Are Treated Celiac Patients at Risk for Mycotoxins? An Italian Case-Study
Abstract
:1. Introduction
2. Results
2.1. Dietary Data
2.2. Mycotoxin Excretion
3. Discussion
4. Conclusions
5. Experimental Section
5.1. Chemicals
5.2. Subjects and Study Design
5.3. Urine Sample Collection and Extraction
5.4. UHPC-ESI-MS/MS Analysis
5.5. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, R.; Le Donne, C.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [PubMed]
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef] [PubMed]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Blaszkewicz, M.; Degen, G.H. Assessment of deoxynivalenol exposure among Bangladeshi and German adults by a biomarker-based approach. Toxicol. Lett. 2016, 258, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Dofkova, M.; Skarkova, J.; Pfohl-Leszkowicz, A.; Ruprich, J. Ochratoxin A Dietary Exposure of Ten Population Groups in the Czech Republic: Comparison with Data over the World. Toxins 2015, 7, 3608–3635. [Google Scholar] [CrossRef] [PubMed]
- López, P.; de Rijk, T.; Srpong, R.C.; Mengelers, M.J.B.; Castenmiller, J.J.M.; Alewijn, M. A mycotoxin-dedicated total diet study in the Netherlands in 2013: Part II—Occurrence. World Mycotoxin J. 2016, 9, 89–108. [Google Scholar] [CrossRef]
- De Boevre, M.; Jacxsens, L.; Lachat, C.; Eeckhout, M.; Di Mavungu, J.D.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; De Meulenaer, B.; et al. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Marchi, D.; Scussel, V.M. Fumonisins B1 and B2 in the corn-milling process and corn-based products, and evaluation of estimated daily intake. Food Addit. Contam. Part A 2016, 33, 339–345. [Google Scholar]
- European Food Safety Authority (EFSA). Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA J. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- D’Arco, G.; Fernández-Franzón, M.; Font, G.; Damiani, P.; Mañes, J. Survey of fumonisins B1, B2 and B3 in conventional and organic retail corn products in Spain and Italy and estimated dietary exposure. Food Addit. Contam. Part B 2009, 2, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Brera, C.; Debegnach, F.; De Santis, B.; Di Ianni, S.; Gregori, E.; Neuhold, S.; Valitutti, F. Exposure assessment to mycotoxins in gluten-free diet for celiac patients. Food Chem. Toxicol. 