.S.; Ingram, J.R.; McGill, A.-T.; Poppitt, S.D. Lipids, CHOs, proteins: Can all macronutrients put a “brake” on eating? Physiol. Behav. 2013, 120, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, S.D.; Braymer, H.D.; Bray, G.A. CD36 mRNA in the gastrointestinal tract is differentially regulated by dietary fat intake in obesity-prone and obesity-resistant rats. Dig. Dis. Sci. 2013, 58. [Google Scholar] [CrossRef] [PubMed]
- Rasoamanana, R.; Darcel, N.; Fromentin, G.; Tomé, D. Nutrient sensing and signalling by the gut. Proc. Nutr. Soc. 2012, 71, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Proserpio, C.; Laureati, M.; Bertoli, S.; Battezzati, A.; Pagliarini, E. Determinants of Obesity in Italian Adults: The Role of Taste Sensitivity, Food Liking, and Food Neophobia. Chem. Senses 2016, 41, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mela, D.J.; Sacchetti, D.A. Sensory preferences for fats: Relationships with diet and body composition. Am. J. Clin. Nutr. 1991, 53, 908–915. [Google Scholar] [PubMed]
- Tucker, R.M.; Nuessle, T.M.; Garneau, N.L.; Smutzer, G.; Mattes, R.D. No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem. Senses 2015, 40, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Chevrot, M.; Passilly-Degrace, P.; Ancel, D.; Bernard, A.; Enderli, G.; Gomes, M.; Robin, I.; Issanchou, S.; Vergès, B.; Nicklaus, S. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am. J. Clin. Nutr. 2014, 99, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.M.; Kaiser, K.A.; Parman, M.A.; George, B.J.; Allison, D.B.; Mattes, R.D. Comparisons of fatty acid taste detection thresholds in people who are lean vs. overweight or obese: A systematic review and meta-analysis. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.P.; Bolhuis, D.P.; Torres, S.J.; Keast, R.S.J. Dietary fat restriction increases fat taste sensitivity in people with obesity. Obesity (Silver Spring) 2016, 24, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Baños, R.; Botella, C.; de la Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Palouzier-paulignan, B.; Lacroix, M.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vanderwoude, E.A.; Sudan, R.; Leopold, D.A.; Thompson, J.S. Gastric bypass does not influence olfactory function in obese patients. Obes. Surg. 2012, 22. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Lai, T.Q.; Nguyen, M.T.T.; Nguyen, T.V. Relationship between body mass index and percent body fat in Vietnamese: Implications for the diagnosis of obesity. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Gemming, L.; Jiang, Y.; Swinburn, B.; Utter, J.; Mhurchu, C.N. Under-reporting remains a key limitation of self-reported dietary intake: An analysis of the 2008/09 New Zealand Adult Nutrition Survey. Eur. J. Clin. Nutr. 2014, 68, 259–264. [Google Scholar] [CrossRef] [PubMed]



