Forthcoming Challenges in Mycotoxins Toxicology Research for Safer Food—A Need for Multi-Omics Approach
Abstract
:1. Introduction: Food Toxicology Branched out from Toxicology
2. Mycotoxins and Myco-Cocktails: A Major Issue in Food Toxicology and Food Safety
3. Pleiotropy of Mycotoxins Action
3.1. Multiple Mycotoxins vs Multiple Targets
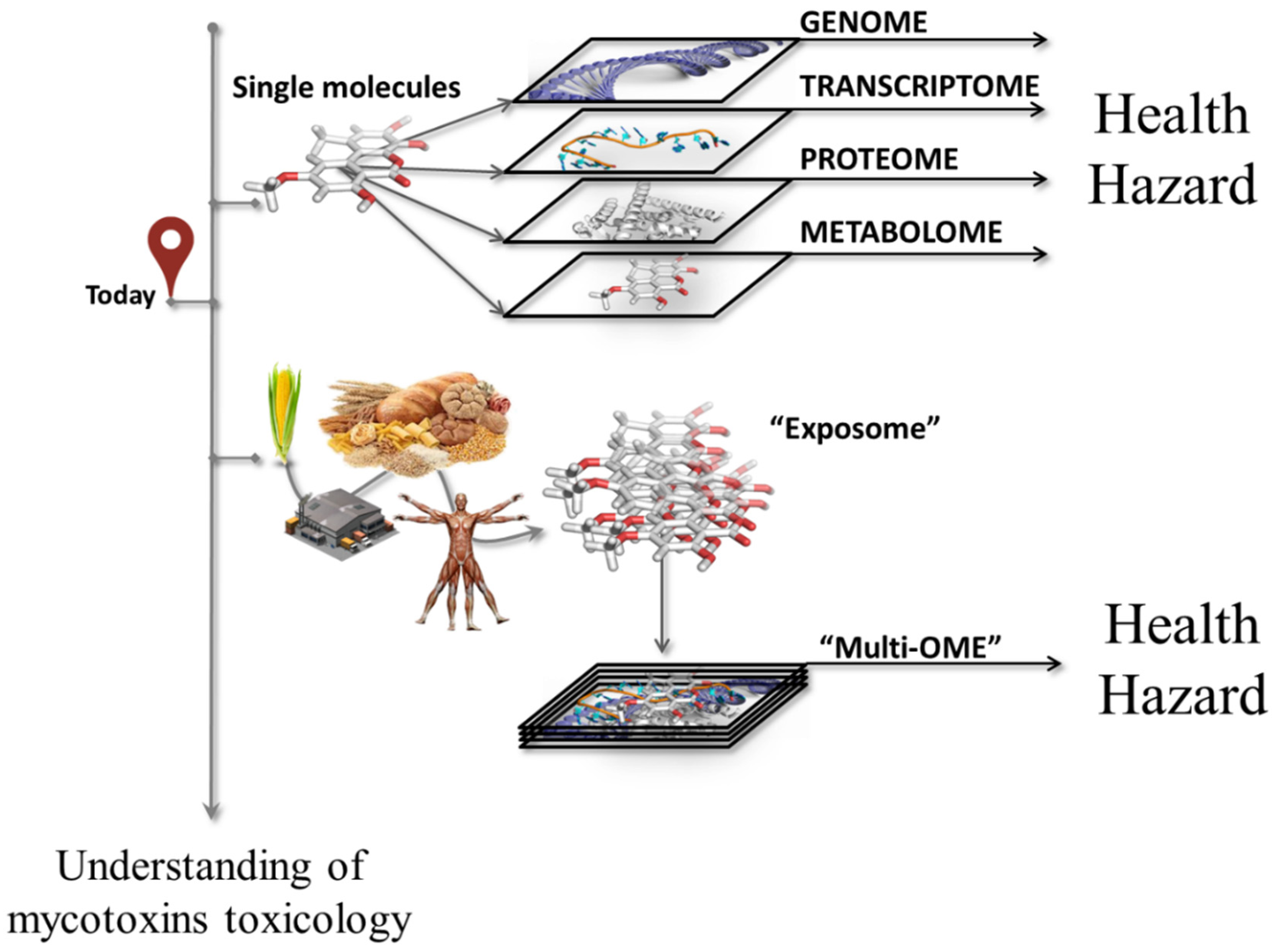
3.2. “Omics” Toxicity of Mycotoxins
3.3. “Omics” Methodologies for Toxicological Research
4. Combined Toxicity of Mycotoxins and Other Xenobiotics
5. Multi-omics Approach as the Toolbox for Unraveling Combined Toxicity
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kotsonis, F.N.; Burdock, G.A. Food Toxicology. In Casarett & Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; Klaassen, C.D., Ed.; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Shaw, I.C. Chemical residues, food additives and natural toxicants in food—The cocktail effect. Int. J. Food Sci. Tech. 2014, 49, 2149–2157. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Vaghini, S.; Cilla, A.; Garcia-Llatas, G.; Lagarda, M.J. Bioaccessibility study of plant sterol-enriched fermented milks. Food Funct. 2016, 7, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Papke, O.; Dellarco, M. Dioxin, dibenzofuran, and PCB congeners in cooked and uncooked food. Organohalogen Comp. 1997, 33, 462–466. [Google Scholar]
- Ouédraogo, O.; Amyot, M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ. Res. 2011, 111, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G. Current issues and perspectives in food safety and risk assessment. Hum. Exp. Toxicol. 2015, 34, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotoxin J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- Bennet, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Susca, A.; Mulé, G.; Logrieco, A.F.; Proctor, R.H. Molecular biodiversity of mycotoxigenic fungi that threaten food safety. Int. J. Food Microbiol. 2013, 167, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Ruiz, M.J.; Font, G.; Berrada, H. Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 2013, 93, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Marr, J.S.; Malloy, C.D. An epidemiologic analysis of the ten plagues of Egypt. Caduceus 1996, 12, 7–24. [Google Scholar] [PubMed]
- Dellafiora, L.; Dall’Asta, C.; Cozzini, P. Ergot alkaloids: From witchcraft till in silico analysis.Multi-receptor analysis of ergotamine metabolites. Toxicol. Rep. 2015, 2, 535–545. [Google Scholar] [CrossRef]
- Alm, T. The witch trials of Finnmark, northern Norway, during the 17th century: Evidence for ergotism as a contributing factor. Econ. Bot. 2003, 57, 403–416. [Google Scholar] [CrossRef]
- Packer, S. Jewish mystical movements and the European ergot epidemics. Israel J. Psychatr. Relat. Sci. 1998, 35, 227–239. [Google Scholar]
- Blount, W.P. Turkey ‘X’ disease. J. Brit. Turkey Fed. 1961, 9, 55–58. [Google Scholar]