2014, 69, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Scarlato, A.P.; Galaverna, G.; Brighenti, F.; Pellegrini, N. Dietary exposure to fumonisins and evaluation of nutrient intake in a group of adult celiac patients on a gluten-free diet. Mol. Nutr. Food Res. 2012, 56, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; Ramos, A.J.; Marín, S.; Sanchis, V. Occurrence of fumonisins in Catalonia (Spain) and an exposure assessment of specific population groups. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 799–808. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; van Egmond, H.P.; Nauta, M.; Rombouts, F.M.; Notermans, S.H. Assessment of human exposure to fumonisin B1. J. Food Prot. 1998, 61, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.-A.; Montes, R.; Navarro, A.; Segarra, R.; Cuesta, G.; Hernández, E. Occurrence of deoxynivalenol and nivalenol in Spanish corn-based food products. J. Food Compos. Anal. 2008, 21, 423–427. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Galaverna, G.; Mangia, M.; Sforza, S.; Dossena, A.; Marchelli, R. Free and bound fumonisins in gluten-free food products. Mol. Nutr. Food Res. 2009, 53, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.; Njobeh, P.B.; Turner, P.C.; Kouanfack, C.; Eyongetah, M.; Dutton, M.; et al. Bio-monitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food Chem. Toxicol. 2013, 62, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wallin, S.; Gambacorta, L.; Kotova, N.; Lemming, E.W.C.; Nälsén, C.; Solfrizzo, M.; Olsen, M. Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem. Toxicol. 2015, 83, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.; Cramer, B.; Humpf, H.U. Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol. Nutr. Food. Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxin 2014, 6, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Tomba, C.; Elli, L.; Sieri, S.; Grioni, S.; Bardella, M.T.; Agostoni, C.; Doneda, L.; et al. Evaluation of a modified Italian EPIC food frequency questionnaire for individuals with celiac disease. J. Acad. Nutr. Diet. 2016, 116, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, B.; Martins, J.C.; Debongnie, P.; Uhlig, S.; Callebaut, A. Fast and sensitive LC-MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch. Toxicol. 