1. Introduction
Aflatoxin B
1 (AFB
1) is a ubiquitous hepatotoxic, hepatocarcinogenic, and immunosuppressive mycotoxin. Poultry and other livestock are exposed to AFB
1 by consuming contaminated feed. Many agricultural feed commodities (corn, cottonseed, peanuts, and sorghum) and other foods (figs, tree nuts, and spices) are at especially high risk of being contaminated [
1]. AFB
1 is practically unavoidable in most feed ingredients, especially corn [
2,
3,
4], and is expected to concomitantly increase with global climate change [
5]. Approximately 25% of the world’s annual food supply is contaminated with mycotoxins, and losses attributed to AFB
1 are significant to the poultry industry [
1].
Poultry are among the most sensitive animals to the toxic effects of AFB
1 [
6,
7]. Domesticated turkeys are among the most sensitive species [
8], but wild turkeys are more resistant [
9]. Turkey sensitivity is historically important because it was instrumental in the discovery of AFB
1 as being responsible for the deaths of domestic turkeys in Europe due to “Turkey X Disease” that was traced to contaminated feed [
10]. AFB
1 is a potent immunotoxin acting to suppress cell-mediated, humoral, and phagocytic functions in chickens and turkeys [
11,
12,
13]. As a result, it has a wide array of toxic effects, including; reduced feed intake, weight gain, and feed efficiency, and increased mortality, hepatotoxicity, GI hemorrhaging, and susceptibility to bacterial and viral diseases. Embryonic exposure to AFB
1 produces dose-related DNA damage [
14] and compromised immune response through suppression of humoral and cellular immunity making hatched chicks more susceptible to disease [
13]. Thus, in addition to being a potent natural toxin, AFB
1 is a powerful “force-multiplier”, amplifying adverse effects of other agents that are detrimental to poultry health.
Aflatoxin B
1 toxicity requires bioactivation by hepatic cytochrome P450s (CYPs) to the electrophilic exo-AFB
1-8,9-epoxide (AFBO). In the absence of GST activity, AFBO can form adducts that bind to DNA, RNA and other macromolecules, causing immunotoxicity, mutations, and aflatoxicosis [
15]. The extreme sensitivity of domesticated turkeys to AFB
1 is associated with efficient epoxidation by cytochromes P4501A5 and 3A37 [
16], both of which have been cloned, heterologously expressed and functionally characterized [
8,
17]. Using anti-peptide antibodies, P4501A5 was found to be the dominant bioactivating and metabolizing enzyme at environmentally relevant AFB
1 concentrations in turkey liver [
8].
While P450-mediated bioactivation plays an important role, the principal determinant of response to AFB
1 is the efficiency of detoxification by hepatic glutathione S-transferases (GSTs), most notably alpha class (GSTAs) [
18]. The α-GST cluster in turkeys includes six genes,
GSTA1.1-
A1.3,
GSTA2,
GSTA3, and
GSTA4 [
19,
20]. Whereas, wild and heritage breed turkeys possess GST-mediated AFBO detoxification activity, livers from domestic turkeys lack detectable activity [
20]. Thus, the most likely mechanism for the extreme sensitivity of domestic turkeys is dysfunction in hepatic GSTs, rendering them unable to detoxify AFB
1 [
21,
22,
23]. As a result, AFBO forms adducts, which can induce DNA mutations, block transcription and alter translation [
24,
25].
To understand the response of the domesticated turkey to AFB
1 exposure, we initiated study of the hepatic transcriptome following dietary AFB
1 challenge. Results of this study identified genes and gene pathways in the liver directly affected by AFB
1 [
26]. Functional analysis found transcripts significantly dis-regulated by toxicity and affecting pathways of cancer, apoptosis, cell cycle, and lipid regulation. These changes reflect the molecular mechanisms of inflammation, proliferation and liver damage in aflatoxicosis. This study was followed by analysis of spleen tissues from the same birds [
27] that found short exposure to AFB
1 suppressed innate immune transcripts, especially from antimicrobial genes that are indicative of either increased cytotoxic potential or activation-induced cell death in the spleen during aflatoxicosis.