- Pitschmann, V. Overall view of chemical and biochemical weapons. Toxins 2014, 6, 1761–1784. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.F. The trichothecenes and their biosynthesis. Prog. Chem. Org. Nat. Prod. 1997, 88, 63–130. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.E.; Løes, A.K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. Mycotoxins: Pathogenicity factors or virulence factors? Mycoses 2008, 51, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathanail, A.V.; Syvahuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Graniczkowska, K.; De Saeger, S. Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol. Lett. 2015, 233, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Generotti, S.; Cirlini, M.; Malachova, A.; Sulyok, M.; Berthiller, F.; Dall’Asta, C.; Suman, M. Deoxynivalenol & Deoxynivalenol-3-Glucoside Mitigation through Bakery Production Strategies: Effective Experimental Design within Industrial Rusk-Making Technology. Toxins 2015, 7, 2773–2790. [Google Scholar] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Humpf, H.U.; Voss, K.A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Feist, M.; Proske, M.; Koch, M.; Nehls, I. Degradation of the Alternaria mycotoxins alternariol, alternariol monomethyl ether, and altenuene upon bread baking. J. Agric. Food Chem. 2010, 58, 9622–9630. [Google Scholar] [CrossRef] [PubMed]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Ehrlich, V.A.; Dellafiora, L.; Mollergues, J.; Dall’Asta, C.; Serrant, P.; Marin-Kuan, M.; Lo Piparo, E.; Schilter, B.; Cozzini, P. Hazard assessment through hybrid in vitro/in silico approach: The case of zearalenone. Altex 2015, 32, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 1987, 7, 253–306. [Google Scholar] [CrossRef]
- Liu, G.T.; Qian, Y.Z.; Zhang, P.; Dong, W.H.; Qi, Y.M.; Guo, H.T. Etiological role of Alternaria alternata in human esophageal cancer. Chin. Med. J. (Engl.) 1992, 105, 394–400. [Google Scholar] [PubMed]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the IIα isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Vejdovszky, K.; Schmidt, V.; Warth, B.; Marko, D. Combinatory estrogenic effects between the isoflavone genistein and the mycotoxins zearalenone and alternariol in vitro. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Vejdovszky, K.; Hahn, K.; Braun, D.; Warth, B.; Marko, D. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Dall’Asta, C.; Cruciani, G.; Galaverna, G.; Cozzini, P. Molecular modelling approach to evaluate poisoning of topoisomerase I by alternariol derivatives. Food Chem. 2015, 189, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Son, H.Y.; Cho, S.W.; Ha, C.S.; Kang, B.H. Zearalenone induces male germ cell apoptosis in rats. Toxicol. Lett. 2003, 138, 185–192. [Google Scholar] [CrossRef]
- Tatay, E.; Font, G.; Ruiz, M.J. Cytotoxic effects of zearalenone and its metabolites and antioxidant cell defense in CHO-K1 cells. Food Chem. Toxicol. 2016, 96, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. In Vitro 2016, 36, 120–132. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization & International Agency for Research on Cancer. International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 2002; pp. 1–601. [Google Scholar]
