The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis
Abstract
:1. Introduction
2. Results
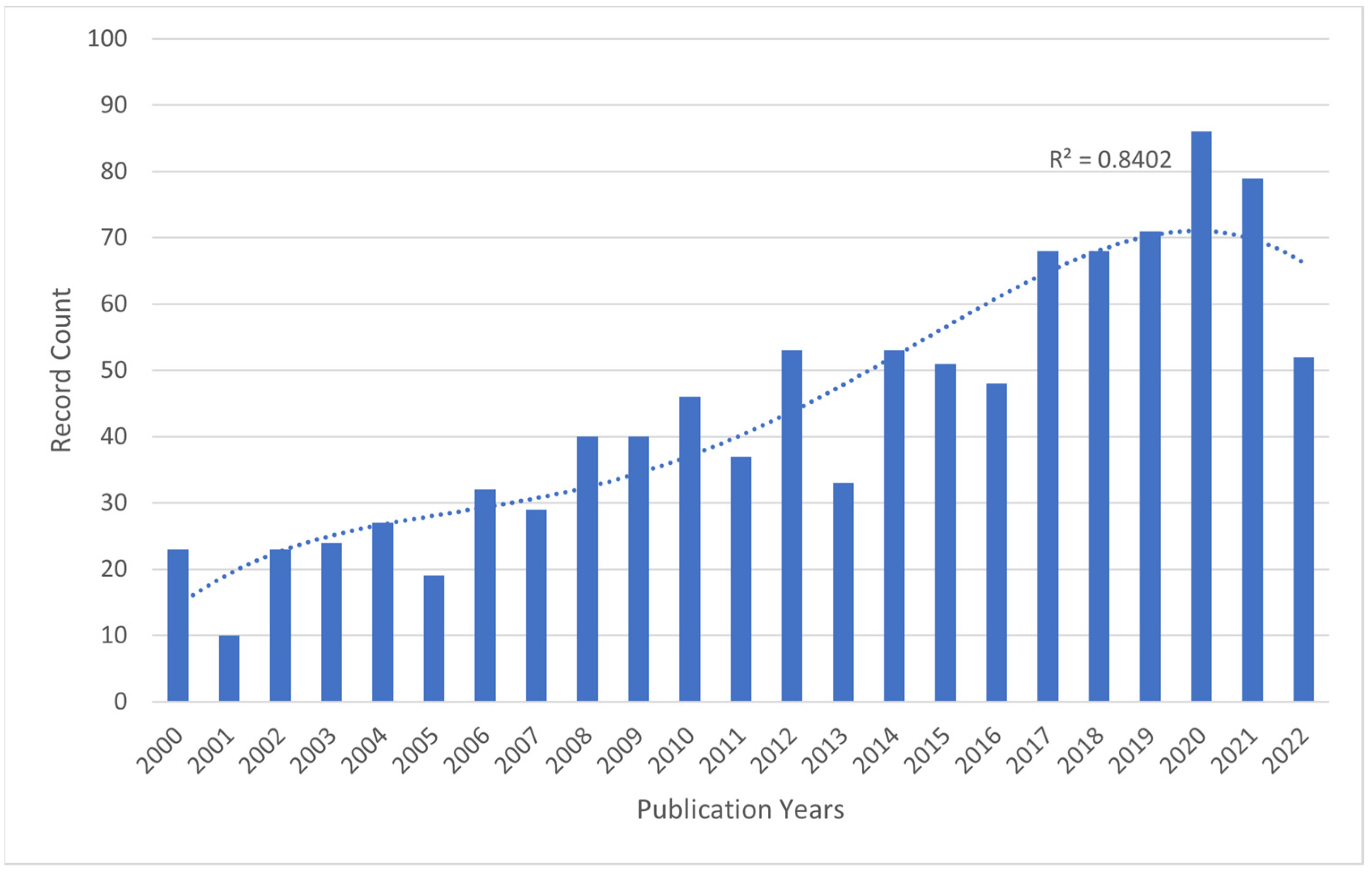
2.1. Publication Outputs and Time Trend
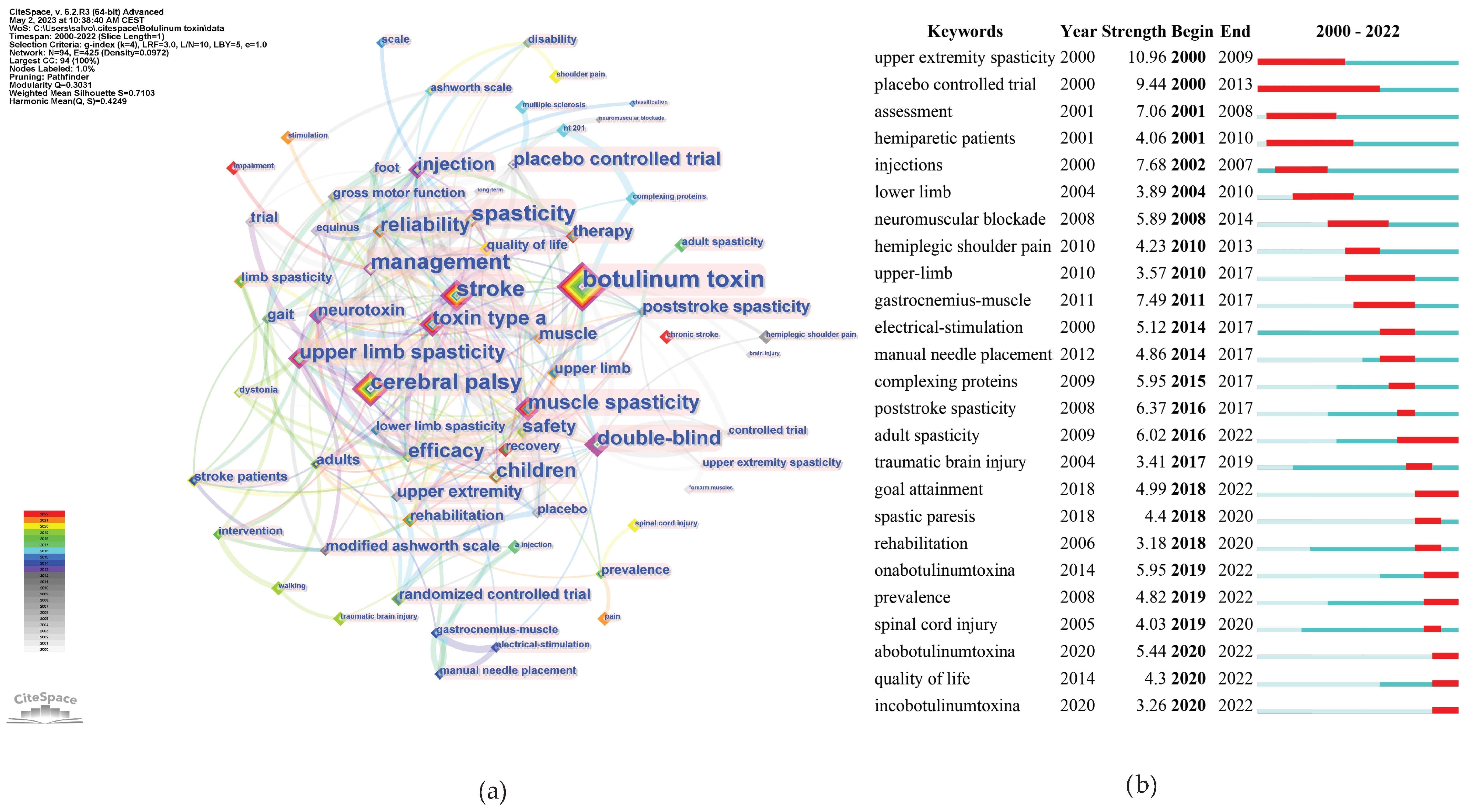
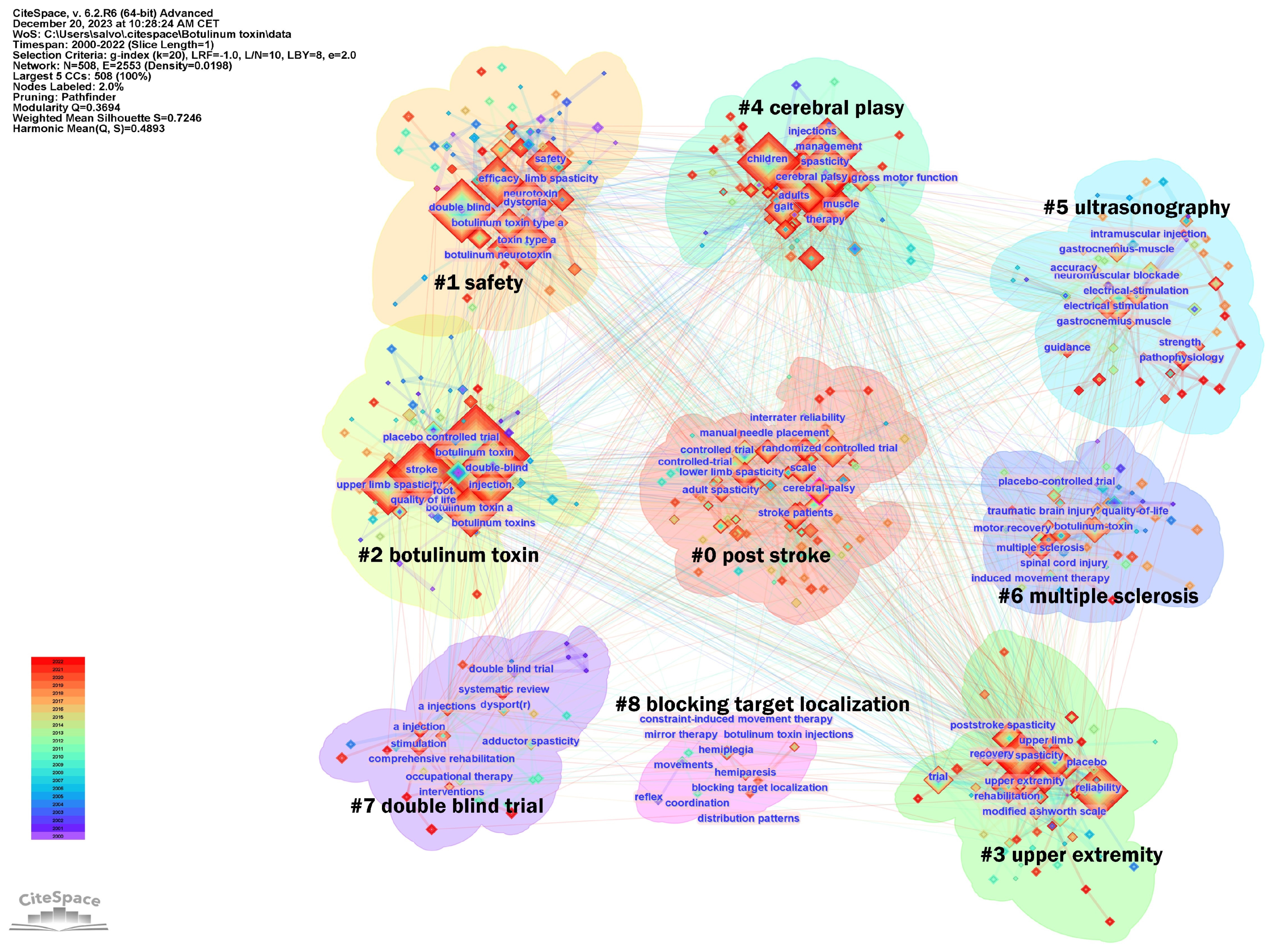
2.2. Hot Topics in Literature Research
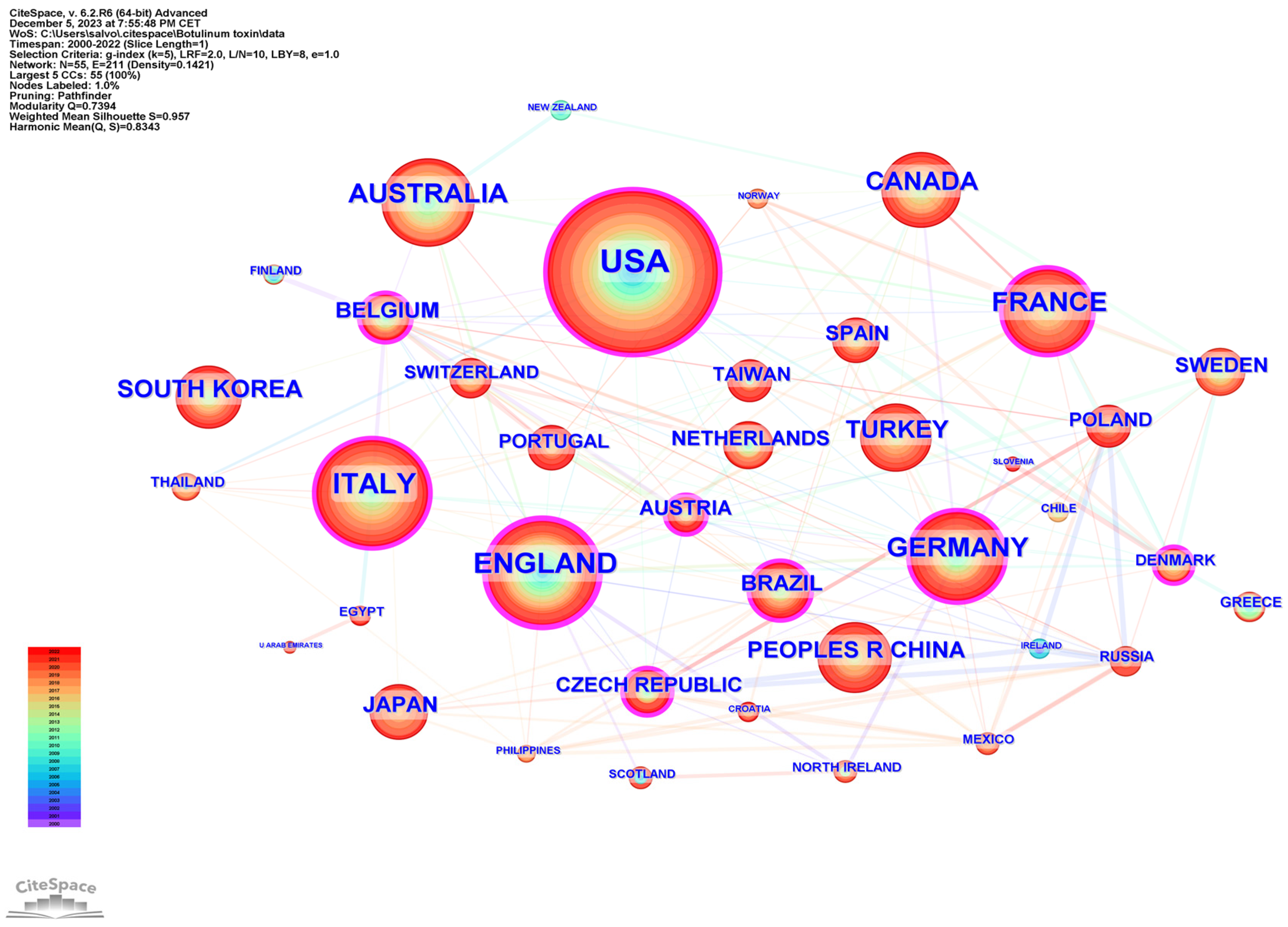
2.3. Country Analysis
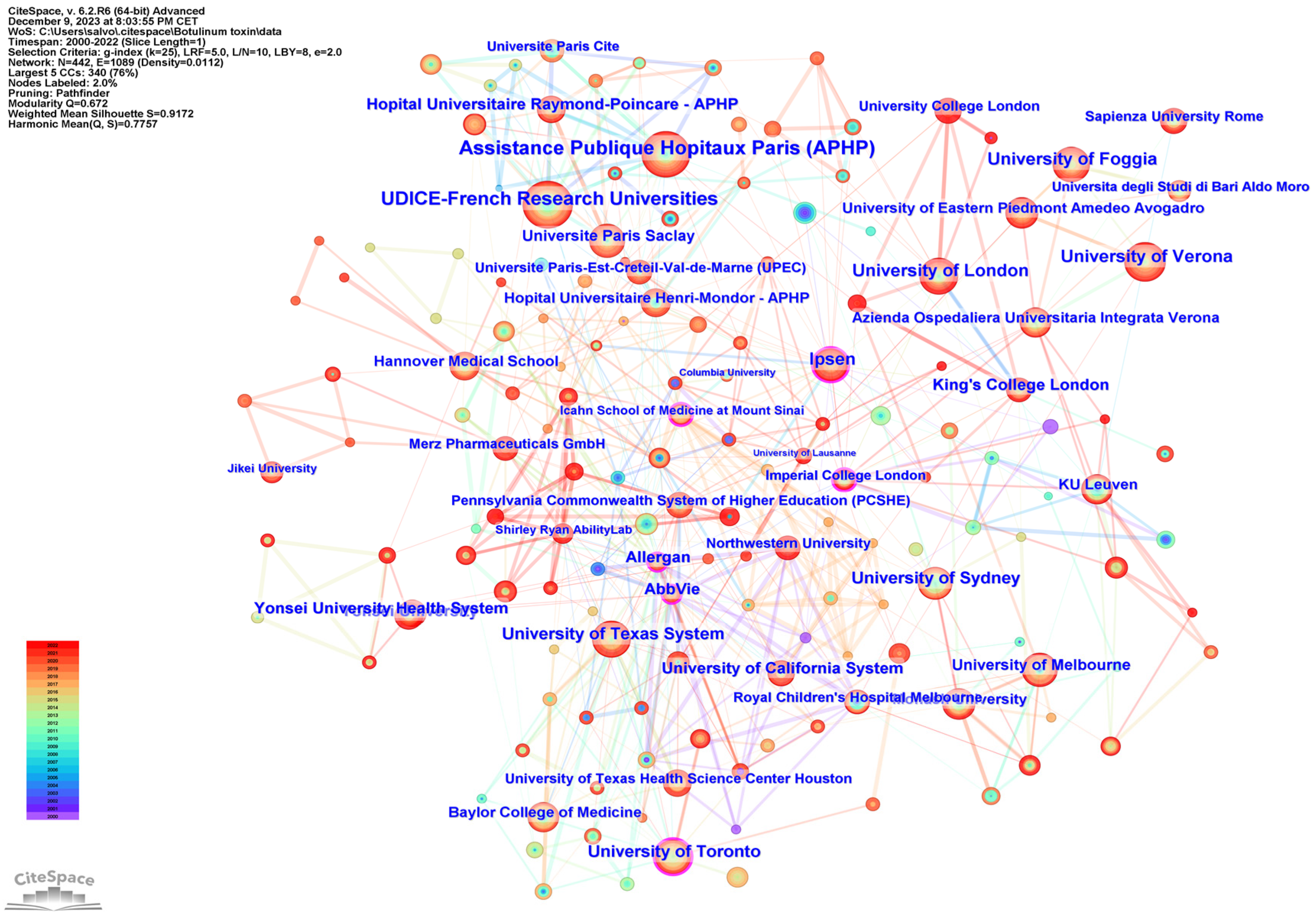
2.4. Institution Analysis
2.5. Journals Analysis
2.6. Authors Analysis
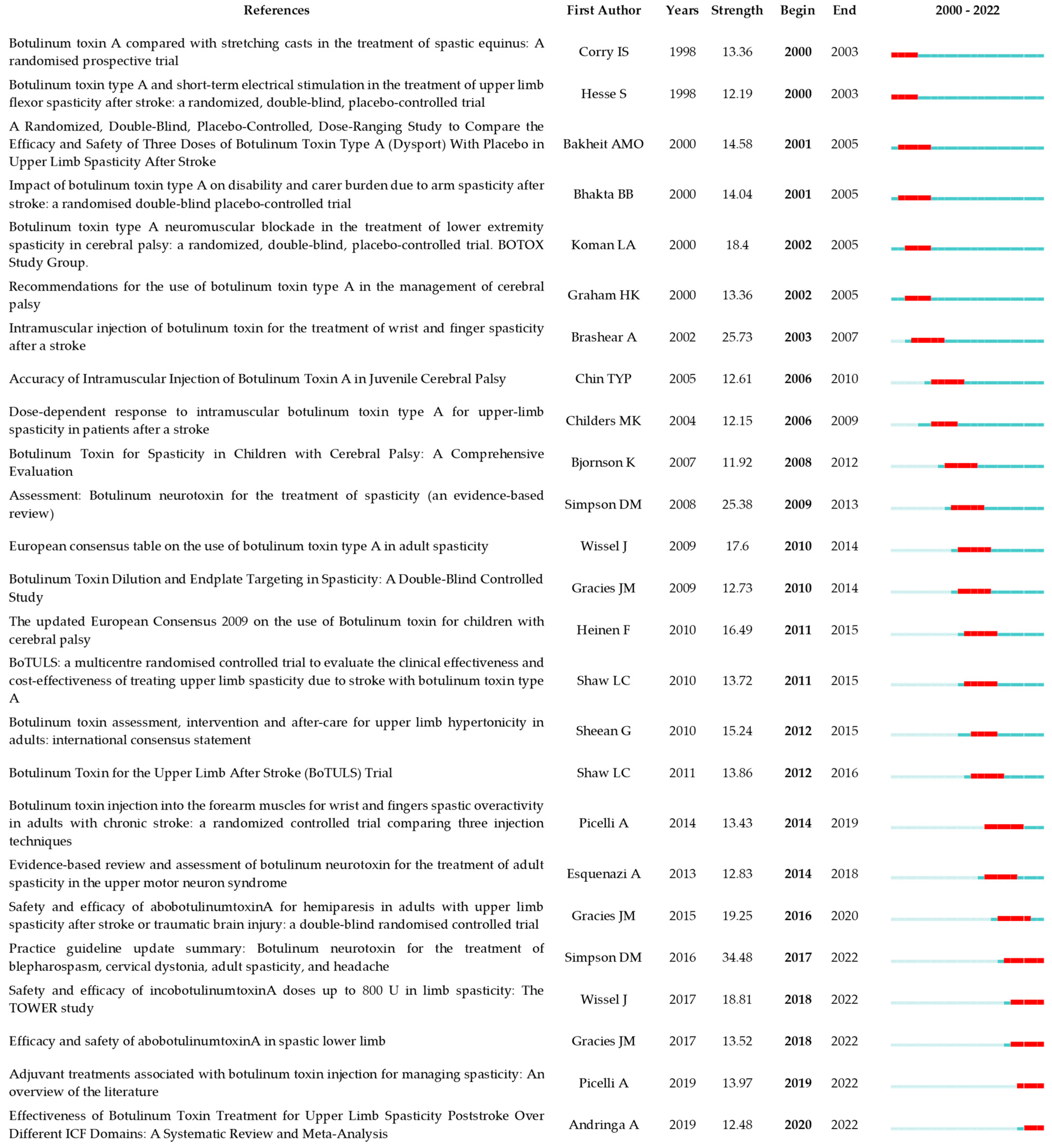
2.7. Analysis of References
2.8. Keywords Analysis
3. Discussion
3.1. Research Status
3.2. Research Hotspots and Trends
3.3. Research Frontiers and Knowledge Bases
3.3.1. Early Adoption and Applications of Botulinum Toxin in Spasticity
3.3.2. The Role of Botulinum Toxin in Pediatric Populations
3.3.3. The Role of Botulinum Toxin in Adult Populations
3.3.4. Efficacy of Botulinum Toxin in Spasticity in Adults
3.3.5. Safety of Botulinum Toxin Injection in Adults
3.3.6. Therapeutic Implications of Adjunctive Therapies and Multimodal Approach in Spasticity Treatment
3.3.7. Innovations in Diagnostics and Treatment Evaluation
3.4. Study Limitations
4. Conclusions and Future Directions
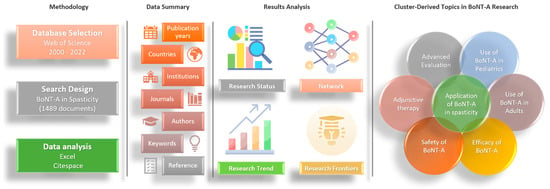
5. Materials and Methods
5.1. Data Collection
5.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Acronyms
| ASIA | American Spinal Injury Association |
| BONT-A | Botulinum Toxin type-A |
| CP | Cerebral Palsy |
| EMG | Electromyography |
| ES | Electrical Stimulation |
| ESWT | Extracorporeal Shock Wave Therapy |
| FDA | Food and Drug Administration |
| GMFCS | Gross Motor Function Classification System |
| HRV | Heart Rate Variability |
| HSP | Hereditary Spastic Paraplegia |
| IF | Impact Factor |
| JCR | Journal Citation Reports |
| J-PURE | phase 3 study of upper limb poststroke spasticity in adults from Japan |
| LLR | Log-Likelihood Ratio |
| LSI | Latent Semantic Indexing |
| MRI | Magnetic Resonance Imaging |
| Nab | Neutralizing Antibody |
| QOL | Quality of Life |
| REFLEX | phase 3 study in adult patients with post-stroke lower limb spasticity |
| SBOTE | The Spasticity treated by Botulinum Toxin and ESWT |
| SCI | Spinal Cord Injury |
| SWE | Shear Wave Elastography |
| TBI | Traumatic Brain Injury |
| TIM | Treatment with IncobotulinumtoxinA in Movement |
| TIMO | Treatment with IncobotulinumtoxinA in Movement Open-Label |
| TOWER | Titration Study in Lower and Upper Limb Spasticity |
References
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical Perceptions, Neurological Realities and Meaningful Measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Lance, J. Symposium Synopsis. In Spasticity: Disordered Motor Control; Felman, R.G., Young, R.R., Koella, W.P., Eds.; Yearbook Medical: Chicago, IL, USA, 1980; pp. 485–494. [Google Scholar]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and Risk Factors for Spasticity After Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 11, 616097. [Google Scholar] [CrossRef] [PubMed]
- Milinis, K.; Tennant, A.; Young, C.A. Spasticity in Multiple Sclerosis: Associations with Impairments and Overall Quality of Life. Mult. Scler. Relat. Disord. 2016, 5, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dragojlovic, N.; Romanoski, N.L.; Verduzco-Gutierrez, M.; Francisco, G.E. Prevalence and Treatment Characteristics of Spastic Hypertonia on First-Time Admission to Acute Inpatient Rehabilitation. Am. J. Phys. Med. Rehabil. 2022, 101, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The Epidemiology of Cerebral Palsy: Incidence, Impairments and Risk Factors. Disabil. Rehabil. 2006, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of Spastic Paresis. I: Paresis and Soft Tissue Changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.M.; Patel, A.T. Spasticity Evaluation and Management Tools. Muscle Nerve 2023, 67, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Kocer, S.; Murie Fernandez, M.; Balcaitiene, J.; Fheodoroff, K. An International Survey of Patients Living with Spasticity. Disabil. Rehabil. 2017, 39, 1428–1434. [Google Scholar] [CrossRef]
- Santamato, A.; Facciorusso, S.; Spina, S.; Cinone, N.; Avvantaggiato, C.; Santoro, L.; Ciritella, C.; Smania, N.; Picelli, A.; Gasperini, G.; et al. Discontinuation of Botulinum Neurotoxin Type-A Treatment during COVID-19 Pandemic: An Italian Survey in Post Stroke and Traumatic Brain Injury Patients Living with Spasticity. Eur. J. Phys. Rehabil. Med. 2021, 57, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Molteni, F.; Santamato, A. May Spasticity-Related Unpleasant Sensations Interfere with Daily Activities in People with Stroke and Traumatic Brain Injury? Secondary Analysis from the CORTOX Study. J. Clin. Med. 2024, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Ward, A.B.; Erztgaard, P.; Bensmail, D.; Hecht, M.J.; Lejeune, T.M.; Schnider, P.; Altavista, M.C.; Cavazza, S.; Deltombe, T.; et al. European Consensus Table on the Use of Botulinum Toxin Type A in Adult Spasticity. J. Rehabil. Med. 2009, 41, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Park, D.M. Effect of Treatment with Botulinum Toxin on Spasticity. Postgrad. Med. J. 1989, 65, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Tsui, J.K.C.; Bhatt, M.H.; Varelas, M.; Hashimoto, S.A.; Calne, D.B. Treatment of Spasticity with Botulinum Toxin—A Double-Blind-Study. Ann. Neurol. 