2015, 89, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, A.B.; Fiates, G.M.; dos Anjos, A.; Teixeira, E. Gluten-free is not enough—Perception and suggestions of celiac consumers. Int. J. Food Sci. Nutr. 2014, 65, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Lattanzio, V.M.T.; Powers, S.; Visconti, A. Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal. Bioanal. Chem. 2011, 401, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC). Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Adam, G.; Frohlich, J.; Krska, R. Assessment of human deoxynivalenol exposure using an LC-MS/MS based biomarker method. Toxicol. Lett. 2012, 211, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Van der Westhuizen, L.; Shephard, G.; Burger, H.M.; Rheeder, J.P.; Gelderblom, W.C.A.; Wild, C.P.; Gong, Y.Y. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol. Biomark. Prev. 2011, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Torres, O.; Showker, J.L.; Zitomer, N.C.; Matute, J.; Voss, K.A.; Gelineau-van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol. Nutr. Food Res. 2012, 56, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- European Institute of Oncology: Food Composition Database for Epidemiological Studies in Italy. 2008. Available online: http://www.ieo.it/bda (accessed on 1 December 2014).
- Mazzeo, T.; Cauzzi, S.; Brighenti, F.; Pellegrini, N. The development of a composition database of gluten-free products. Public Health Nutr. 2015, 18, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Njumbe Ediage, E.; Wu, A.; De Saeger, S. Development and application of salting-out assisted liquid/liquid extraction for multi-mycotoxin biomarkers analysis in pig urine with high performance liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1292, 111–120. [Google Scholar] [CrossRef] [PubMed]
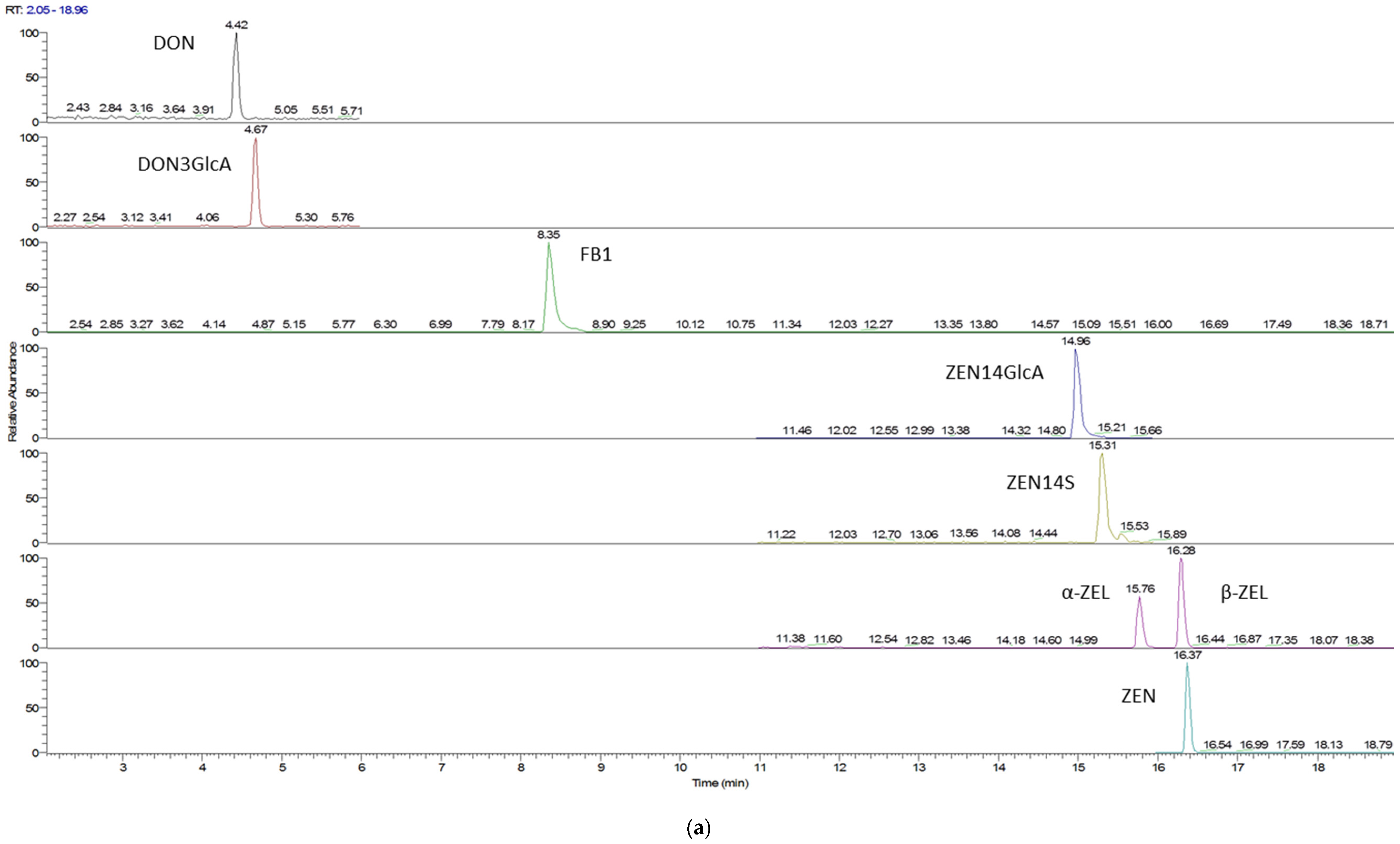
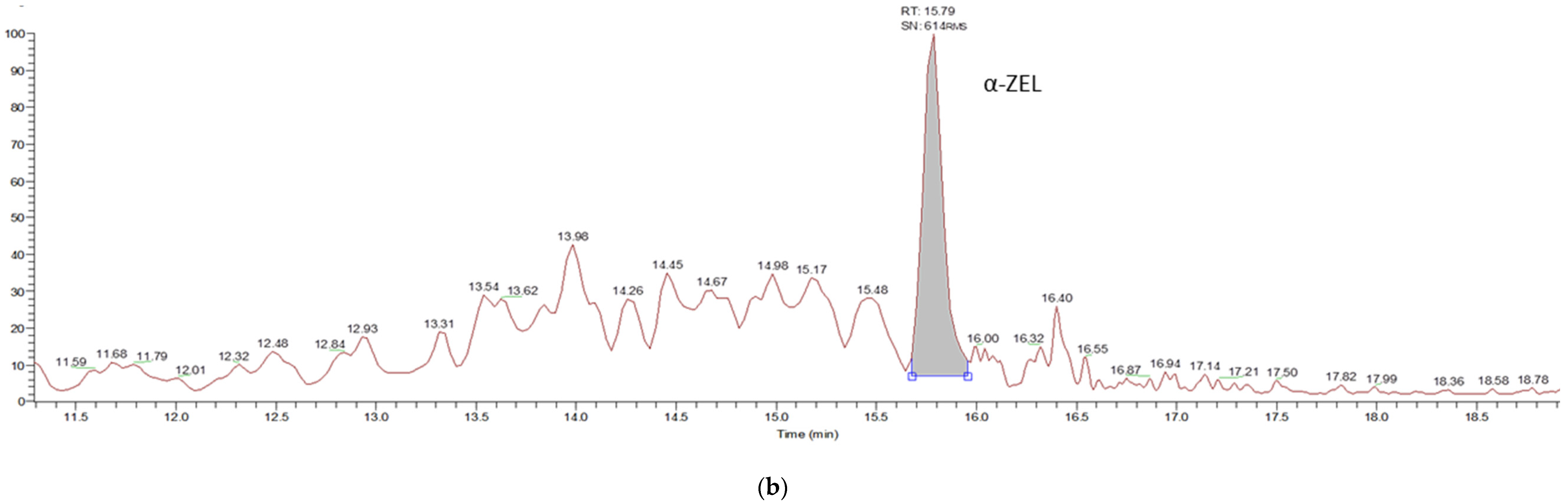
| Characteristics | Celiac Patients (n = 55) | Control Subjects (n = 50) |
|---|---|---|
| Age a, year | 43.2 ± 13.2 | 38.4 ± 13.6 |
| Body Mass Index a, kg/m2 | 22.8 ± 4.1 | 22.5 ± 3.9 |
| Energy Intake a, kcal/day | 2130 ± 281 | 2132 ± 309 |
| Female, n (%) | 42 (76) | 37 (74) |
| Food Categories (g/day) | Celiac Patients (n = 55) | Control Subjects (n = 50) | p-Value a | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Median | Range | Mean ± SE | Median | Range | ||