- Besaratinia, A.; Kim, S.I.; Hainaut, P.; Pfeifer, G.P. In vitro recapitulating of TP53 mutagenesis in hepatocellular carcinoma associated with dietary aflatoxin B1 exposure. Gastroenterology 2009, 137, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Huang, Y.; Yang, Y.; Wang, S. Identification of AFB1-interacting proteins and interactions between RPSA and AFB1. J. Hazard Mater. 2016, 301, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Supriya, C.; Girish, B.P.; Reddy, P.S. Aflatoxin B1-Induced Reproductive Toxicity in Male Rats: Possible Mechanism of Action. Int. J. Toxicol. 2014, 33, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.J.; Vidal, N.; di Scala, C.; Varini, K.; Maresca, M. The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia 2011. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Katika, M.R.; Hendriksen, P.J.; Shao, J.; Van Loveren, H.; Peijnenburg, A. Transcriptome analysis of the human T lymphocyte cell line Jurkat and human peripheral blood mononuclear cells exposed to deoxynivalenol (DON): New mechanistic insights. Toxicol. Appl. Pharmacol. 2012, 264, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Wang, S.B.; Wang, R.G.; Zhang, W.; Wang, P.L.; Su, X.O. Phosphoproteome Analysis Reveals the Molecular Mechanisms Underlying Deoxynivalenol-Induced Intestinal Toxicity in IPEC-J2 Cells. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; di Pasquale, E.; Perrier, J.; Oswald, I.P.; Maresca, M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Pujol, A.; Nicoletti, C.; Pinton, P.; Armand, L.; di Pasquale, E.; Oswald, I.P.; Perrier, J.; Maresca, M. The Food-Associated Ribotoxin Deoxynivalenol Modulates Inducible NO Synthase in Human Intestinal Cell Model. Toxicol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Whitten, D.A.; Wilkerson, C.G.; Pestka, J.J. Dynamic changes in ribosome-associated proteome and phosphoproteome during deoxynivalenol-induced translation inhibition and ribotoxic stress. Toxicol Sci. 2014, 138, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Dinu, D.; Bodea, G.O.; Ceapa, C.D.; Munteanu, M.C.; Roming, F.I.; Serban, A.I.; Hermenean, A.; Costache, M.; Zarnescu, O.; Dinischiotu, A. Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon 2011, 57, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Hofstad, A.N.; Nussbaumer, T.; Akhunov, E.; Shin, S.; Kugler, K.G.; Kistler, H.C.; Mayer, K.F.; Muehlbauer, G.J. Examining the Transcriptional Response in Wheat Near-Isogenic Lines to Infection and Deoxynivalenol Treatment. Plant Gen. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer, T.; Warth, B.; Sharma, S.; Ametz, C.; Bueschl, C.; Parich, A.; Pfeifer, M.; Siegwart, G.; Steiner, B.; Lemmens, M.; et al. Joint Transcriptomic and Metabolomic Analyses Reveal Changes in the Primary Metabolism and Imbalances in the Subgenome Orchestration in the Bread Wheat Molecular Response to Fusarium graminearum. G3 (Bethesda) 2015, 5, 2579–2592. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, J.F.; Lombard, M.J.; Du Plessis, L.H.; Zandberg, L. Evaluation of the cytotoxic properties, gene expression profiles and secondary signalling responses of cultured cells exposed to fumonisin B1, deoxynivalenol and zearalenone mycotoxins. Arch. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, J.; Zhang, X.; Bian, X. MicroRNAs as key mediators of hepatic detoxification. Toxicology 2016, 10, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Bolleyn, J.; De Kock, J.; Rodrigues, R.M.; Vinken, M.; Rogiers, V.; Vanhaecke, T. MicroRNAs as key regulators of xenobiotic biotransformation and drug response. Arch. Toxicol. 2015, 89, 1523–1541. [Google Scholar] [CrossRef] [PubMed]