1990, 28, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Aoki, K.R.; Autti-Rämö, I.; Boyd, R.N.; Delgado, M.R.; Gaebler-Spira, D.J.; Gormley, M.E.; Guyer, B.M.; Heinen, F.; Holton, A.F.; et al. Recommendations for the Use of Botulinum Toxin Type A in the Management of Cerebral Palsy. Gait Posture 2000, 11, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Chu, W.H.; Howell, S.; Chakraborty, S.; Koblar, S.; Visvanathan, R.; Cameron, I.; Wilson, D. A Systematic Review: Efficacy of Botulinum Toxin in Walking and Quality of Life in Post-Stroke Lower Limb Spasticity. Syst. Rev. 2018, 7, 1. [Google Scholar] [CrossRef]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G.; et al. The Updated European Consensus 2009 on the Use of Botulinum Toxin for Children with Cerebral Palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef]
- Mingers, J.; Leydesdorff, L. A Review of Theory and Practice in Scientometrics. Eur. J. Oper. Res. 2015, 246, 1–19. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Sommerfeld, D.K.; Eek, E.U.B.; Svensson, A.K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after Stroke—Its Occurrence and Association with Motor Impairments and Activity Limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Lee, C.H.; Jenkins, S.; Turkel, C.; Study, B.P.S.S. Intramuscular Injection of Botulinum Toxin for the Treatment of Wrist and Finger Spasticity after a Stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Smith, B.P.; Shilt, J.S. Cerebral Palsy. Lancet 2004, 363, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M. Pathophysiology of Spastic Paresis. II: Emergence of Muscle Overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef]
- Simpson, D.M.; Gracies, J.M.; Graham, H.K.; Miyasaki, J.M.; Naumann, M.; Russman, B.; Simpson, L.L.; So, Y. Assessment: Botulinum Neurotoxin for the Treatment of Spasticity (an Evidence-Based Review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurologysymbol. Neurology 2008, 70, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Botulinum Toxin in Clinical Practice. J. Neurol. Neurosurg. Psychiatry 2004, 75, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of Botulinum Toxin Type A on Disability and Carer Burden Due to Arm Spasticity after Stroke: A Randomised Double Blind Placebo Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2000, 69, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M.O.; Thilmann, A.F.; Ward, A.B.; Poewe, W.; Wissel, J.; Muller, J.; Benecke, R.; Collin, C.; Muller, F.; Ward, C.D.; et al. A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Compare the Efficacy and Safety of Three Doses of Botulinum Toxin Type A (Dysport) with Placebo in Upper Limb Spasticity after Stroke. Stroke 2000, 31, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Mcintyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.A.; Goldsmith, S. A Systematic Review of Interventions for Children with Cerebral Palsy: State of the Evidence. Dev. Med. Child Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice Guideline Update Summary: Botulinum Neurotoxin for the Treatment of Blepharospasm, Cervical Dystonia, Adult Spasticity, and Headache Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after Stroke: Physiology, Assessment and Treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Zorowitz, R.D.; Gillard, P.J.; Brainin, M. Poststroke Spasticity Sequelae and Burden on Stroke Survivors and Caregivers. Neurology 2013, 80, S45–S52. [Google Scholar] [CrossRef]
- Gracies, J.; Brashear, A.; Jech, R.; McAllister, P.; Banach, M.; Valkovic, P.; Walker, H.; Marciniak, C.; Deltombe, T.; Skoromets, A.; et al. Safety and Efficacy of AbobotulinumtoxinA for Hemiparesis in Adults with Upper Limb Spasticity after Stroke or Traumatic Brain Injury: A Double-Blind Randomised. Lancet Neurol. 2015, 14, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Gripenstedt, U.; Welmer, A.K. Spasticity after Stroke: An Overview of Prevalence, Test Instruments, and Treatments. Am. J. Phys. Med. Rehabil. 2012, 91, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D. Clinical Applications of Botulinum Toxin. Curr. Opin. Microbiol. 2012, 15, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E. New Insights into the Pathophysiology of Post-Stroke Spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef]
- Wheeler, A.; Smith, H.S. Botulinum Toxins: Mechanisms of Action, Antinociception and Clinical Applications. Toxicology 2013, 306, 124–146. [Google Scholar] [CrossRef] [PubMed]