To better examine the differences between wild and domesticated birds, we developed an
in ovo exposure model to provide controlled AFB
1 exposure to developing embryos [
28]. RNA-seq analysis found AFB
1 effects were dependent on both length of exposure and turkey type (domesticated vs. wild), confirming significant differences in the response to AFB
1 attributed to genetic background [
28]. Transcriptome responses to AFB
1 occurred more rapidly in domesticated birds (1 day post-exposure), and led to the up regulation in cell cycle regulators, Nrf2-mediated response genes and coagulation factors. Expression changes in the embryonic liver also suggested cellular responses to oxidative stress and xenobiotics were initiated by AFB
1 exposure. In contrast, the response in wild turkey embryos occurred later (five days post-exposure). Combined, these studies demonstrated that GST-mediated hepatic detoxification of AFBO is largely responsible for the differences in resistance between turkey types, but other processes and pathways (i.e., apoptosis, cellular regulation, immune responses) are also important. Whereas, understanding the effects of AFB
1 on developing embryos is important in poultry production, the manifestation of AFB
1 toxicity is likely to be different in more mature birds with fully developed gastrointestinal systems. The purpose of this study was to compare the hepatic transcriptome response to dietary AFB
1 in juvenile (three weeks of age), susceptible (domesticated), and more resistant (wild) turkeys. We hypothesized that transcriptome responses in juvenile birds would reflect the more mature status of the gastrointestinal and antioxidant systems than those of embryos.
3. Discussion
When compared to their domestic relatives, wild turkeys are relatively resistant to aflatoxicosis. This difference is largely due to functional hepatic GSTA-mediated detoxification activity of the bioactive electrophilic AFBO intermediate that is completely lacking in domesticated birds [
20]. The present data indicates other pathways may also account for difference in AFB
1 susceptibility, such as cellular regulation, modulation of apoptosis, inflammatory responses, and other pathways relevant to AFB
1 pathogenesis. The liver is the principal organ of AFB
1 bioactivation and detoxification [
6,
21,
22,
24,
45]. In turkeys, AFB
1 causes reduced feed intake, weight gain, and immunological function in a dose-dependent fashion [
46,
47]. Dietary exposure in poultry causes lipid accumulation, resulting in hepatomegaly and increases in liver:body weight ratios [
48,
49,
50]. During the 14 day exposure, decreased relative liver mass initially occurred in both EW and DT consistent with that observed in chickens [
49] and wild turkeys [
9].
Numerous significant DEGs occurring in the livers of AFB
1-treated birds have potential roles in lipid metabolism or accumulation. AFB
1 is known to alter lipid metabolism and increase lipid content resulting in pale or yellowed pigmentation [
46]. Dietary AFB
1 primarily down regulated several hepatic apolipoprotein genes (cofactors in lipid binding and transport) in the turkey, and dis-regulation of genes, such as
ANGTPL3, would have direct effects on lipids. Significant up regulation of
ANGTPL3 was observed for both EW and DT birds treated with AFB
1. This would likely stimulate synthesis of plasma triglycerides (TG) via the inhibition of lipoprotein lipase (LPL) activity. In both AFB
1-treated groups,
LPL was significantly down regulated (log
2FC = −2.905 and −6.032 in EW and DT birds, respectively). LPL functions in the hydrolysis of triglycerides in lipoproteins and is essential to lipid metabolism and storage. Significant down regulation of
LPL was also observed in our previous analyses of AFB
1-treated domesticated Orlopp turkeys [
26] and decreased expression of LPL occurs in AFB
1-treated chickens [
50].