| Bread and substitutes | 84.5 ± 7.0 | 76.0 | 1.4–215.7 | 125.9 ± 7.6 | 112.8 | 15.0–268.6 | 0.000 |
| Whole-meal bread and substitutes | 3.3 ± 1.7 | 0.0 | 0.0–72.9 | 10.6 ± 2.7 | 0.0 | 0.0–74.3 | 0.000 |
| Flour | 3.7 ± 1.0 | 0.0 | 0.0–31.4 | 8.8 ± 1.7 | 3.6 | 0.0–48.0 | 0.003 |
| Pasta | 47.5 ± 4.4 | 44.3 | 0.0–167.1 | 51.1 ± 4.3 | 47.9 | 0.0–131.4 | 0.460 |
| Whole-meal pasta | 0.8 ± 0.5 | 0.0 | 0.0–22.9 | 2.20 ± 1.1 | 0.0 | 0.0–41.4 | 0.361 |
| Rice | 24.2 ± 2.8 | 20.9 | 0.0–81.4 | 13.7 ± 2.0 | 10.0 | 0.0–52.9 | 0.010 |
| Brown rice | 1.0 ± 0.6 | 0.0 | 0.0–22.9 | 1.11 ± 0.5 | 0.0 | 0.0–17.1 | 0.419 |
| Breakfast cereals | 9.5 ± 2.9 | 0.0 | 0.0–98.6 | 5.3 ± 1.9 | 0.0 | 0.0–83.6 | 0.402 |
| Biscuits | 23.7 ± 3.2 | 18.1 | 0.0–105.0 | 21.2 ± 3.1 | 12.0 | 0.0–107.1 | 0.744 |
| Whole-meal biscuits | 0.8 ± 0.5 | 0.0 | 0.0–20.0 | 2.0 ± 0.8 | 0.0 | 0.0–32.9 | 0.060 |
| Cakes | 32.1 ± 5.2 | 17.1 | 0.0–180.0 | 34.0 ± 4.0 | 26.5 | 0.0–114.3 | 0.259 |
| Other cereals | 19.8 ± 3.7 | 5.7 | 0.0–112.9 | 11.0 ± 2.7 | 0.0 | 0.0–75.7 | 0.043 |
| Total cereal-based food | 252.9 ± 9.1 | 248.3 | 123.3–411.3 | 286.1 ± 9.3 | 273.9 | 189.8–442.3 | 0.021 |
| Groups | DON | DON-GlcA | ZEN | α+βZEL | ZEN14GlcA | FB1 | |
|---|---|---|---|---|---|---|---|
| Control subjects | Mean ± SE (µg/day) | 0.92 ± 0.42 | 1.37 ± 0.79 | 0.01 ± 0.01 | 0.06 ± 0.04 | 0.06 ± 0.06 | 0.19 ± 0.12 |
| Mean ± SE (µg/L) | 0.17 ± 0.08 | 0.23 ± 0.13 | 0.03 ± 0.00 | 0.09 ± 0.01 | 0.02 ± 0.01 | 0.07 ± 0.05 | |
| Median (µg/day) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Median (µg/L) | 0.00 | 0.00 | 0.000 | 0.002 | 0.000 | 0.00 | |
| Range (min–max) (µg/day) | LOD *–3.91 | LOD *–8.75 | LOD *–0.08 | LOD *–0.50 | LOD *–0.83 | LOD *–1.52 | |
| Range (min–max) (µg/L) | LOD *–2.41 | LOD *–5.84 | LOD *–0.05 | LOD *–0.25 | LOD *–0.83 | LOD *–2.54 | |
| Freq. (%) (positive/total) | 10 (5/50) | 12 (6/50) | 10 (5/50) | 4 (2/50) | 4 (2/50) | 5 (3/50) | |
| Celiac patients | Mean ± SE (µg/day) | 1.09 ± 0.76 | 1.42 ± 0.72 | 0.04 ± 0.01 | 0.02 ± 0.12 | 0.00 | 0.00 |
| Mean ± SE (µg/L) | 0.22 ± 0.17 | 0.22 ± 0.13 | 0.01 ± 0.00 | 0.06 ± 0.04 | 0.02 ± 0.01 | 0.07 ± 0.05 | |
| Median (µg/day) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Median (µg/L) | 0.00 | 0.00 | 0.002 | 0.002 | 0.000 | 0.00 | |