- Corry, I.S.; Cosgrove, A.P.; Duffy, C.M.; McNeill, S.; Taylor, T.C.; Graham, H.K. Botulinum Toxin A Compared with Stretching Casts in the Treatment of Spastic Equinus: A Randomised Prospective Trial. J. Pediatr. Orthop. 1998, 18, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Reiter, F.; Konrad, M.; Jahnke, M.T. Botulinum Toxin Type A and Short-Term Electrical Stimulation in the Treatment of Upper Limb Flexor Spasticity after Stroke: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Rehabil. 1998, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Mooney, J.F.; Smith, B.P.; Walker, F.; Leon, J.M.; Grp, B.S. Botulinum Toxin Type A Neuromuscular Blockade in the Treatment of Lower Extremity Spasticity in Cerebral Palsy: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Pediatr. Orthop. 2000, 20, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.Y.P.; Nattrass, G.R.; Selber, P.; Graham, H.K. Accuracy of Intramuscular Injection of Botulinum Toxin A in Juvenile Cerebral Palsy: A Comparison between Manual Needle Placement and Placement Guided by Electrical Stimulation. J. Pediatr. Orthop. 2005, 25, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Childers, M.K.; Brashear, A.; Jozefczyk, P.; Reding, M.; Alexander, D.; Good, D.; Walcott, J.M.; Jenkins, S.W.; Turkel, C.; Molloy, P.T. Dose-Dependent Response to Intramuscular Botulinum Toxin Type A for Upper-Limb Spasticity in Patients after a Stroke. Arch. Phys. Med. Rehabil. 2004, 85, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Bjornson, K.; Hays, R.; Graubert, C.; Price, R.; Won, F.; McLaughlin, J.F.; Cohen, M. Botulinum Toxin for Spasticity in Children with Cerebral Palsy: A Comprehensive Evaluation. Pediatrics 2007, 120, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum Toxin Dilution and Endplate Targeting in Spasticity: A Double-Blind Controlled Study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16.e2. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A Multicentre Randomized Controlled Trial to Evaluate the Clinical Effectiveness and Cost-Effectiveness of Treating Upper Limb Spasticity Due to Stroke with Botulinum Toxin Type A. Health Technol. Assess. 2010, 14, 1–113. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B.J. Botulinum Toxin Assessment, Intervention and after-Care for Upper Limb Hypertonicity in Adults: International Consensus Statement. Eur. J. Neurol. 2010, 17, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.C.; Price, C.I.M.; Van Wijck, F.M.J.; Shackley, P.; Steen, N.; Barnes, M.P.; Ford, G.A.; Graham, L.A.; Rodgers, H. Botulinum Toxin for the Upper Limb after Stroke (BoTULS) Trial: Effect on Impairment, Activity Limitation, and Pain. Stroke 2011, 42, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Lobba, D.; Midiri, A.; Prandi, P.; Melotti, C.; Baldessarelli, S.; Smania, N. Botulinum Toxin Injection into the Forearm Muscles for Wrist and Fingers Spastic Overactivity in Adults with Chronic Stroke: A Randomized Controlled Trial Comparing Three Injection Techniques. Clin. Rehabil. 2014, 28, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Albanese, A.; Chancellor, M.B.; Elovic, E.; Segal, K.R.; Simpson, D.M.; Smith, C.P.; Ward, A.B. Evidence-Based Review and Assessment of Botulinum Neurotoxin for the Treatment of Adult Spasticity in the Upper Motor Neuron Syndrome. Toxicon 2013, 67, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Bensmail, D.; Ferreira, J.J.; Molteni, F.; Satkunam, L.; Moraleda, S.; Rekand, T.; McGuire, J.; Scheschonka, A.; Flatau-Baqué, B.; et al. Safety and Efficacy of IncobotulinumtoxinA Doses up to 800 U in Limb Spasticity the TOWER Study. Neurology 2017, 88, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, C.; McAllister, P.; Walker, H.; Brashear, A.; Edgley, S.; Deltombe, T.; Khatkova, S.; Banach, M.; Gul, F.; Vilain, C.; et al. Efficacy and Safety of AbobotulinumtoxinA (Dysport) for the Treatment of Hemiparesis in Adults with Upper Limb Spasticity Previously Treated with Botulinum Toxin: Subanalysis from a Phase 3 Randomized Controlled Trial. Phys. Med. Rehabil. 2017, 9, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Santamato, A.; Chemello, E.; Cinone, N.; Cisari, C.; Gandolfi, M.; Ranieri, M.; Smania, N.; Baricich, A. Adjuvant Treatments Associated with Botulinum Toxin Injection for Managing Spasticity: An Overview of the Literature. Ann. Phys. Rehabil. Med. 2019, 62, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef] [PubMed]
- Frevert; Dressler Complexing Proteins in Botulinum Toxin Type A Drugs: A Help or a Hindrance? Biologics 2010, 4, 325–332. [CrossRef]
- Mathevon, L.; Declemy, A.; Laffont, I.; Perennou, D. Immunogenicity Induced by Botulinum Toxin Injections for Limb Spasticity: A Systematic Review. Ann. Phys. Rehabil. Med. 2019, 62, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.W.; Jain, N.; Sublett, J.W. Immunogenicity of Botulinum Toxin Formulations: Potential Therapeutic Implications. Adv. Ther. 2021, 38, 5046–5064. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Botulinum Toxin: State of the Art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration Website Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (accessed on 19 December 2023).