As expected, the significant hepatic DEGs included the Phase I and II detoxifying enzymes that we have shown are relevant to AFB
1 exposure in turkeys (
Table 6). Previous studies have demonstrated efficient epoxidation by hepatic turkey cytochromes CYP1A5 and CYP3A37 [
16]. At environmentally-relevant hepatic concentrations (<50 uM) CYP1A5 bioactivates the majority (~98%) of AFB
1 [
17,
21], whereas CYP3A37 predominates at much higher substrate concentrations unlikely to be achieved in the livers of exposed animals [
16]. Based on RNA-seq, it is clear that dietary AFB
1 significantly down regulated
CYP1A5 in both EW and DT birds, but more significantly so in DT. This result is at odds with our earlier findings in another strain of DT (Orlopp) where almost no expression change was observed for
CYP1A5 and
CYP3A37, and where none of the transcripts associated with CYP genes had significant DE as a result of AFB
1 treatment [
26]. Significant down regulation of
CYP1A5 in response to AFB
1 was also observed in ducks, another avian species with high AFB
1 susceptibility [
51]. Several other P450 genes in addition to
1A5 and
3A37 had significant DE in the present study (
Table 6), including both
CYP2W1 and
CYP2K1. Interestingly, these genes have been shown in other species to activate AFB
1 into cytotoxic products [
52,
53]. We have found
CYP2W1-like transcripts to have significant DE in DT embryos challenged with AFB
1 [
28]. Down regulation of
CYP1A5 in both EW and DT birds could affect their overall ability to bioactivate AFB
1. However, as this expression change was seen in both bird types, it does not account for the differences seen in AFB
1 susceptibility [
16].
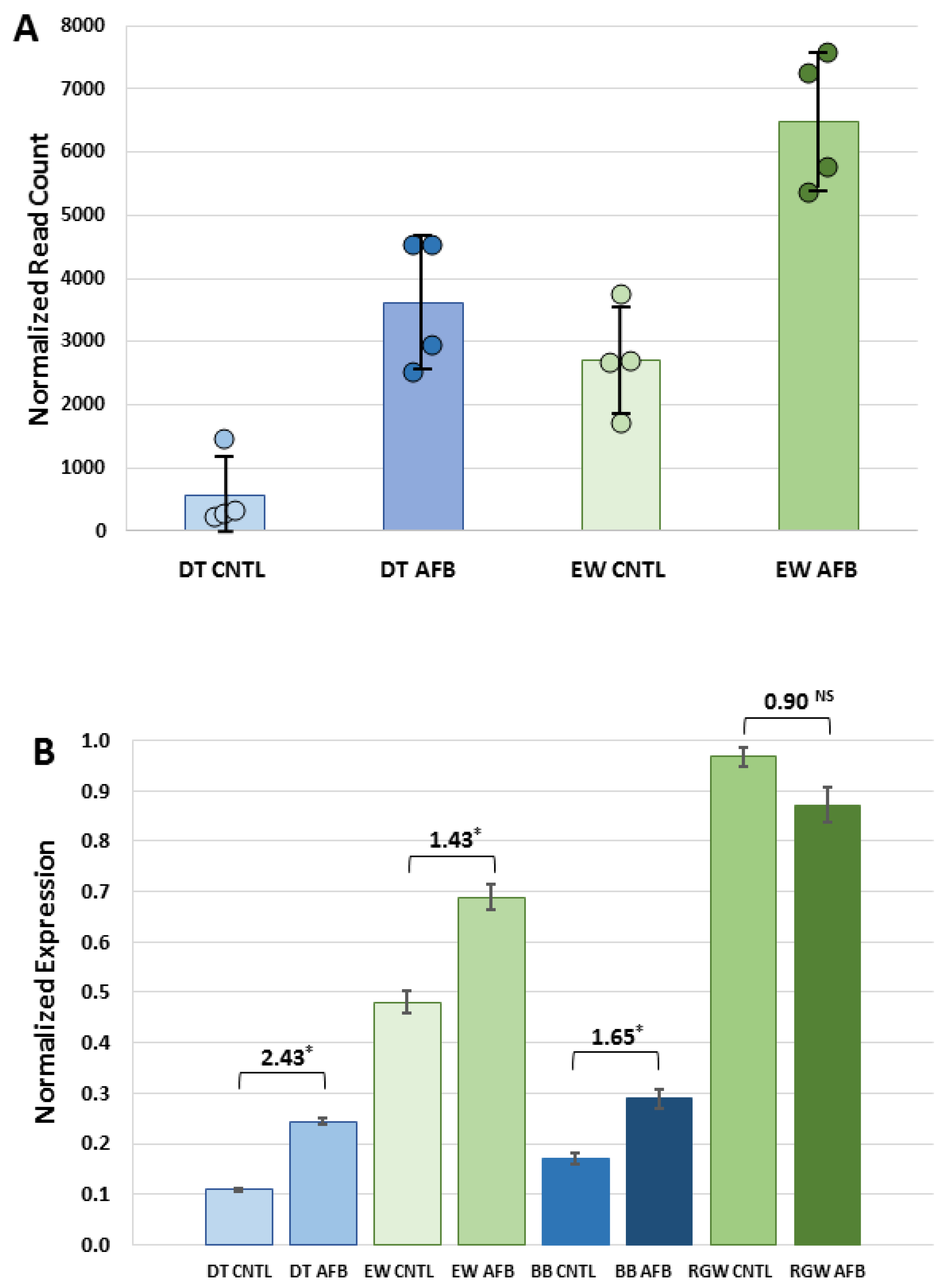
Expression of GSTs with affinity toward AFBO is a known predictor of relative AFB
1 resistance [
20]. Constitutive expression of
GSTA3, the ortholog to the putative AFB
1-protective GSTA3 isoform in mice [
18] was significantly higher in EW than in DT birds. Dietary AFB
1 caused significant down-regulation of hepatic α-class GSTs, with the exception of
GSTA3, where increased expression of this isoform was observed in the AFB
1-treated DT group. This pattern was also observed in the qRT experiments of other wild (RGW) and domesticated (BB) turkeys. A similar pattern of
GSTA3 expression in response to AFB