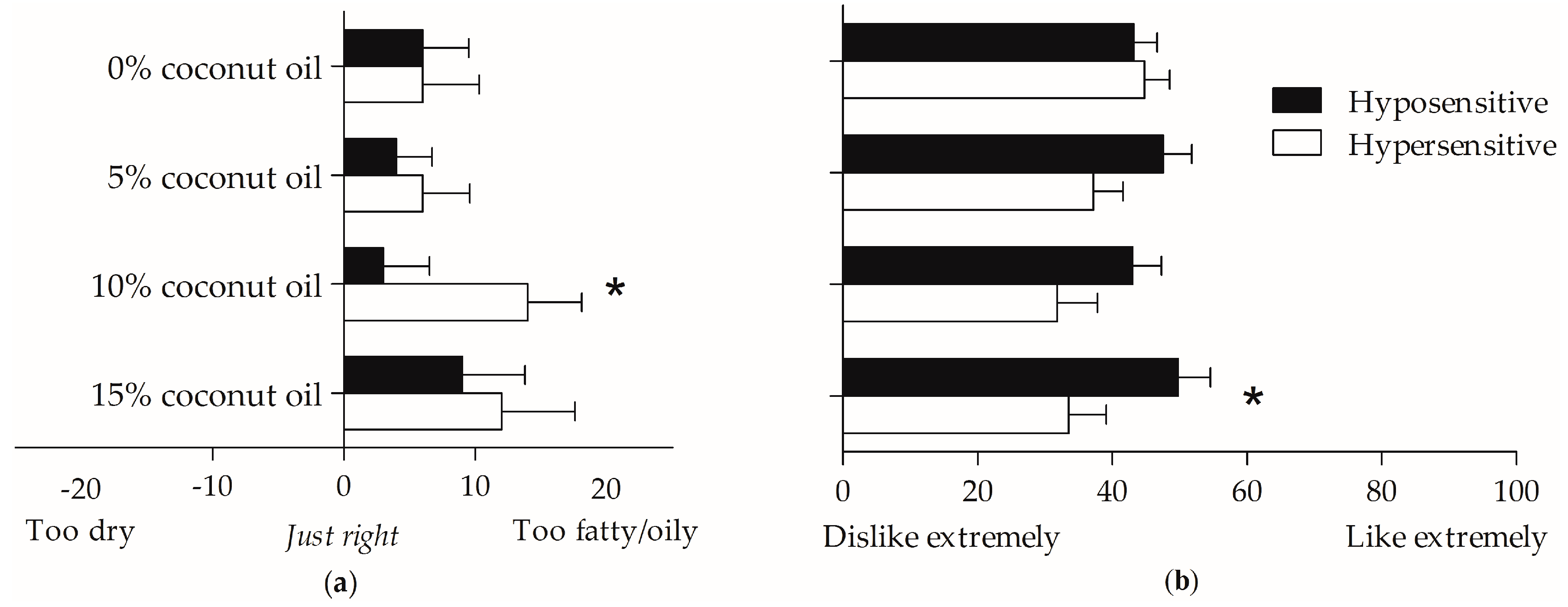
| Measurement | Methods | Reference | Equipment | Outcomes |
|---|---|---|---|---|
| Body composition profile | Anthropometric measurements (height, weight) and BIA | Ling et al., 2011; von Hurst et al., 2015 [35,42] | Direct segmental measurement (DSM) BIA (InBody230, Biospace Co. Ltd., Seoul, Korea). Stadiometer | Body composition -BMI profiling (height and weight) -fat and lean body mass |
| Taste perception oleic acid (C18:1) | 3-AFC procedure ascending method with six correct responses (three at the same concentration and three at consecutively higher concentrations) | Developed in this study with reference to Haryono et al., 2014; Mattes, 2007; Keast et al., 2014; Running, 2014; Stewart et al., 2010; Stewart, Feinle-Bisset, and Keast, 2011; Stewart, Newman, et al., 2011; Tucker and Mattes, 2013 [9,10,36,37,43,44,45,46] | Silverson homogeniser (L4RT) | Sensitivity to oleic acid (C18:1) threshold measurement. Identification of “hypo” or “hypersensitivity” |
| Olfactory perception oleic acid (C18:1) | 3-AFC procedure. Maximum of seven concentration levels | Developed in this study with reference to Boesveldt and Lundström, 2014; Hummel, Sekinger, Wolf, Pauli, and Kobal, 1997; Kallas and Halpern, 2011 [13,47,48] | - | Sensitivity to oleic acid (C18:1) olfactory threshold measurement |
| n-butanol olfactory perception | 3-AFC procedure. 16 concentration levels presented in rising order (pens 16, 14, 12, etc.) | Denzer et al., 2014; Hummel et al., 2007, 1997 [39,40] | Burghart Sniffin’ Sticks smell test | Sensitivity to n-butanol (Sniffin’ Sticks) olfactory threshold |
| Mouthfeel perception | Subjective hedonic and intensity linear scales, JAR scales | Developed in this study with reference to Ares, Barreiro, and Giménez, 2009; Keller et al., 2012; Martínez-Ruiz et al., 2014; Popper, 2014; Worch, Lê, Punter, and Pagès, 2012 [21,49,50,51,52] | - | Subjective rating of mouthfeel (intensity, liking, etc.) |
| Dietary intake | 220-item FFQ | Kruger et al., 2015; Houston, 2014 [34,53] | Analysis using Foodworks 7 2012 (Xyris Software Pty Ltd., Kenmore Hills, Queensland, Australia). Questionnaire completed on SurveyMonkey™ online platform | Daily energy, macronutrient and food group intake |