- Rosales, R.L.; Bigalke, H.; Dressler, D. Pharmacology of Botulinum Toxin: Differences between Type A Preparations. Eur. J. Neurol. 2006, 13, 2–10. [Google Scholar] [CrossRef] [PubMed]
- McLellan, K.; Gaines Das, R.E.; Ekong, T.A.N.; Sesardic, D. Therapeutic Botulinum Type a Toxin: Factors Affecting Potency. Toxicon 1996, 34, 975–985. [Google Scholar] [CrossRef]
- Brin, M.F.; James, C.; Maltman, J. Botulinum Toxin Type A Products Are Not Interchangeable: A Review of the Evidence. Biologics 2014, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Love, S.C.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O’Flaherty, S.; Graham, H.K. Botulinum Toxin Assessment, Intervention and after-Care for Lower Limb Spasticity in Children with Cerebral Palsy: International Consensus Statement. Eur. J. Neurol. 2010, 17, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Hirtz, D.; Aisen, M.; Ashwal, S.; Fehlings, D.L.; McLaughlin, J.; Morrison, L.A.; Shrader, M.W.; Tilton, A.; Vargus-Adams, J. Practice Parameter: Pharmacologic Treatment of Spasticity in Children and Adolescents with Cerebral Palsy (an Evidence-Based Review) Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child. Neurology 2010, 74, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.M. Safety of High-Dose Botulinum Toxin Type A Therapy for the Treatment of Pediatric Spasticity. J. Child Neurol. 2006, 21, 189–192. [Google Scholar] [CrossRef]
- Naumann, M.; Albanese, A.; Heinen, F.; Molenaers, G.; Relja, M. Safety and Efficacy of Botulinum Toxin Type A Following Long-Term Use. Eur. J. Neurol. 2006, 13, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.; Smith, K.; Sheedy, M.; Adair, B.; Yu, X.; Graham, H.K. Systemic Adverse Events Following Botulinum Toxin A Therapy in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2010, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Apkon, S.D.; Cassidy, D. Safety Considerations in the Use of Botulinum Toxins in Children with Cerebral Palsy. Phys. Med. Rehabil. 2010, 2, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy. Toxins 2015, 7, 1629–1648. [Google Scholar] [CrossRef] [PubMed]
- Tilton, A.; Russman, B.; Aydin, R.; Dincer, U.; Escobar, R.G.; Kutlay, S.; Lipczyk, Z.; Velez, J.C.; Grandoulier, A.S.; Tse, A.; et al. AbobotulinumtoxinA (Dysport®) Improves Function According to Goal Attainment in Children with Dynamic Equinus Due to Cerebral Palsy. J. Child Neurol. 2017, 32, 482–487. [Google Scholar] [CrossRef]
- Delgado, M.R.; Bonikowski, M.; Carranza, J.; Dabrowski, E.; Matthews, D.; Russman, B.; Tilton, A.; Velez, J.C.; Grandoulier, A.S.; Picaut, P. Safety and Efficacy of Repeat Open-Label AbobotulinumtoxinA Treatment in Pediatric Cerebral Palsy. J. Child. Neurol. 2017, 32, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, E.; Bonikowski, M.; Gormley, M.; Volteau, M.; Picaut, P.; Delgado, M.R. AbobotulinumtoxinA Efficacy and Safety in Children with Equinus Foot Previously Treated with Botulinum Toxin. Pediatr. Neurol. 2018, 82, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Tilton, A.; Russman, B.; Benavides, O.; Bonikowski, M.; Carranza, J.; Dabrowski, E.; Dursun, N.; Gormley, M.; Jozwiak, M.; et al. AbobotulinumtoxinA for Equinus Foot Deformity in Cerebral Palsy: A Randomized Controlled Trial. Pediatrics 2016, 137, e20152830. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.R.; Tilton, A.; Carranza-Del Río, J.; Dursun, N.; Bonikowski, M.; Aydin, R.; Maciag-Tymecka, I.; Oleszek, J.; Dabrowski, E.; Grandoulier, A.S.; et al. Efficacy and Safety of AbobotulinumtoxinA for Upper Limb Spasticity in Children with Cerebral Palsy: A Randomized Repeat-Treatment Study. Dev. Med. Child. Neurol. 2021, 63, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, J.; Tilton, A.; del Rio, J.C.; Dursun, N.; Bonikowski, M.; Dabrowski, E.; Page, S.; Regnault, B.; Thompson, C.; Delgado, M.R.; et al. Muscle Selection and Dosing in a Phase 3, Pivotal Study of AbobotulinumtoxinA Injection in Upper Limb Muscles in Children with Cerebral Palsy. Front. Neurol. 2021, 12, 728615. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Mooney, J.F.; Smith, B.P.; Goodman, A.; Mulvaney, T. Management of Spasticity in Cerebral Palsy with Botulinum-a Toxin: Report of a Preliminary, Randomized, Double-Blind Trial. J. Pediatr. Orthop. 1994, 14, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, R.; Kim, H.; Meilahn, J.; Chambers, H.G.; Racette, B.A.; Bonikowski, M.; Park, E.S.; McCusker, E.; Liu, C.C.; Brin, M.F. Efficacy and Safety of OnabotulinumtoxinA with Standardized Physiotherapy for the Treatment of Pediatric Lower Limb Spasticity: A Randomized, Placebo-Controlled, Phase III Clinical Trial. NeuroRehabilitation 2022, 50, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, R.; McCusker, E.; Gormley, M.; Fehlings, D.; Alter, K.E.; Greaves, S.; Liu, C.; Brin, M.F. Efficacy and Safety of OnabotulinumtoxinA with Standardized Occupational Therapy for Treatment of Pediatric Upper Limb Spasticity: Phase III Placebo-Controlled Randomized Trial. NeuroRehabilitation 2021, 49, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Heinen, F.; Kanovsky, P.; Schroeder, A.S.; Chambers, H.G.; Dabrowski, E.; Geister, T.L.; Hanschmann, A.; Martinez-Torres, F.J.; Pulte, I.; Banach, M.; et al. IncobotulinumtoxinA for the Treatment of Lower-Limb Spasticity in Children and Adolescents with Cerebral Palsy: A Phase 3 Study. J. Pediatr. Rehabil. Med. 2021, 14, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, E.; Chambers, H.G.; Gaebler-Spira, D.; Banach, M.; Kanovsky, P.; Dersch, H.; Althaus, M.; Geister, T.L.; Heinen, F. IncobotulinumtoxinA Efficacy/Safety in Upper-Limb Spasticity in Pediatric Cerebral Palsy: Randomized Controlled Trial. Pediatr. Neurol. 2021, 123, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kanovsky, P.; Heinen, F.; Schroeder, A.S.; Chambers, H.G.; Dabrowski, E.; Geister, T.L.; Hanschmann, A.; Martinez-Torres, F.J.; Pulte, I.; Banach, M.; et al. Safety and Efficacy of Repeat Long-Term IncobotulinumtoxinA Treatment for Lower Limb or Combined Upper/Lower Limb Spasticity in Children with Cerebral Palsy. J. Pediatr. Rehabil. Med. 2022, 15, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.M.; Mohammed, M.O.; EL-Sobky, T.A.; ElKadery, N.A.; ElZohiery, A.K. Botulinum Toxin A Injection in Treatment of Upper Limb Spasticity in Children with Cerebral Palsy A Systematic Review of Randomized Controlled Trials. JBJS Rev. 2020, 8, e0119. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Gouron, R.; Barbier, V. Effects of Botulinum Toxin Injections in the Upper Limbs of Children with Cerebral Palsy: A Systematic Review of the Literature. Orthop. Traumatol. Surg. Res. 2023, 103578. [Google Scholar] [CrossRef] [PubMed]
- Blumetti, F.C.; Belloti, J.C.; Tamaoki, M.J.S.; Pinto, J.A. Botulinum Toxin Type A in the Treatment of Lower Limb Spasticity in Children with Cerebral Palsy. Cochrane Database Syst. Rev. 2019, 2019, CD001408. [Google Scholar] [CrossRef] [PubMed]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Pediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Multani, I.; Manji, J.; Tang, M.J.; Herzog, W.; Howard, J.J.; Graham, H.K. Sarcopenia, Cerebral Palsy, and Botulinum Toxin Type A. JBJS Rev. 2019, 7, e4. [Google Scholar] [CrossRef] [PubMed]
- Kaya Keles, C.S.; Ates, F. Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles. Toxins 2022, 14, 772. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, B.B. Management of Spasticity in Stroke. Br. Med. Bull. 2000, 56, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Brashear, A.; Kaelin, D.; Liu, J.Y.; Millis, S.R.; Barron, R.; Turkel, C. Repeated Treatments with Botulinum Toxin Type a Produce Sustained Decreases in the Limitations Associated with Focal Upper-Limb Poststroke Spasticity for Caregivers and Patients. Arch. Phys. Med. Rehabil. 2008, 89, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum Neurotoxins for Post-Stroke Spasticity in Adults: A Systematic Review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Esquenazi, A.; Brashear, A.; Banach, M.; Kocer, S.; Jech, R.; Khatkova, S.; Benetin, J.; Vecchio, M.; McAllister, P.; et al. Efficacy and Safety of AbobotulinumtoxinA in Spastic Lower Limb: Randomized Trial and Extension. Neurology 2017, 89, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Hu, G.C. Post-Stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Yablon, S.A.; Agana, B.T.; Ivanhoe, C.B.; Boake, C. Botulinum Toxin in Severe Upper Extremity Spasticity among Patients with Traumatic Brain Injury: An Open-Labeled Trial. Neurology 1996, 47, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Verplancke, D.; Snape, S.; Salisbury, C.F.; Jones, P.W.; Ward, A.B. A Randomized Controlled Trial of Botulinum Toxin on Lower Limb Spasticity Following Acute Acquired Severe Brain Injury. Clin. Rehabil. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Safarpour, Y.; Mousavi, T.; Jabbari, B. Botulinum Toxin Treatment in Multiple Sclerosis-a Review. Curr. Treat. Options Neurol. 2017, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Hyman, N.; Barnes, M.; Bhakta, B.; Cozens, A.; Bakheit, M.; Kreczy-Kleedorfer, B.; Poewe, W.; Wissel, J.; Bain, P.; Glickman, S.; et al. Botulinum Toxin (Dysport®) Treatment of Hip Adductor Spasticity in Multiple Sclerosis: A Prospective, Randomised, Double Blind, Placebo Controlled, Dose Ranging Study. J. Neurol. Neurosurg. Psychiatry 2000, 68, 707–712. [Google Scholar] [CrossRef]