1 was also observed in turkey embryos early after exposure, where small increases were observed in DT [
28] and in ducks [
51].
Expression of
GSTA3 mRNA in turkeys is not correlated with AFB
1 sensitivity in that domesticated birds lack hepatic GST-mediated AFBO conjugating activity [
19], despite expression of
GSTA3. Hepatic cytosols isolated from wild turkeys possess functional AFBO-trapping GSTs [
20]. While hepatic GSTs in DT lack detoxification activity, with or without AFB
1 treatment, increased
GSTA3 expression in DT in response to AFB
1 may reflect a greater inflammatory response or perhaps an indicator of hepatocyte injury. Although GSTs are toxicologically important for their role in “trapping” electrophilic intermediates by conjugating with the nucleophilic GSH, they may also play a role in cell signaling through binding of non-substrate ligands to mediate cell proliferation and cell death [
54]. Up regulation of
GSTAs may also reflect antioxidant functions as AFB
1, exposure in poultry can lead to oxidative stress and lipid peroxidation [
55,
56]. When combined, these results support the hypothesis that the greater ability of wild turkeys to detoxify AFB
1 is related to higher constitutive expression of
GSTA3, coupled with an inherited (genetic) difference in functional expression in domesticated birds. Expression in these
CYP and
GSTAs suggests that the physiological response to AFB
1 is mediated through genes not experimentally linked in the turkey to AFB
1 metabolism.
Up regulation of transcription factors and metabolic inhibitors characterized the shared response to AFB
1. Taken together, these are genes that comprise the molecular mechanisms underlying aflatoxicosis. Recurrent themes amongst the many DEGs of AFB
1-treated birds are linked by functional analysis to inflammation, apoptosis, the cell cycle (cancer), or lipid regulation, suggesting common underlying regulation. For example, recent studies of AFB
1-induced hepatocellular carcinoma have examined regulatory ncRNAs (miRNA and lncRNA) [
57,
58]. Studies in the rat, another AFB
1-susceptible species, have found coincident DE of transcripts that are related to these same functions and specific lncRNAs in hepatocellular carcinomas [
59,
60]. Our study of miRNA expression in the same turkey liver tissues used in the present study is currently underway (Coulombe, unpublished).