- Mattes, W.B.; Pettit, S.D.; Sansone, S.A.; Bushel, P.R.; Waters, M.D. Database development in toxicogenomics: Issues and efforts. Environ. Health Perspect. 2004, 112, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.; Afriyie-Gyawu, E.; Huebner, H.J.; Porter, W.; Wang, J.S.; Jolly, P.E.; Phillips, T.D. Noninvasive identification of interindividual variation in xenobiotic-metabolizing enzymes: Implications for cancer epidemiology and biomarker studies. J. Toxicol. Environ. Health A 2006, 69, 1203–1216. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, A.; Raggi, M.E.; Marabotti, A.; Milanesi, L. Ochratoxin A as possible factor trigging autism and its male prevalence via epigenetic mechanism. Nutr. Neurosci. 2016, 19, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Buick, J.K.; Moffat, I.; Williams, A.; Swartz, C.D.; Recio, L.; Hyduke, D.R.; Li, H.H.; Fornace, A.J., Jr.; Aubrecht, J.; Yauk, C.L. Integration of metabolic activation with a predictive toxicogenomics signature to classify genotoxic versus nongenotoxic chemicals in human TK6 cells. Environ. Mol. Mutagen. 2015, 56, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Föllmann, W.; Ali, N.; Blaszkewicz, M.; Degen, G.H. Biomonitoring of Mycotoxins in Urine: Pilot Study in Mill Workers. J. Toxicol. Environ. Health A 2016, 79, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, C.; Ellmer, D.; Mikula, H.; Pahlke, G.; Warth, B.; Gehrke, H.; Zimmermann, K.; Heiss, E.; Fröhlich, J.; Marko, D. Impact of phase I metabolism on uptake, oxidative stress and genotoxicity of the emerging mycotoxin alternariol and its monomethyl ether in esophageal cells. Arch. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Svingen, T.; Lund Hansen, N.; Taxvig, C.; Vinggaard, A.M.; Jensen, U.; Have Rasmussen, P. Enniatin B and beauvericin are common in Danish cereals and show high hepatotoxicity on a high-content imaging platform. Environ. Toxicol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, C.; Frizzell, C.; Shannon, M.; Ruiz, M.J.; Connolly, L. An in vitro investigation on the cytotoxic and nuclear receptor transcriptional activity of the mycotoxins fumonisin B1 and beauvericin. Toxicol. Lett. 2016, 257, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Connolly, L.; Frizzell, C.; Elliott, C.T. High content analysis: A sensitive tool to detect and quantify the cytotoxic, synergistic and antagonistic effects of chemical contaminants in foods. Toxicol. Lett. 2015, 233, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-Term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; Oswald, I.P. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Dall'Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; MacDonald, S.; Lloyd, A.; Tarbin, J. Masked trichothecene and zearalenone mycotoxins withstand digestion and absorption in the upper GI tract but are efficiently hydrolyzed by human gut microbiota in vitro. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Barilli, A.; Galaverna, G.; Michlmayr, H.; Adam, G.; Berthiller, F.; Dall'Asta, C. Study on the uptake and deglycosylation of the masked forms of zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2016, 98, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Galaverna, G.; Righi, F.; Cozzini, P.; Dall'Asta, C. Assessing the hydrolytic fate of the masked mycotoxin zearalenone-14-glucoside - A warning light for the need to look at the “maskedome”. Food Chem. Toxicol. 2017, 99, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 cytotoxicity againsthuman intestinal Caco-2 cells is enhanced in the presence of other mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E.; López De Cerain, A. Co-occurrence of aflatoxins, ochratoxin A and zearalenone in barley from a northern region of Spain. Food Chem. 2012, 132, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sancho, G.; González-Arias, C.A.; Ramos, A.J.; Sanchis, V.; Fernández-Cruz, M.L. Cytotoxicity of the mycotoxins deoxynivalenol and ochratoxin A on Caco-2 cell line in presence of resveratrol. Toxicol. In Vitro 2015, 29, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G.; Beisl, J.; Marko, D. Genistein and delphinidin antagonize the genotoxic effects of the mycotoxin alternariol in human colon carcinoma cells. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gramec Skledar, D.; Peterlin Mašič, L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, J.; Wu, Y.; Shao, B. Analysis of bisphenol A and alkylphenols in cereals by automated on-line solid-phase extraction and liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 6116–6122. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Garsou, S.; Schaut, A.; De Saeger, S.; Pussemier, L.; Van Peteghem, C.; Larondelle, Y.; Schneider, Y.J. Differential modulation of ochratoxin A absorption across Caco-2 cells by dietary polyphenols, used at realistic intestinal concentrations. Toxicol. Lett. 2005, 159, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; De Saeger, S.; Van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dellafiora, L.; Dall’Asta, C. Forthcoming Challenges in Mycotoxins Toxicology Research for Safer Food—A Need for Multi-Omics Approach. Toxins 2017, 9, 18. https://doi.org/10.3390/toxins9010018
Dellafiora L, Dall’Asta C. Forthcoming Challenges in Mycotoxins Toxicology Research for Safer Food—A Need for Multi-Omics Approach. Toxins. 2017; 9(1):18. https://doi.org/10.3390/toxins9010018
Chicago/Turabian StyleDellafiora, Luca, and Chiara Dall’Asta. 2017. "Forthcoming Challenges in Mycotoxins Toxicology Research for Safer Food—A Need for Multi-Omics Approach" Toxins 9, no. 1: 18. https://doi.org/10.3390/toxins9010018
APA StyleDellafiora, L., & Dall’Asta, C. (2017). Forthcoming Challenges in Mycotoxins Toxicology Research for Safer Food—A Need for Multi-Omics Approach. Toxins, 9(1), 18. https://doi.org/10.3390/toxins9010018