- Marciniak, C.; Rader, L.; Gagnon, C. The Use of Botulinum Toxin for Spasticity after Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2008, 87, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Lui, J.; Sarai, M.; Mills, P.B. Chemodenervation for Treatment of Limb Spasticity Following Spinal Cord Injury: A Systematic Review. Spinal Cord. 2015, 53, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Palazón-García, R.; Alcobendas-Maestro, M.; Esclarin-de Ruz, A.; Benavente-Vaidepeñas, A.M. Treatment of Spasticity in Spinal Cord Injury with Botulinum Toxin. J. Spinal Cord Med. 2019, 42, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Diniz de Lima, F.; Faber, I.; Servelhere, K.R.; Bittar, M.F.R.; Martinez, A.R.M.; Piovesana, L.G.; Martins, M.P.; Martins, C.R.; Benaglia, T.; de Sá Carvalho, B.; et al. Randomized Trial of Botulinum Toxin Type A in Hereditary Spastic Paraplegia—The SPASTOX Trial. Mov. Disord. 2021, 36, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Van Lith, B.J.H.; Den Boer, J.; van de Warrenburg, B.P.C.; Weerdesteyn, V.; Geurts, A.C. Functional Effects of Botulinum Toxin Type A in the Hip Adductors and Subsequent Stretching in Patients with Hereditary Spastic Paraplegia. J. Rehabil. Med. 2019, 51, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Paparella, G.; Vavla, M.; Bernardi, L.; Girardi, G.; Stefan, C.; Martinuzzi, A. Efficacy of a Combined Treatment of Botulinum Toxin and Intensive Physiotherapy in Hereditary Spastic Paraplegia. Front. Neurosci. 2020, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- De Niet, M.; De Bot, S.T.; Van De Warrenburg, B.P.C.; Weerdesteyn, V.; Geurts, A.C. Functional Effects of Botulinum Toxin Type-A Treatment and Subsequent Stretching of Spastic Calf Muscles: A Study in Patients with Hereditary Spastic Paraplegia. J. Rehabil. Med. 2015, 47, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Baricich, A.; Battaglia, M.; Cuneo, D.; Cosenza, L.; Millevolte, M.; Cosma, M.; Filippetti, M.; Dalise, S.; Azzollini, V.; Chisari, C.; et al. Clinical Efficacy of Botulinum Toxin Type A in Patients with Traumatic Brain Injury, Spinal Cord Injury, or Multiple Sclerosis: An Observational Longitudinal Study. Front. Neurol. 2023, 14, 1133390. [Google Scholar] [CrossRef] [PubMed]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic Literature Review of AbobotulinumtoxinA in Clinical Trials for Adult Upper Limb Spasticity. Am. J. Phys. Med. Rehabil. 2015, 94, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dashtipour, K.; Chen, J.J.; Walker, H.W.; Lee, M.Y. Systematic Literature Review of AbobotulinumtoxinA in Clinical Trials for Lower Limb Spasticity. Medicine 2016, 95, e2468. [Google Scholar] [CrossRef] [PubMed]
- Wein, T.; Esquenazi, A.; Jost, W.H.; Ward, A.B.; Pan, G.; Dimitrova, R. OnabotulinumtoxinA for the Treatment of Poststroke Distal Lower Limb Spasticity: A Randomized Trial. Phys. Med. Rehabil. 2018, 10, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.T.; Ward, A.B.; Geis, C.; Jost, W.H.; Liu, C.C.; Dimitrova, R. Impact of Early Intervention with OnabotulinumtoxinA Treatment in Adult Patients with Post-Stroke Lower Limb Spasticity: Results from the Double-Blind, Placebo-Controlled, Phase 3 REFLEX Study. J. Neural Transm. 2020, 127, 1619–1629. [Google Scholar] [CrossRef]
- Simpson, D.M.; Alexander, D.N.; O’Brien, C.F.; Tagliati, M.; Aswad, A.S.; Leon, J.M.; Gibson, J.; Mordaunt, J.M.; Monaghan, E.P. Botulinum Toxin Type A in the Treatment of Upper Extremity Spasticity: A Randomized, Double-Blind, Placebo-Controlled Trial. Neurology 1996, 46, 1306. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Osako, Y.; Suyama, K.; Maeda, T.; Uechi, Y.; Iwasaki, M.; Grp, G.S.S. Botulinum Toxin Type A in Post-Stroke Upper Limb Spasticity. Curr. Med. Res. Opin. 2010, 26, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Kanovsky, P.; Slawek, J.; Denes, Z.; Platz, T.; Sassin, I.; Comes, G.; Grafe, S. Efficacy and Safety of Botulinum Neurotoxin NT 201 in Poststroke Upper Limb Spasticity. Clin. Neuropharmacol. 2009, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Elovic, E.P.; Munin, M.C.; Kanovsky, P.; Hanschmann, A.; Hiersemenzel, R.; Marciniak, C. Randomized, Placebo-Controlled Trial of Incobotulinumtoxina for Upper-Limb Post-Stroke Spasticity. Muscle Nerve 2016, 53, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, C.; Munin, M.C.; Brashear, A.; Rubin, B.S.; Patel, A.T.; Slawek, J.; Hanschmann, A.; Hiersemenzel, R.; Elovic, E.P. IncobotulinumtoxinA Efficacy and Safety in Adults with Upper-Limb Spasticity Following Stroke: Results from the Open-Label Extension Period of a Phase 3 Study. Adv. Ther. 2019, 36, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Masakado, Y.; Abo, M.; Kondo, K.; Saeki, S.; Saitoh, E.; Dekundy, A.; Hanschmann, A.; Kaji, R.; Grp, J.P.S. Efficacy and Safety of IncobotulinumtoxinA in Post-Stroke Upper-Limb Spasticity in Japanese Subjects: Results from a Randomized, Double-Blind, Placebo-Controlled Study (J-PURE). J. Neurol. 2020, 267, 2029–2041. [Google Scholar] [CrossRef]
- Lagalla, G.; Danni, M.; Reiter, F.; Ceravolo, M.G.; Provinciali, L. Post-Stroke Spasticity Management with Repeated Botulinum Toxin Injections in the Upper Limb. Am. J. Phys. Med. Rehabil. 2000, 79, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Jacinto, J.; Fheodoroff, K.; Brashear, A.; Maisonobe, P.; Lysandropoulos, A.; Ashford, S.; Grp, U.-I.S. Longitudinal Goal Attainment with Integrated Upper Limb Spasticity Management Including Repeat Injections of Botulinum Toxin A: Findings from the Prospective, Observational Upper Limb International Spasticity (Ulis-Iii) Cohort Study. J. Rehabil. Med. 2021, 53, jrm00157. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.M.; Jech, R.; Valkovic, P.; Marque, P.; Vecchio, M.; Denes, Z.; Vilain, C.; Delafont, B.; Picaut, P. When Can Maximal Efficacy Occur with Repeat Botulinum Toxin Injection in Upper Limb Spastic Paresis? Brain Commun. 2020, 3, fcaa201. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Puce, L.; Pelosin, E.; Serrati, C.; Fattapposta, F.; Rinalduzzi, S.; Abbruzzese, G.; Curra, A. Do Flexible Inter-Injection Intervals Improve the Effects of Botulinum Toxin A Treatment in Reducing Impairment and Disability in Patients with Spasticity? Med. Hypotheses 2017, 102, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Ojardias, E.; Ollier, E.; Lafaie, L.; Celarier, T.; Giraux, P.; Bertoletti, L. Time Course Response after Single Injection of Botulinum Toxin to Treat Spasticity after Stroke: Systematic Review with Pharmacodynamic Model-Based Meta-Analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101579. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saheri, F.; Reis Barbosa, E. Botulinum Toxin: Mechanisms of Action. Arq. Neuropsiquiatr. 2005, 63, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Samizadeh, S.; De Boulle, K. Botulinum Neurotoxin Formulations: Overcoming the Confusion. Clin. Cosmet. Investig. Dermatol. 2018, 11, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Efendy, F.; Teleg, E.S.A.; Delos Santos, M.M.D.; Rosales, M.C.E.; Ostrea, M.; Tanglao, M.J.; Ng, A.R. Botulinum Toxin as Early Intervention for Spasticity after Stroke or Non-Progressive Brain Lesion: A Meta-Analysis. J. Neurol. Sci. 2016, 371, 6–14. [Google Scholar] [CrossRef]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.T.; Delgado-De Los Santos, M.M.; Chua, K.S.G.; Abdullah, S.J.B.F.; Zakine, B.; Maisonobe, P.; et al. Botulinum Toxin Injection for Hypertonicity of the Upper Extremity within 12 Weeks after Stroke: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef]
- Rosales, R.L.; Balcaitiene, J.; Berard, H.; Maisonobe, P.; Goh, K.J.; Kumthornthip, W.; Mazlan, M.; Latif, L.A.; Delos Santos, M.M.D.; Chotiyarnwong, C.; et al. Early AbobotulinumtoxinA (Dysport®) in Post-Stroke Adult Upper Limb Spasticity: ONTIME Pilot Study. Toxins 2018, 10, 253. [Google Scholar] [CrossRef]
- Wissel, J.; Fheodoroff, K.; Hoonhorst, M.; Müngersdorf, M.; Gallien, P.; Meier, N.; Hamacher, J.; Hefter, H.; Maisonobe, P.; Koch, M. Effectiveness of AbobotulinumtoxinA in Post-Stroke Upper Limb Spasticity in Relation to Timing of Treatment. Front. Neurol. 2020, 11, 104. [Google Scholar] [CrossRef]
- Picelli, A.; Santamato, A.; Cosma, M.; Baricich, A.; Chisari, C.; Millevolte, M.; Del Prete, C.; Mazzù, I.; Girardi, P.; Smania, N. Early Botulinum Toxin Type A Injection for Post-Stroke Spasticity: A Longitudinal Cohort Study. Toxins 2021, 13, 374. [Google Scholar] [CrossRef]
- Lindsay, C.; Ispoglou, S.; Helliwell, B.; Hicklin, D.; Sturman, S.; Pandyan, A. Can the Early Use of Botulinum Toxin in Post Stroke Spasticity Reduce Contracture Development? A Randomised Controlled Trial. Clin. Rehabil. 2021, 35, 399–409. [Google Scholar] [CrossRef]
- Wissel, J.; Kivi, A. Post-Stroke Spastic Movement Disorder and Botulinum Toxin A Therapy: Early Detection and Early Injection. Ann. Rehabil. Med.-ARM 2023, 47, 326–336. [Google Scholar] [CrossRef]
- Sunnerhagen, K.S.; Opheim, A.; Alt Murphy, M. Onset, Time Course and Prediction of Spasticity after Stroke or Traumatic Brain Injury. Ann. Phys. Rehabil. Med. 2019, 62, 431–434. [Google Scholar] [CrossRef]
- Wissel, J.; Ri, S.J. Assessment, Goal Setting, and Botulinum Neurotoxin a Therapy in the Management of Post-Stroke Spastic Movement Disorder: Updated Perspectives on Best Practice. Expert. Rev. Neurother. 2022, 22, 27–42. [Google Scholar] [CrossRef]