Transcriptome analysis not only includes genes responding to the presence of AFB
1, but also reveals genes dis-regulated as a response to toxic insult. Significant up regulation was seen for several vasoactive peptides, including, neuropeptide Y (
NPY), somatostatin (
SST), substance P (tachykinin,
TAC1), and vasoactive intestinal peptide (
VIP), suggesting altered sinusoidal blood flow with AFB
1 treatment. Also, affected were extracellular matrix proteins including glycoproteins (e.g., HAPLN1 and HAPLN3), protein receptors (
KERA,
LAMB3,
LUM,
LRRN2, and
LRRN3), proteinase inhibitors (
TIMP4), signaling molecules (
SFRP1,
Wnt6 and
7a), and structural proteins (
COL10A1,
FRAS1). Expression of the majority of the ADAM metallopeptidases was altered in AFB
1 treatment (
Table S2). Some of these proteases are thought to be involved in regulating matrix degradation [
61]. Unique response in the EW birds was seen in genes that negatively regulate cellular processes, components of the extracellular matrix and accumulation of coagulation factors. DT birds showed greater up regulation of genes responding to inflammation, which was likely due to the reduced ability to detoxify AFBO. Dis-regulation of extracellular matrix proteins is a resulting effect of chronic liver injury [
32]. Aflatoxin inhibits cell-mediated immunity in domestic poults [
47,
62] with the suppression of lymophoblastogenesis [
9], T-helper, or cytotoxic T-cell activity [
63].
Multiple genes involved in pathways of coagulation (
FGA,
FGB,
FGG,
F9,
HRG,
SERPINA10, and
SERPINC1) were expressed at higher levels in EW as compared to DT, where they were among the genes with the highest negative fold change. Lower expression of coagulation factors was also seen in livers of domesticated turkey embryos after just 5 days of exposure to AFB
1 [
28]. AFB
1 has been shown to increase blood clotting times in poultry [
64,
65] and activities of coagulation factors, such as F9, were reduced by dietary AFB
1 in chickens [
50,
65]. Effects on hemostasis are more dramatic in turkeys than chickens [
66]. In comparison, only small non-significant increases in prothrombin times were seen in wild turkeys exposed to AFB
1 [
9], which is consistent with the gene expression patterns observed in the liver transcriptomes.
In a previous comparison of EW and DT after
in ovo exposure [
28], we used RNA-seq to examine gene expression responses to AFB
1 in the embryonic hepatic transcriptomes and identified gene expression effects dependent on exposure time and turkey type. Most notable in turkey embryos was the more rapid response of the DT, which was likely due to their lack of GST activity towards the AFBO-epoxide. The present study was designed to contrast gene expression responses in the hepatic transcriptome of growing domesticated and wild turkeys during AFB
1 exposure. In conclusion, our findings emphasize the differential response of these genetically distinct birds, demonstrating significant differences in expression of Phase I and Phase II genes and in genes important in cellular regulation, modulation of apoptosis, and inflammatory responses. The molecular basis for the differences in AFB
1 detoxification observed between EW and DT birds, and the mechanism of GSTA silencing in DT remain under investigation.
4. Materials and Methods
This study used two turkey subspecies previously demonstrated to vary in AFB
1-detoxifying GST activity. Eggs from domesticated (DT = Nicholas) and a wild subspecies (Eastern wild = EW,
Meleagris gallopavo silvestris) were obtained from Privett Hatchery (Portales, NM, USA) and hatched at Utah State University. Birds were sexed by PCR [
67]. Male turkey poults were maintained on an
ad libitum standard grow-up soy-based diet and acclimated to the facility for two weeks. At the end of this period, males from each line (
n = 8 for EW and
n = 10 for DT) were equally assigned to one of two treatment groups and subjected to a short-term AFB
1-treatment protocol [
21,
68]. For the AFB
1 treatment, the diet of challenge birds was amended beginning on day 15 with 320 ppb AFB
1 (Sigma-Aldrich, Inc., St. Louis, MO, USA) that continued for 14 days. Control birds continued on the standard diet with AFB
1 levels below detection limits (<10 ppb), based on testing of 50 g of feed extracted and cleaned using Mycosep 112 AflaZON cleanup columns (Romer Labs., Union, MO, USA), and examined by HPLC. Birds were weighed three times per week and feed and water availability checked daily. At the conclusion of the 14 day challenge period, birds were sacrificed by CO
2 asphyxiation and blood collected by cardiac puncture for DNA and serological analysis. Livers were removed, examined, weighed, sampled, and fixed in neutral buffered formalin for histological examination. Portions of the liver tissues infused with RNAlater (ThermoFisher Scientific, Waltham, MA, USA) for RNA-Seq analysis. All of the procedures were under the authority and institutional approval of Utah State University’s Animal Use and Care Committee. Ethical approval code: 2670, Date of approval: 26 September 2016.