| Eating behaviour | TFEQ | Stunkard and Messick, 1985 [54] | Questionnaire completed on SurveyMonkey™ online platform | Restraint, disinhibition, and hunger measurement |
| Variable | All (n = 50) |
|---|---|
| Age (year) 1 | 26 (22, 32) |
| Height (cm) 2 | 166 ± 6 |
| Weight (kg) 1 | 67 (57, 76) |
| BMI (kg/m2) 1 | 24 (21, 28) |
| PBF (%) 2 | 30 ± 8 |
| Variable | Hypersensitive (n = 22) | Hyposensitive (n = 28) | p-Value |
|---|---|---|---|
| Detection rate 1 | 3.36 mM (2.14, 5.53) | 12.12 mM (8.91, 19.37) | <0.001 |
| Detection threshold 2 [10,36,37] | 2.58 mM (1.47, 3.35) | 11.10 mM (6.07, 12.73) | <0.001 |
| Hypersensitive (n = 22) | Hyposensitive (n = 28) | TOTAL (n = 50) | p-Value | |
|---|---|---|---|---|
| Oleic acid olfactory detection rate 1 (mM) 2 | 24.2 (11, 61) | 97.3 (24, 181) | 45.4 (16, 158) | 0.041 4 |
| n-butanol threshold score 3 | 9.5 ± 1.8 | 8.1 ± 2.3 | 8.7 ± 2.2 | 0.029 4 |
| Cognitive dietary restraint 2 | 8.0 (4, 11) | 10 (7, 12) | 9.0 (5, 11) | 0.232 |
| Flexible restraint 2 | 3.0 (1, 4) | 3.5 (2, 5) | 3.0 (1.8, 4) | 0.159 |
| Rigid restraint 2 | 2.0 (1, 3) | 3.0 (1.5, 4) | 3.0 (1, 4) | 0.133 |
| Disinhibition 2 | 4.0 (3, 6) | 6.5 (3, 10) | 5.0 (3, 9) | 0.046 4 |
| Habitual susceptibility 2 | 0.0 (0, 1) | 0.5 (0, 2) | 0.0 (0, 1) | 0.197 |
| Emotional susceptibility 2 | 0.0 (0, 1) | 2.0 (0, 3) | 1.0 (0, 2) | 0.029 4 |
| Situational susceptibility 2 | 2.0 (2, 4) | 3.0 (1, 4) | 3.0 (1, 4) | 0.538 |
| Hunger 2 | 3.5 (2, 6) | 4.0 (2, 7.5) | 4.0 (2, 6.3) | 0.313 |
| Internal locus 2 | 2.0 (0, 3) | 2.0 (1, 3) | 2.0 (0, 3) | 0.638 |
| External locus 2 | 1.0 (0, 2) | 2.0 (1, 4) | 1.5 (0.8, 3) | 0.125 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindleysides, S.; Beck, K.L.; Walsh, D.C.I.; Henderson, L.; Jayasinghe, S.N.; Golding, M.; Breier, B.H. Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour. Nutrients 2017, 9, 879. https://doi.org/10.3390/nu9080879
Kindleysides S, Beck KL, Walsh DCI, Henderson L, Jayasinghe SN, Golding M, Breier BH. Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour. Nutrients. 2017; 9(8):879. https://doi.org/10.3390/nu9080879
Chicago/Turabian StyleKindleysides, Sophie, Kathryn L. Beck, Daniel C. I. Walsh, Lisa Henderson, Shakeela N. Jayasinghe, Matt Golding, and Bernhard H. Breier. 2017. "Fat Sensation: Fatty Acid Taste and Olfaction Sensitivity and the Link with Disinhibited Eating Behaviour" Nutrients 9, no. 8: 879. https://doi.org/10.3390/nu9080879