- Urban, P.P.; Wolf, T.; Uebele, M.; Marx, J.J.; Vogt, T.; Stoeter, P.; Bauermann, T.; Weibrich, C.; Vucurevic, G.D.; Schneider, A.; et al. Occurence and Clinical Predictors of Spasticity after Ischemic Stroke. Stroke 2010, 41, 2016–2020. [Google Scholar] [CrossRef]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-Stroke Spasticity: Predictors of Early Development and Considerations for Therapeutic Intervention. Phys. Med. Rehabil. 2015, 7, 60–67. [Google Scholar] [CrossRef]
- Smith, M.-C.; Ackerley, S.J.; Barber, P.A.; Byblow, W.D.; Stinear, C.M. PREP2 Algorithm Predictions Are Correct at 2 Years Poststroke for Most Patients. Neurorehabil. Neural Repair 2019, 33, 635–642. [Google Scholar] [CrossRef]
- Esquenazi, A.; Stoquart, G.; Hedera, P.; Jacinto, L.J.; Dimanico, U.; Constant-Boyer, F.; Brashear, A.; Grandoulier, A.S.; Vilain, C.; Picaut, P.; et al. Efficacy and Safety of AbobotulinumtoxinA for the Treatment of Hemiparesis in Adults with Lower Limb Spasticity Previously Treated with Other Botulinum Toxins: A Secondary Analysis of a Randomized Controlled Trial. Phys. Med. Rehabil. 2020, 12, 853–860. [Google Scholar] [CrossRef]
- Esquenazi, A.; Bavikatte, G.; Bandari, D.S.; Jost, W.H.; Munin, M.C.; Tang, S.F.T.; Largent, J.; Adams, A.M.; Zuzek, A.; Francisco, G.E. Long-Term Observational Results from the ASPIRE Study: OnabotulinumtoxinA Treatment for Adult Lower Limb Spasticity. Phys. Med. Rehabil. 2021, 13, 1079–1093. [Google Scholar] [CrossRef]
- Francisco, G.E.; Feng, W.; Munin, M.C.; Ngo, K.; Schwartz, M.; Sadeghi, M.; Zuzek, A.; Esquenazi, A. Individualized OnabotulinumtoxinA Treatment of Upper Limb Spasticity in US Clinical Practices: Analysis of Practice Patterns from the ASPIRE Study. Toxicon 2022, 214, S62–S63. [Google Scholar] [CrossRef]
- Naumann, M.; Jankovic, J. Safety of Botulinum Toxin Type A: A Systematic Review and Meta-Analysis. Curr. Med. Res. Opin. 2004, 20, 981–990. [Google Scholar] [CrossRef]
- Baizabal-Carvallo, J.F.; Jankovic, J.; Pappert, E. Flu-like Symptoms Following Botulinum Toxin Therapy. Toxicon 2011, 58, 1–7. [Google Scholar] [CrossRef]
- Ahsanuddin, S.; Roy, S.; Nasser, W.; Povolotskiy, R.; Paskhover, B. Adverse Events Associated with Botox as Reported in a Food and Drug Administration Database. Aesthetic Plast. Surg. 2021, 45, 1201–1209. [Google Scholar] [CrossRef]
- Coté, T.R.; Mohan, A.K.; Polder, J.A.; Walton, M.K.; Braun, M.M. Botulinum Toxin Type A Injections: Adverse Events Reported to the US Food and Drug Administration in Therapeutic and Cosmetic Cases. J. Am. Acad. Dermatol. 2005, 53, 407–415. [Google Scholar] [CrossRef]
- Pittock, S.J.; Moore, A.P.; Hardiman, O.; Ehler, E.; Kovac, M.; Bojakowski, J.; al Khawaja, I.; Brozman, M.; Kanovsky, P.; Skorometz, A.; et al. A Double-Blind Randomised Placebo-Controlled Evaluation of Three Doses of Botulinum Toxin Type A (Dysport®) in the Treatment of Spastic Equinovarus Deformity after Stroke. Cerebrovasc. Dis. 2003, 15, 289–300. [Google Scholar] [CrossRef]
- Dressler, D.; Saberi, F.A.; Kollewe, K.; Schrader, C. Safety Aspects of IncobotulinumtoxinA High-Dose Therapy. J. Neural Transm. 2015, 122, 327–333. [Google Scholar] [CrossRef]
- Santamato, A.; Panza, F.; Ranieri, M.; Frisardi, V.; Micello, M.F.; Filoni, S.; Fortunato, F.; Intiso, D.; Basciani, M.; Logroscino, G.; et al. Efficacy and Safety of Higher Doses of Botulinum Toxin Type A NT 201 Free from Complexing Proteins in the Upper and Lower Limb Spasticity after Stroke. J. Neural Transm. 2013, 120, 469–476. [Google Scholar] [CrossRef]
- Santamato, A.; Ranieri, M.; Solfrizzi, V.; Lozupone, M.; Vecchio, M.; Daniele, A.; Greco, A.; Seripa, D.; Logroscino, G.; Panza, F. High Doses of IncobotulinumtoxinA for the Treatment of Post-Stroke Spasticity: Are They Safe and Effective? Expert Opin. Drug Metab. Toxicol. 2016, 12, 843–846. [Google Scholar] [CrossRef]
- Santamato, A.; Panza, F.; Intiso, D.; Baricich, A.; Picelli, A.; Smania, N.; Fortunato, F.; Seripa, D.; Fiore, P.; Ranieri, M. Long-Term Safety of Repeated High Doses of IncobotulinumtoxinA Injections for the Treatment of Upper and Lower Limb Spasticity after Stroke. J. Neurol. Sci. 2017, 378, 182–186. [Google Scholar] [CrossRef]
- Baricich, A.; Grana, E.; Carda, S.; Santamato, A.; Cisari, C.; Invernizzi, M. High Doses of OnabotulinumtoxinA in Post-Stroke Spasticity: A Retrospective Analysis. J. Neural Transm. 2015, 122, 1283–1287. [Google Scholar] [CrossRef]
- Baricich, A.; Grana, E.; Carda, S.; Santamato, A.; Molinari, C.; Cisari, C.; Invernizzi, M. Heart Rate Variability Modifications Induced by High Doses of IncobotulinumtoxinA and OnabotulinumtoxinA in Hemiplegic Chronic Stroke Patients: A Single Blind Randomized Controlled, Crossover Pilot Study. Toxicon 2017, 138, 145–150. [Google Scholar] [CrossRef]
- Kirshblum, S.; Solinsky, R.; Jasey, N.; Hampton, S.; Didesch, M.; Seidel, B.; Botticello, A. Adverse Event Profiles of High Dose Botulinum Toxin Injections for Spasticity. Phys. Med. Rehabil. 2020, 12, 349–355. [Google Scholar] [CrossRef]
- Mejia, N.I.; Dat Vuong, K.; Jankovic, J. Long-Term Botulinum Toxin Efficacy, Safety, and Immunogenicity. Mov. Disord. 2005, 20, 592–597. [Google Scholar] [CrossRef]
- Sheean, G. Botulinum Treatment of Spasticity: Why Is It so Difficult to Show a Functional Benefit? Curr. Opin. Neurol. 2001, 14, 771–776. [Google Scholar] [CrossRef]
- Fabbri, M.; Leodori, G.; Fernandes, R.M.; Bhidayasiri, R.; Marti, M.J.; Colosimo, C.; Ferreira, J.J. Neutralizing Antibody and Botulinum Toxin Therapy: A Systematic Review and Meta-Analysis. Neurotox. Res. 2016, 29, 105–117. [Google Scholar] [CrossRef]
- Gordon, M.F.; Brashear, A.; Elovic, E.; Kassicieh, D.; Marciniak, C.; Liu, J.; Turkel, C. Repeated Dosing of Botulinum Toxin Type A for Upper Limb Spasticity Following Stroke. Neurology 2004, 63, 1971–1973. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Jansen, A.; Brauns, R.; Ürer, B.; Bigalke, H.; Hartung, H.P.; Meuth, S.G.; Lee, J.I.; Albrecht, P.; et al. Significantly Lower Antigenicity of Incobotulinumtoxin than Abo- or Onabotulinumtoxin. J. Neurol. 2023, 270, 788–796. [Google Scholar] [CrossRef]
- Jankovic, J.; Carruthers, J.; Naumann, M.; Ogilvie, P.; Boodhoo, T.; Attar, M.; Gupta, S.; Singh, R.; Soliman, J.; Yushmanova, I.; et al. Neutralizing Antibody Formation with OnabotulinumtoxinA (BOTOX®) Treatment from Global Registration Studies across Multiple Indications: A Meta-Analysis. Toxins 2023, 15, 342. [Google Scholar] [CrossRef]
- Mills, P.B.; Finlayson, H.; Sudol, M.; O’Connor, R. Systematic Review of Adjunct Therapies to Improve Outcomes Following Botulinum Toxin Injection for Treatment of Limb Spasticity. Clin. Rehabil. 2016, 30, 537–548. [Google Scholar] [CrossRef]
- Carda, S.; Invernizzi, M.; Baricich, A.; Cisari, C. Casting, Taping or Stretching after Botulinum Toxin Type A for Spastic Equinus Foot: A Single-Blind Randomized Trial on Adult Stroke Patients. Clin. Rehabil. 2011, 25, 1119–1127. [Google Scholar] [CrossRef]
- Allart, E.; Mazevet, D.; Idée, S.; Boyer, F.C.; Bonan, I. Adjunct Therapies after Botulinum Toxin Injections in Spastic Adults: Systematic Review and SOFMER Recommendations. Ann. Phys. Rehabil. Med. 2022, 65, 101544. [Google Scholar] [CrossRef]
- Santamato, A.; Notarnicola, A.; Panza, F.; Ranieri, M.; Micello, M.F.; Manganotti, P.; Moretti, B.; Fortunato, F.; Filoni, S.; Fiore, P. Sbote Study: Extracorporeal Shock Wave Therapy Versus Electrical Stimulation After Botulinum Toxin Type A Injection for Post-Stroke Spasticity-A Prospective Randomized Trial. Ultrasound Med. Biol. 2013, 39, 283–291. [Google Scholar] [CrossRef]
- Mihai, E.E.; Popescu, M.N.; Iliescu, A.N.; Berteanu, M. A Systematic Review on Extracorporeal Shock Wave Therapy and Botulinum Toxin for Spasticity Treatment: A Comparison on Efficacy. Eur. J. Phys. Rehabil. Med. 2022, 58, 565. [Google Scholar] [CrossRef]
- Duan, H.; Li, Z.; Liu, F. The Effects of Botulinum Toxin Type A Combined with Extracorporeal Shock Wave Therapy on Triceps Spasticity in Stroke Patients. Ann. Phys. Rehabil. Med. 2018, 61, e365. [Google Scholar] [CrossRef]
- Kwon, D.R.; Kwon, D.G. Botulinum Toxin a Injection Combined with Radial Extracorporeal Shock Wave Therapy in Children with Spastic Cerebral Palsy: Shear Wave Sonoelastographic Findings in the Medial Gastrocnemius Muscle, Preliminary Study. Children 2021, 8, 1059. [Google Scholar] [CrossRef]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonzaiuti, D. How Could Robotic Training and Botolinum Toxin Be Combined in Chronic Post Stroke Upper Limb Spasticity? A Pilot Study. Eur. J. Phys. Rehabil. Med. 2015, 51, 381–387. [Google Scholar]
- Leong, B. The Vegetative and Minimally Conscious States in Children: Spasticity, Muscle Contracture and Issues for Physiotherapy Treatment. Brain Inj. 2002, 16, 217–230. [Google Scholar] [CrossRef]
- He, J.; Luo, A.; Yu, J.; Qian, C.; Liu, D.; Hou, M.; Ma, Y. Quantitative Assessment of Spasticity: A Narrative Review of Novel Approaches and Technologies. Front. Neurol. 2023, 14, 1121323. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Johnson, G.R.; Price, C.I.M.; Curless, R.H.; Barnes, M.P.; Rodgers, H. A Review of the Properties and Limitations of the Ashworth and Modified Ashworth Scales as Measures of Spasticity. Clin. Rehabil. 1999, 13, 373–383. [Google Scholar] [CrossRef]
- Haugh, A.; Pandyan, A.; Johnson, G. A Systematic Review of the Tardieu Scale for the Measurement of Spasticity. Disabil. Rehabil. 2006, 28, 899–907. [Google Scholar] [CrossRef]