4.1. RNA Isolation and Sequencing
Total RNA was isolated from each sample by TRIzol extraction (Ambion, Inc., Foster City, CA), DNase-treated (Turbo DNA-freeTM Kit, Ambion, Inc.), and stored at −80 °C. Initial RNA concentration and quality was assessed by spectrophotometry (Nanodrop 8000). RNA samples were submitted for library preparation and sequencing at the University of Minnesota Genomics Center (UMGC). Replicate samples were sequenced from each treatment group (n = 4). Each sample was quantified by RiboGreen assay (Invitrogen Corp., Carlsbad, CA, USA) and RNA integrity confirmed on a 2100 Bioanalyzer (Aligent Technologies, Santa Clara, CA, USA). Each sample had clear 18S and 28S peak separation on the electropherograms and an average RNA Integrity Number (RIN) of 6.3. Indexed libraries (n = 16) were constructed with 1 μg of total RNA/sample with the TruSeq RNA Sample Preparation Kit version 2 (Illumina, Inc., San Diego, CA, USA) and size selected for approximately 200 bp inserts. Libraries were multiplexed, pooled, and sequenced over two lanes on the HiSeq 2000 using v3 chemistry (Illumina, Inc.) to produce 101-bp paired-end reads. Data are deposited in the NCBI Gene Expression Omnibus (GEO) repository as part of SRA BioProject 346253.
4.2. RNA-seq Data Analyses
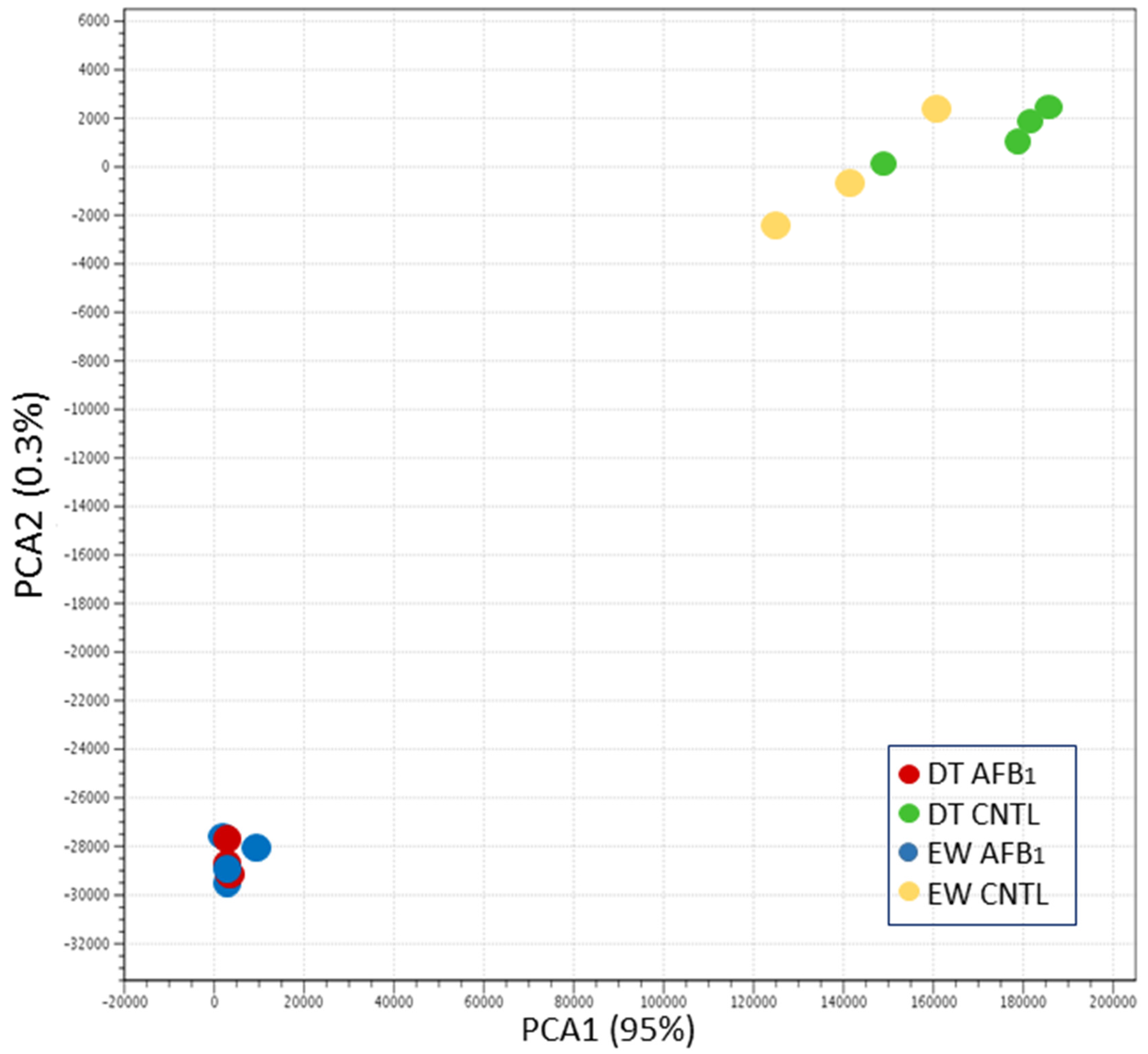
Sequence reads were groomed (Trimmomatic, [
69]) and quality checked (FastQC, [
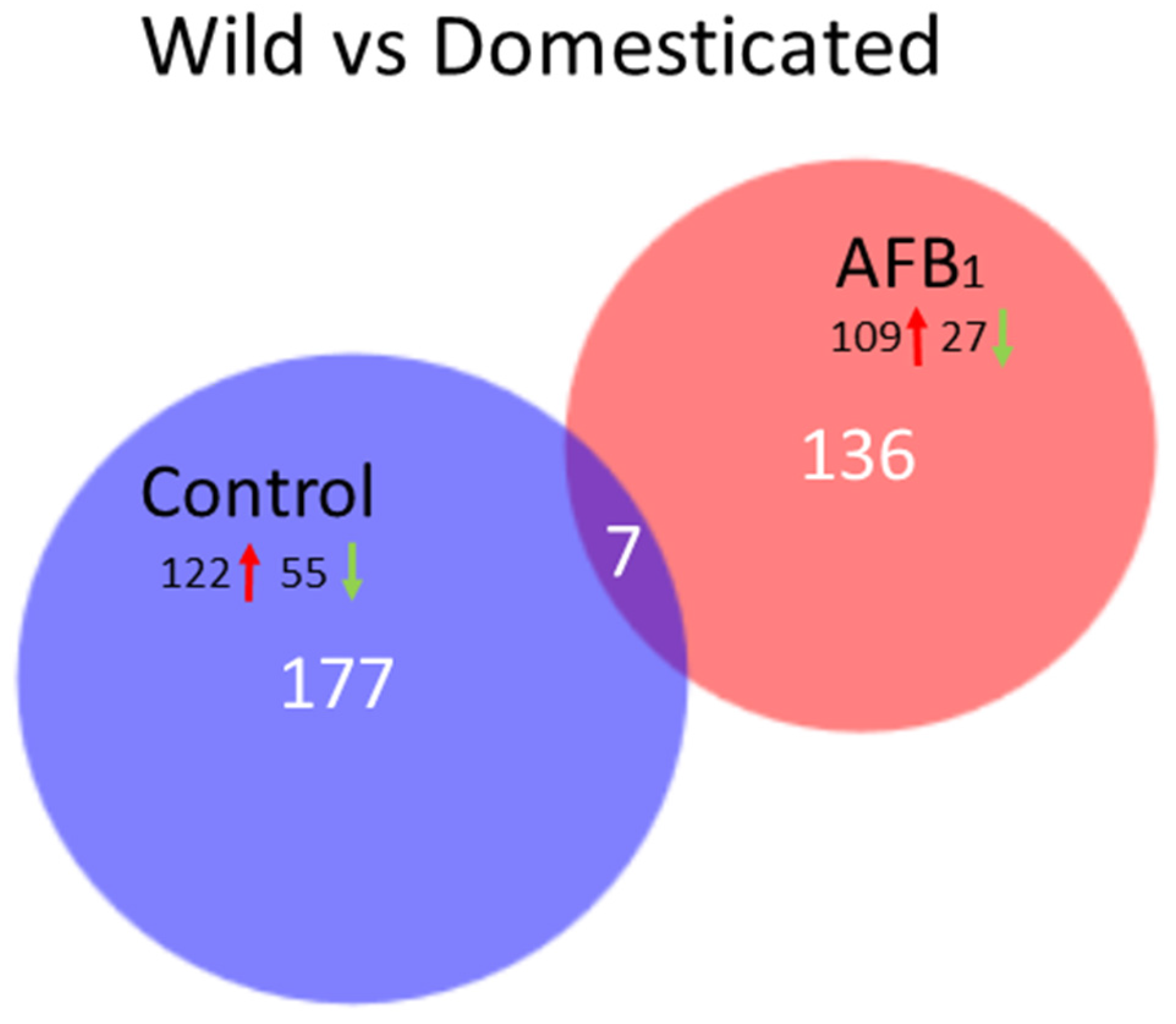