| Range (min–max) (µg/day) | LOD *–14.31 | LOD *–10.98 | LOD *–0.22 | LOD *–2.19 | - | - | |
| Range (min–max) (µg/L) | LOD *–8.94 | LOD *–5.59 | LOD *–0.10 | LOD *–2.19 | - | - | |
| Freq. (%) (positive/total) | 7 (4/55) | 9 (5/55) | 25 (14/55) | 9 (5/55) | 0 (0/55) | 0 (0/55) |
| Group | Lower Bound | Upper Bound | |||||
|---|---|---|---|---|---|---|---|
| DON (µmol eq/day) | ZEN (µmol eq/day) | FB1 (nmol eq/day) | DON (µmol eq/day) | ZEN (µmol eq/day) | FB1 (nmol eq/day) | ||
| Control subjects | Mean ± SE | 0.22 ± 0.14 | 0.03 ± 0.02 | 0.07 ± 0.05 | 0.39 ± 0.17 | 0.09 ± 0.01 | 0.14 ± 0.04 |
| Median | 0.00 | 0.00 | 0.00 | 0.12 | 0.07 | 0.07 | |
| Range (min–max) | 0.00–5.50 | 0.00–0.54 | 0.00–2.11 | 0.11–7.21 | 0.07–0.59 | 0.07–2.11 | |
| Celiac patients | Mean ± SE | 0.26 ± 0.16 | 0.07 ± 0.04 | 0.00 ± 0.00 | 0.44 ± 0.21 | 0.14 ± 0.04 | 0.07 ± 0.00 |
| Median | 0.00 | 0.00 | 0.00 | 0.11 | 0.07 | 0.07 | |
| Range (min–max) | 0.00–6.89 | 0.00–2.17 | - | 0.11–9.03 | 0.07–2.24 | 0.07–0.07 | |
| p-value a | n.s. | n.s. | 0.033 | 0.000 | 0.000 | 0.033 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirlini, M.; Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Elli, L.; Bardella, M.T.; Agostoni, C.; Doneda, L.; Brighenti, F.; Dall’Asta, C.; et al. Are Treated Celiac Patients at Risk for Mycotoxins? An Italian Case-Study. Toxins 2017, 9, 11. https://doi.org/10.3390/toxins9010011
Cirlini M, Mazzeo T, Roncoroni L, Lombardo V, Elli L, Bardella MT, Agostoni C, Doneda L, Brighenti F, Dall’Asta C, et al. Are Treated Celiac Patients at Risk for Mycotoxins? An Italian Case-Study. Toxins. 2017; 9(1):11. https://doi.org/10.3390/toxins9010011
Chicago/Turabian StyleCirlini, Martina, Teresa Mazzeo, Leda Roncoroni, Vincenza Lombardo, Luca Elli, Maria T. Bardella, Carlo Agostoni, Luisa Doneda, Furio Brighenti, Chiara Dall’Asta, and et al. 2017. "Are Treated Celiac Patients at Risk for Mycotoxins? An Italian Case-Study" Toxins 9, no. 1: 11. https://doi.org/10.3390/toxins9010011
APA StyleCirlini, M., Mazzeo, T., Roncoroni, L., Lombardo, V., Elli, L., Bardella, M. T., Agostoni, C., Doneda, L., Brighenti, F., Dall’Asta, C., & Pellegrini, N. (2017). Are Treated Celiac Patients at Risk for Mycotoxins? An Italian Case-Study. Toxins, 9(1), 11. https://doi.org/10.3390/toxins9010011