- Mirbagheri, M.M.; Barbeau, H.; Kearney, R.E. Intrinsic and Reflex Contributions to Human Ankle Stiffness: Variation with Activation Level and Position. Exp. Brain Res. 2000, 135, 423–436. [Google Scholar] [CrossRef]
- Williams, S.A.; Reid, S.; Elliott, C.; Shipman, P.; Valentine, J. Muscle Volume Alterations in Spastic Muscles Immediately Following Botulinum Toxin Type-A Treatment in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2013, 55, 813–820. [Google Scholar] [CrossRef]
- Fortuna, R.; Vaz, M.A.; Sawatsky, A.; Hart, D.A.; Herzog, W. A Clinically Relevant BTX-A Injection Protocol Leads to Persistent Weakness, Contractile Material Loss, and an Altered MRNA Expression Phenotype in Rabbit Quadriceps Muscles. J. Biomech. 2015, 48, 1700–1706. [Google Scholar] [CrossRef]
- Elwischger, K.; Kasprian, G.; Weber, M.; Meyerspeer, M.; Linder, C.; Auff, E.; Prayer, D.; Sycha, T.; Kranz, G. Intramuscular Distribution of Botulinum Toxin-Visualized by MRI. J. Neurol. Sci. 2014, 344, 76–79. [Google Scholar] [CrossRef]
- Spina, S.; Facciorusso, S.; Botticelli, C.; Intiso, D.; Ranieri, M.; Colamaria, A.; Fiore, P.; Ciritella, C.; Genêt, F.; Santamato, A. Ultrasonographic Evaluation of Three Approaches for Botulinum Toxin Injection into Tibialis Posterior Muscle in Chronic Stroke Patients with Equinovarus Foot: An Observational Study. Toxins 2021, 13, 829. [Google Scholar] [CrossRef]
- Westhoff, B.; Seller, K.; Wild, A.; Jaeger, M.; Krauspe, R. Ultrasound-Guided Botulinum Toxin Injection Technique for the Iliopsoas Muscle. Dev. Med. Child Neurol. 2003, 45, 829–832. [Google Scholar] [CrossRef]
- Walter, U.; Dressler, D. Ultrasound-Guided Botulinum Toxin Injections in Neurology: Technique, Indications and Future Perspectives. Expert Rev. Neurother. 2014, 14, 923–936. [Google Scholar] [CrossRef]
- Moreta, M.C.; Fleet, A.; Reebye, R.; McKernan, G.; Berger, M.; Farag, J.; Munin, M.C. Reliability and Validity of the Modified Heckmatt Scale in Evaluating Muscle Changes with Ultrasound in Spasticity. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100071. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, Z.; Bian, R.H.; Lo, W.L.A.; Xie, X.Y.; Wang, R.L.; Huang, D.F.; Li, L. Alterations of Elastic Property of Spastic Muscle with Its Joint Resistance Evaluated from Shear Wave Elastography and Biomechanical Model. Front. Neurol. 2019, 10, 736. [Google Scholar] [CrossRef]
- Lehoux, M.C.; Sobczak, S.; Cloutier, F.; Charest, S.; Bertrand-Grenier, A. Shear Wave Elastography Potential to Characterize Spastic Muscles in Stroke Survivors: Literature Review. Clin. Biomech. 2020, 72, 84–93. [Google Scholar] [CrossRef]
- Jardon, M.; Nguyen, J.; Casaletto, E.; Ko, L.; Wolff, A.; Daluiski, A.; Nwawka, O.K. Utilization of Shear Wave Elastography to Quantify and Predict Response to Upper Extremity Botulinum Toxin Injections in Patients with Cerebral Palsy: A Pilot Study. Clin. Neurol. Neurosurg. 2023, 230, 107798. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Niimi, M.; Hara, T.; Sakurai, Y.; Soshi, S.; Udaka, J.; Abo, M. Shear Wave Velocity to Evaluate the Effect of Botulinum Toxin on Post-Stroke Spasticity of the Lower Limb. Toxins 2022, 15, 14. [Google Scholar] [CrossRef]
- Bertan, H.; Oncu, J.; Vanli, E.; Alptekin, K.; Sahillioglu, A.; Kuran, B.; Yilmaz, F. Use of Shear Wave Elastography for Quantitative Assessment of Muscle Stiffness After Botulinum Toxin Injection in Children with Cerebral Palsy. J. Ultrasound Med. 2020, 39, 2327–2337. [Google Scholar] [CrossRef]
- Choi, S.; Shin, Y.B.; Kim, S.Y.; Kim, J. A Novel Sensor-Based Assessment of Lower Limb Spasticity in Children with Cerebral Palsy. J. Neuroeng. Rehabil. 2018, 15, 45. [Google Scholar] [CrossRef]
- Cinone, N.; Letizia, S.; Santoro, L.; Facciorusso, S.; Armiento, R.; Picelli, A.; Ranieri, M.; Santamato, A. Combined Effects of Isokinetic Training and Botulinum Toxin Type a on Spastic Equinus Foot in Patients with Chronic Stroke: A Pilot, Single-Blind, Randomized Controlled Trial. Toxins 2019, 11, 210. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Davies, L.; McCrory, P.; Hughes, A. Goal Attainment Scaling in the Evaluation of Treatment of Upper Limb Spasticity with Botulinum Toxin: A Secondary Analysis from a Double-Blind Placebo-Controlled Randomized Clinical Trial. J. Rehabil. Med. 2010, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.F. Injection Techniques for Botulinum Toxin Using Electromyography and Electrical Stimulation. Muscle Nerve 1997, 20, 176–180. [Google Scholar] [CrossRef]
- Grigoriu, A.I.; Dinomais, M.; Rémy-Néris, O.; Brochard, S. Impact of Injection-Guiding Techniques on the Effectiveness of Botulinum Toxin for the Treatment of Focal Spasticity and Dystonia: A Systematic Review. Arch. Phys. Med. Rehabil. 2015, 96, 2067–2078. [Google Scholar] [CrossRef]
- Walker, H.W.; Lee, M.Y.; Bahroo, L.B.; Hedera, P.; Charles, D. Botulinum Toxin Injection Techniques for the Management of Adult Spasticity. Phys. Med. Rehabil. 2015, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Tamburin, S.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Santilli, V.; Smania, N. Botulinum Toxin Type A Injection into the Gastrocnemius Muscle for Spastic Equinus in Adults with Stroke A Randomized Controlled Trial Comparing Manual Needle Placement, Electrical Stimulation and Ultrasonography-Guided Injection Techniques. Am. J. Phys. Med. Rehabil. 2012, 91, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Santamato, A.; Micello, M.F.; Panza, F.; Fortunato, F.; Baricich, A.; Cisari, C.; Pilotto, A.; Logroscino, G.; Fiore, P.; Ranieri, M. Can Botulinum Toxin Type A Injection Technique Influence the Clinical Outcome of Patients with Post-Stroke Upper Limb Spasticity? A Randomized Controlled Trial Comparing Manual Needle Placement and Ultrasound-Guided Injection Techniques. J. Neurol. Sci. 2014, 347, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ploumis, A.; Varvarousis, D.; Konitsiotis, S.; Beris, A. Effectiveness of Botulinum Toxin Injection with and without Needle Electromyographic Guidance for the Treatment of Spasticity in Hemiplegic Patients: A Randomized Controlled Trial. Disabil. Rehabil. 2014, 36, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Buyukavci, R.; Akturk, S.; Ersoy, Y. Evaluating the Functional Outcomes of Ultrasound-Guided Botulinum Toxin Type A Injections Using the Euro-Musculus Approach for Upper Limb Spasticity Treatment in Post-Stroke Patients: An Observational Study. Eur. J. Phys. Rehabil. Med. 2018, 54, 738–744. [Google Scholar] [CrossRef]
- Asimakidou, E.; Sidiropoulos, C. A Bayesian Network Meta-Analysis and Systematic Review of Guidance Techniques in Botulinum Toxin Injections and Their Hierarchy in the Treatment of Limb Spasticity. Toxins 2023, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Leydesdorff, L.; Vaughan, L. Co-Occurrence Matrices and Their Applications in Information Science: Extending ACA to the Web Environment. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 1616–1628. [Google Scholar] [CrossRef]
- Braam, R.R.; Moed, H.F.; van Raan, A.F.J. Mapping of Science by Combined Co-Citation and Word Analysis. II: Dynamical Aspects. J. Am. Soc. Inf. Sci. 1991, 42, 252–266. [Google Scholar] [CrossRef]
- Chen, C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Mapp. Sci. Front. Quest Knowl. Vis. 2003, 59, 364–369. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and Community Structure in Networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.C. Centrality in Social Networks Conceptual Clarification. Soc. Netw. 1978, 1, 215–239. [Google Scholar] [CrossRef]
- Kleinberg, J. Bursty and Hierarchical Structure in Streams. In Proceedings of the Eighth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Edmonton, AL, Canada, 23–26 July 2002; Association for Computing Machinery: New York, NY, USA, 2002; pp. 91–101. [Google Scholar]
- Deerwester, S.; Dumais, S.T.; Furnas, G.W.; Landauer, T.K.; Harshman, R. Indexing by Latent Semantic Analysis. J. Am. Soc. Inf. Sci. 1990, 41, 391–407. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to Conduct a Bibliometric Analysis: An Overview and Guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]

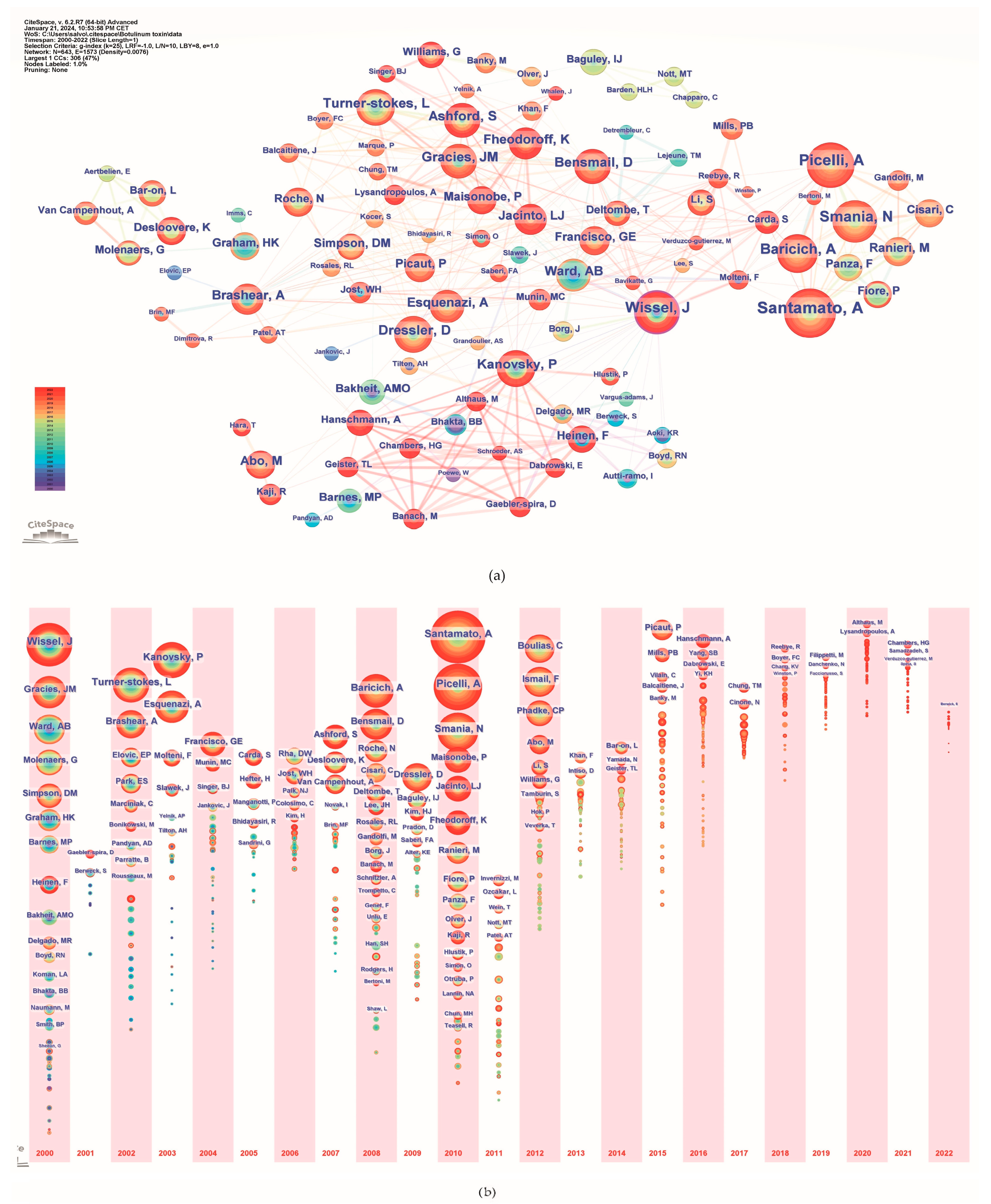
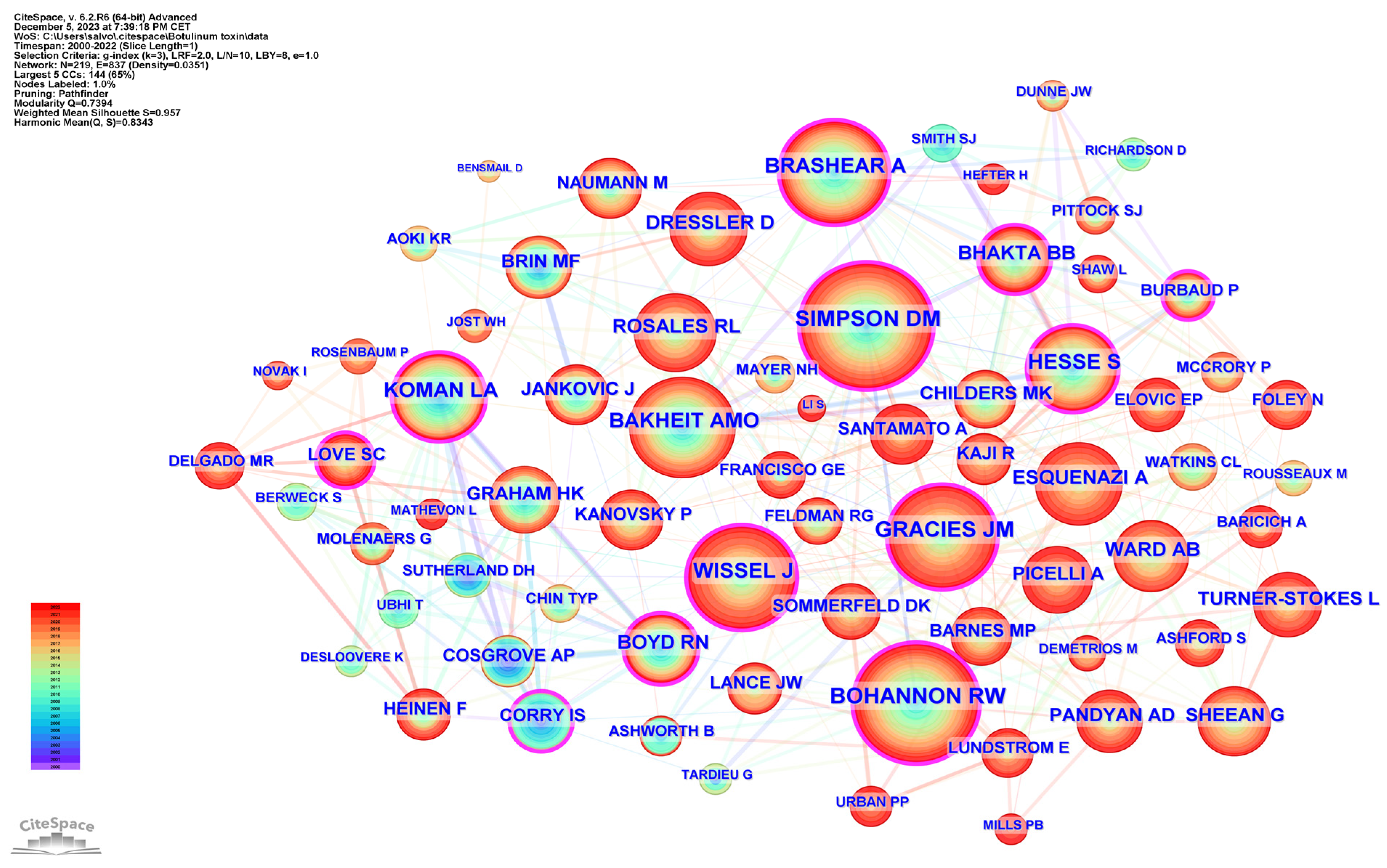
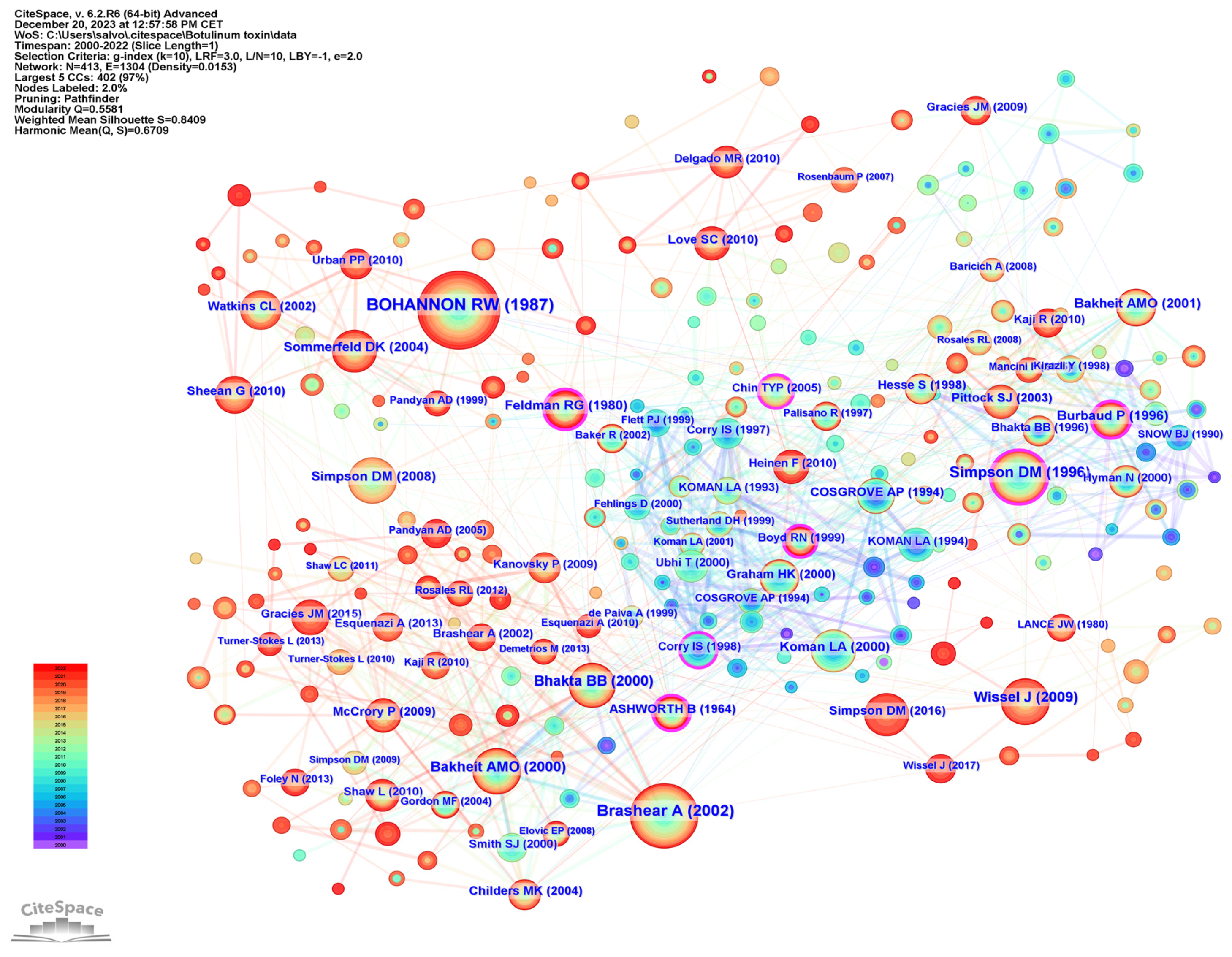
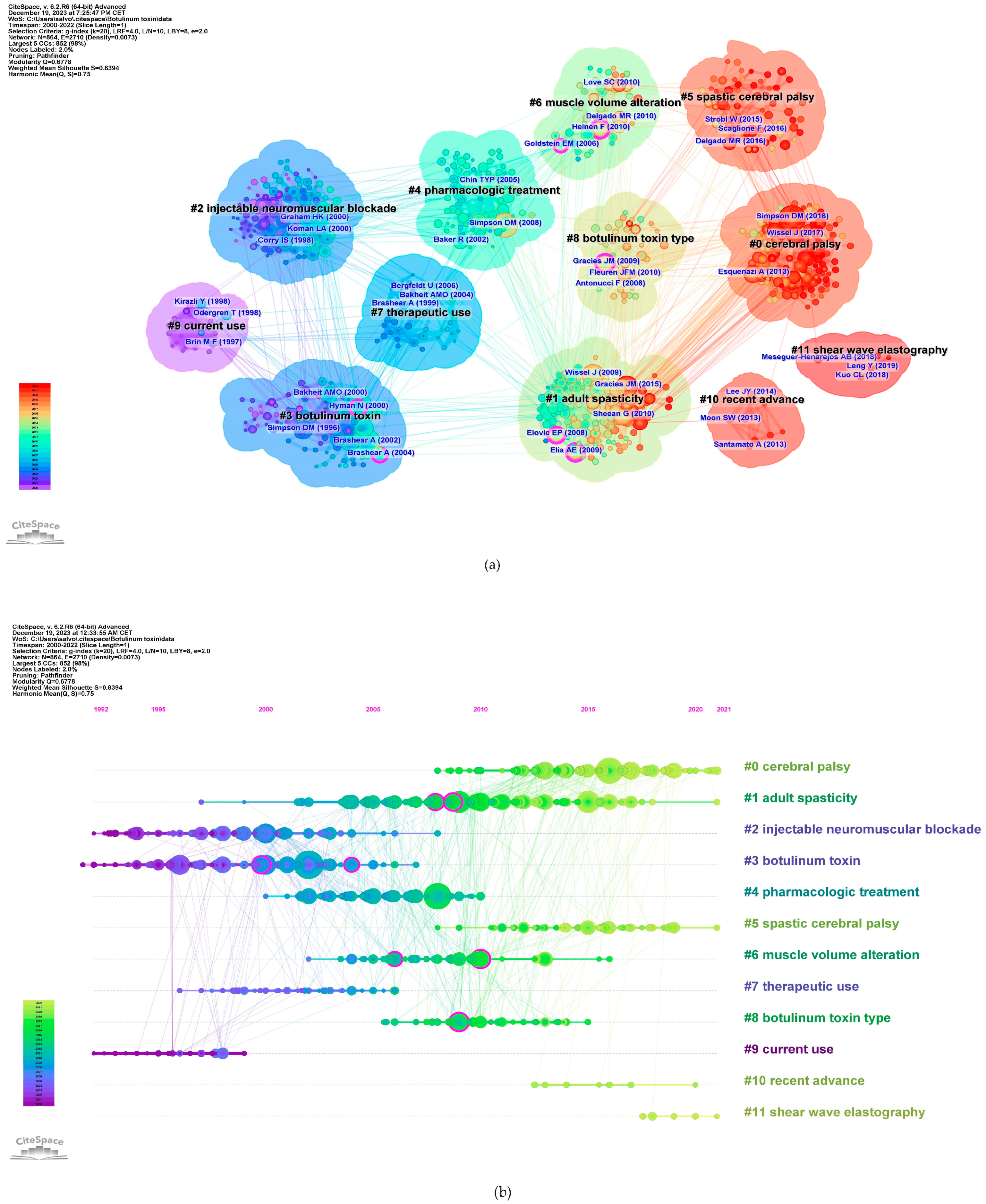

| Rank | Country Region | Publications | Country Region | Centrality |
|---|---|---|---|---|
| 1 | United States | 359 | United Kingdom | 0.20 |
| 2 | United Kingdom | 170 | United States | 0.15 |
| 3 | Italy | 169 | Italy | 0.10 |
| 4 | Germany | 148 | France | 0.09 |
| 5 | France | 146 | Taiwan | 0.09 |
| 6 | Australia | 127 | Germany | 0.08 |
| 7 | Canada | 96 | Morocco | 0.08 |
| 8 | South Korea | 83 | South Korea | 0.07 |
| 9 | Turkey | 81 | Turkey | 0.07 |
| 10 | Peoples R China | 66 | Poland | 0.07 |
| Rank | Institution | Country | Publications |
|---|---|---|---|
| 1 | Assistance Publique Hopitaux Paris (APHP) | France | 68 |
| 2 | UDICE-French Research Universities | France | 65 |
| 3 | University of London | United Kingdom | 53 |
| 4 | University of Verona | Italy | 50 |
| 5 | Universitè Paris Cite | France | 42 |
| 6 | University of Texas System | USA | 42 |
| 7 | University of Toronto | Canada | 42 |
| 8 | University of Foggia | Italy | 41 |
| 9 | University of Sydney | Australia | 41 |
| 10 | Yonsei University | Korea | 36 |
| Rank | Journal | P | IF | Co-Cited Journal | Cit | IF |