70]) prior to read mapping (Bowtie v2.2.4.0) on the turkey genome (UMD 5.0, NCBI Annotation 101). Read counts were normalized in CLC Genomics Workbench (CLCGWB v. 8.0.2, CLC Bio, Aarhus, Denmark) by dividing the total read counts by the group sample sum with the results being expressed as reads per 12.2 M. Hierarchical clustering of samples was performed (based on Euclidean sample distances with single linkage) in CLCGWB. Principal component analysis (PCA), Volcano plots, and Venn diagrams were used to visualize expression data and the results of significance testing. Empirical analysis of differential gene expression and ANOVA were performed in CLCGWB on EdgeR-normalized read counts. Pair-wise comparisons between treatment groups were made in CLCGWB following the standard workflow Wald test with multi-comparison
p-values < 0.05 being considered as significant (Bonferroni and FDR corrected). In each pair-wise comparison, significant DE genes were used to investigate affected gene pathways using Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA, USA). Gene enrichment tests were performed using the PANTHER Overrepresentation Test (GO Consortium release 20150430, [
38]).
4.3. Quantitative Real-Time PCR
To more broadly examine the expression profile response of GSTA3 to dietary AFB1, quantitative real-time PCR (qRT-PCR) was performed on both domesticated and wild turkey liver samples. Samples included AFB1-treated and control animals (six per group) from the domesticated Nicholas turkey (DT) and Eastern Wild (EW) experiment, plus AFB1-treated and control animals (six per group) of a parallel experiment that included domesticated Broad Breasted White (BB), and birds of the Rio Grande subspecies (RGW, M. g. intermedia) of wild turkey. Four of the six samples for the DT and EW groups were in common with the RNA-seq study.