|---|---|---|---|---|---|---|
| 1 | Toxins | 81 | 5.075 | Archives of Physical Medicine and Rehabilitation | 964 | 4.060 |
| 2 | Journal of RehabilitationMedicine | 67 | 3.959 | Neurology | 870 | 12.258 |
| 3 | Archives of Physical Medicine and Rehabilitation | 48 | 4.060 | European Journal of Neurology | 840 | 6.288 |
| 4 | Developmental Medicine and Child Neurology | 44 | 4.864 | Clinical Rehabilitation | 729 | 2.884 |
| 5 | American Journal of Physical Medicine Rehabilitation | 43 | 3.412 | Journal of Neurology, Neurosurgery, and Psychiatry | 701 | 13.654 |
| 6 | Disability and Rehabilitation | 40 | 2.439 | American Journal of Physical Medicine Rehabilitation | 688 | 3.412 |
| 7 | Clinical Rehabilitation | 35 | 2.884 | Developmental Medicine and Child Neurology | 631 | 4.864 |
| 8 | PM&R | 33 | 2.218 | Muscle Nerve | 622 | 3.852 |
| 9 | European Journal of Physical and Rehabilitation Medicine | 25 | 5.313 | Physical Therapy | 598 | 3.140 |
| 10 | Frontiers in Neurology | 25 | 4.086 | Journal of Rehabilitation Medicine | 595 | 3.959 |
| Rank | Authors | Country | Institution | Centrality | P | H-Index |
|---|---|---|---|---|---|---|
| 1 | Santamato Andrea | Italy | Università degli Studi di Foggia | 0.02 | 41 | 33 |
| 2 | Picelli Alessandro | Italy | Università degli Studi di Verona | 0.07 | 36 | 35 |
| 3 | Wissel Jörg | Germany | Vivantes Klinikum-Spandau, | 0.12 | 34 | 48 |
| 4 | Smania Nicola | Italy | Università degli Studi di Verona | 0.02 | 30 | 51 |
| 5 | Kanovsky Peter | Czech Republic | Univerzita Palackého v Olomouci | 0.05 | 28 | 38 |
| 6 | Turner-Stokes Lynne | United Kingdom | Northwick Park Hospital | 0.05 | 27 | 47 |
| 7 | Baricich Alessio | Italy | Azienda Ospedaliera Maggiore della Carita di Novara | 0.00 | 26 | 23 |
| 8 | Esquenazi Alberto | USA | Moss Rehabilitation Research Institute | 0.03 | 25 | 38 |
| 9 | Gracies Jean Michel | France | Université Paris-Est Créteil | 0.01 | 25 | 40 |
| 10 | Bensmail Djamel | France | Université Paris-Saclay | 0.04 | 24 | 28 |
| 11 | Ismail Farooq | Canada | University of Toronto | 0.00 | 23 | 13 |
| 12 | Ward Anthony B. | United Kingdom | Haywood Community Hospital | 0.05 | 23 | 34 |
| 13 | Boulias Chris | Canada | University of Toronto | 0.00 | 22 | 13 |
| 14 | Brashear Allison | USA | University of California | 0.04 | 22 | 40 |
| 15 | Dressler Dirk | Germany | Hannover Medical School | 0.02 | 22 | 54 |
| Rank | Authors | Country | Institution | F | H-Index |
|---|---|---|---|---|---|
| 1 | Simpson David M. | USA | Icahn School of Medicine at Mount Sinai | 442 | 69 |
| 2 | Bohannon Richard W. | USA | Physical Therapy Consultants | 411 | 73 |
| 3 | Gracies Jean Michel | France | Université Paris-Est Créteil | 350 | 40 |
| 4 | Wissel Jörg | Germany | Vivantes Klinikum-Spandau, | 333 | 48 |
| 5 | Bakheit Abdel Magid O. | United Kingdom | Moseley Hall Hospital, Birmingham | 322 | 26 |
| 6 | Brashear Allison | USA | University of California | 317 | 40 |
| 7 | Koman L. Andrew | USA | Wake Forest University Health Sciences | 270 | 38 |
| 8 | Hesse Stefan | Germany | Medical Park Berlin Humboldtmühle | 265 | 51 |
| 9 | Esquenazi Alberto | USA | Moss Rehabilitation Research Institute | 210 | 38 |
| 10 | Bhakta Bipinchandra B. | United Kingdom | University of Leeds, School of Medicine | 207 | 34 |
| 11 | Rosales Raymond L. | Philippines | University of Santo Tomas Hospital | 199 | 29 |
| 12 | Dressler Dirk | Germany | Hannover Medical school | 198 | 54 |
| 13 | Boyd Roslyn N. | Australia | The University of Queensland | 195 | 64 |
| 14 | Ward Anthony B. | United Kingdom | Haywood Community Hospital | 193 | 34 |
| 15 | Jancovic Joseph | USA | Baylor College of Medicine | 181 | 157 |
| Rank | Title | Citations | First Author | Journal | Publication Year |
|---|---|---|---|---|---|
| 1 | Spasticity after stroke—Its occurrence and association with motor impairments and activity limitations [20] | 460 | Sommerfeld, DK | Stroke | 2004 |
| 2 | Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke [21] | 418 | Brashear, A | New England Journal Of Medicine | 2002 |
| 3 | CP [22] | 327 | Koman, l. | Lancet | 2004 |
| 4 | Pathophysiology of spastic paresis. II: Emergence of muscle overactivity [23] | 300 | Gracies, JM | Muscle & Nerve | 2005 |
| 5 | Recommendations for the use of BoNT-A in the management of CP [15] | 260 | Graham, HK | Gait & Posture | 2000 |
| 6 | Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review)—Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology [24] | 259 | Simpson, DM | Neurology | 2008 |
| 7 | Botulinum toxin in clinical practice [25] | 256 | Jancovic, J | Journal of Neurology Neurosurgery and Psychiatry | 2004 |
| 8 | European consensus table on the use of BoNT-A in adult spasticity [12] | 246 | Wissel, J | Journal Of Rehabilitation Medicine | 2009 |
| 9 | Impact of BoNT-A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial [26] | 236 | Bhakta, BB | Journal Of Neurology Neurosurgery And Psychiatry | 2000 |
| 10 | A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of BoNT-A (Dysport) with placebo in upper limb spasticity after stroke [27] | 232 | Bakheit, AMO | Stroke | 2000 |
| Rank | Title | Citations | First Author | Journal | Publication Year |
|---|---|---|---|---|---|
| 1 | A systematic review of interventions for children with CP: state of the evidence [28] | 757 | Novak, I | Developmental Medicine And Child Neurology | 2013 |
| 2 | Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache Report of the Guideline Development Subcommittee of the American Academy of Neurology [29] | 297 | Simpson, DM | Neurology | 2016 |
| 3 | Spasticity after stroke: Physiology, assessment and treatment [30] | 221 | Thibaut, A | Brain Injury | 2013 |
| 4 | Poststroke spasticity Sequelae and burden on stroke survivors and caregivers [31] | 150 | Zorowitz, RD | Neurology | 2015 |
| 5 | Safety and efficacy of AbobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double-blind randomised controlled trial [32] | 114 | Gracies, JM | Lancet Neurology | 2015 |
| 6 | Spasticity After Stroke An Overview of Prevalence, Test Instruments, and Treatments [33] | 113 | Sommerfeld, DK | American Journal of Physical Medicine & Rehabilitation | 2012 |
| 7 | Clinical applications of botulinum toxin [34] | 109 | Dressler, D | Current Opinion in Microbiology | 2012 |
| 8 | Spasticity, Motor Recovery, and Neural Plasticity after Stroke [35] | 108 | Li, S | Frontiers in Neurology | 2017 |
| 9 | New insights into the pathophysiology of post-stroke spasticity [36] | 107 | Li, S | Frontiers in Neuroscience | 2015 |
| 10 | Botulinum toxins: Mechanisms of action, antinociception and clinical applications [37] | 106 | Wheeler, A | Toxicology | 2013 |
| Category | Research Gap | Description |
|---|---|---|
| Clinical Efficacy and Safety | Long-term Efficacy and Safety | More studies are needed on the long-term effects of BoNT-A, especially in pediatric populations and various formulations. |
| Comparison Across Formulations | Limited research comparing the effectiveness and side effects of different BoNT-A formulations. | |
| Pharmacology and Treatment approaches | Dose Optimization | Research is required to optimize dosing for different patient groups and conditions. Find the correct Dosage/Timing ratio in different stages of disease and related economic aspects. |
| Specific Patient Populations | Need for focused research on BoNT-A’s use in specific populations, such as TBI, SCI, multiple sclerosis, or HSP. | |
| Early Intervention | Exploration is needed on the role and timing of BoNT-A treatment in early stages of conditions like stroke or cerebral palsy. | |
| Patient-Centered Research | Mechanisms of Action | Further investigation into the molecular and physiological mechanisms of BoNT-A’s therapeutic potential and limitations. |
| Development of Resistance | Investigate the development of resistance to BoNT-A, particularly in relation to neutralizing antibodies. | |
| Quality of Life and Functional Outcomes | Studies focusing on the impact of BoNT-A on quality of life and functional outcomes in different patient populations. | |
| Technology and Multimodal Approaches | Emerging Technologies | Research on integrating new technologies (like robotic therapy or brain stimulation) with BoNT-A treatment. |
| Adjunct Therapies and Multimodal Approaches | Detailed studies on the synergistic effects of adjunct therapies and multimodal treatments with BoNT-A are scarce. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Francisco, G.E.; Molteni, F.; Wissel, J.; Santamato, A. The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis. Toxins 2024, 16, 184. https://doi.org/10.3390/toxins16040184
Facciorusso S, Spina S, Picelli A, Baricich A, Francisco GE, Molteni F, Wissel J, Santamato A. The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis. Toxins. 2024; 16(4):184. https://doi.org/10.3390/toxins16040184
Chicago/Turabian StyleFacciorusso, Salvatore, Stefania Spina, Alessandro Picelli, Alessio Baricich, Gerard E. Francisco, Franco Molteni, Jörg Wissel, and Andrea Santamato. 2024. "The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis" Toxins 16, no. 4: 184. https://doi.org/10.3390/toxins16040184
APA StyleFacciorusso, S., Spina, S., Picelli, A., Baricich, A., Francisco, G. E., Molteni, F., Wissel, J., & Santamato, A. (2024). The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis. Toxins, 16(4), 184. https://doi.org/10.3390/toxins16040184