Briefly, cDNA was synthesized from DNase-treated liver mRNA (TRIzol extracted) using Invitrogen Super Script IV First-strand synthesis kit (Invitrogen, Carlsbad, CA, USA). Expression analysis of gene-specific amplicons was performed with the iTaq Universal SYBR Green Supermix (BioRad, Hercules, CA, SA) with the CFX96 touch real time detection system (BioRad, Hercules, CA, USA). Primers were designed within Primer3 software (
http://www.ncbi.nlm.nih.gov/tools/primer-blast) from accessioned genomic DNA sequence (NM_001303157.1) to span an exon/exon junction and at least one intron in the amplicon. RefFinder software was utilized to determine the most stable reference gene. Several normalizing genes were tested for uniformity between treatments and lines and RNA polymerase II subunit D (
POLR2D, XM_003208947) was found to have the highest stability value (0.848). Target gene reactions were conducted in triplicate, and
POLR2D, no template and gDNA controls were run in duplicate. All of the reactions included a disassociation curve to confirm a single product and to preclude the possibility of dimers amplifying. Expression in each RNA sample was normalized first to the control gene
POLR2D. Results were interpreted using the Double Delta Ct Analysis (ΔΔCt, [
71]) and a comparative Ct approach. Expression analysis was performed within the Biorad CFX Maestro software package following the standard ΔΔCt workflow.