Fabrication and Polishing Performance of Diamond Self-Sharpening Gel Polishing Disk
Abstract
:1. Introduction
2. Self-Sharpening Mechanism and Preparation of a Polishing Disk
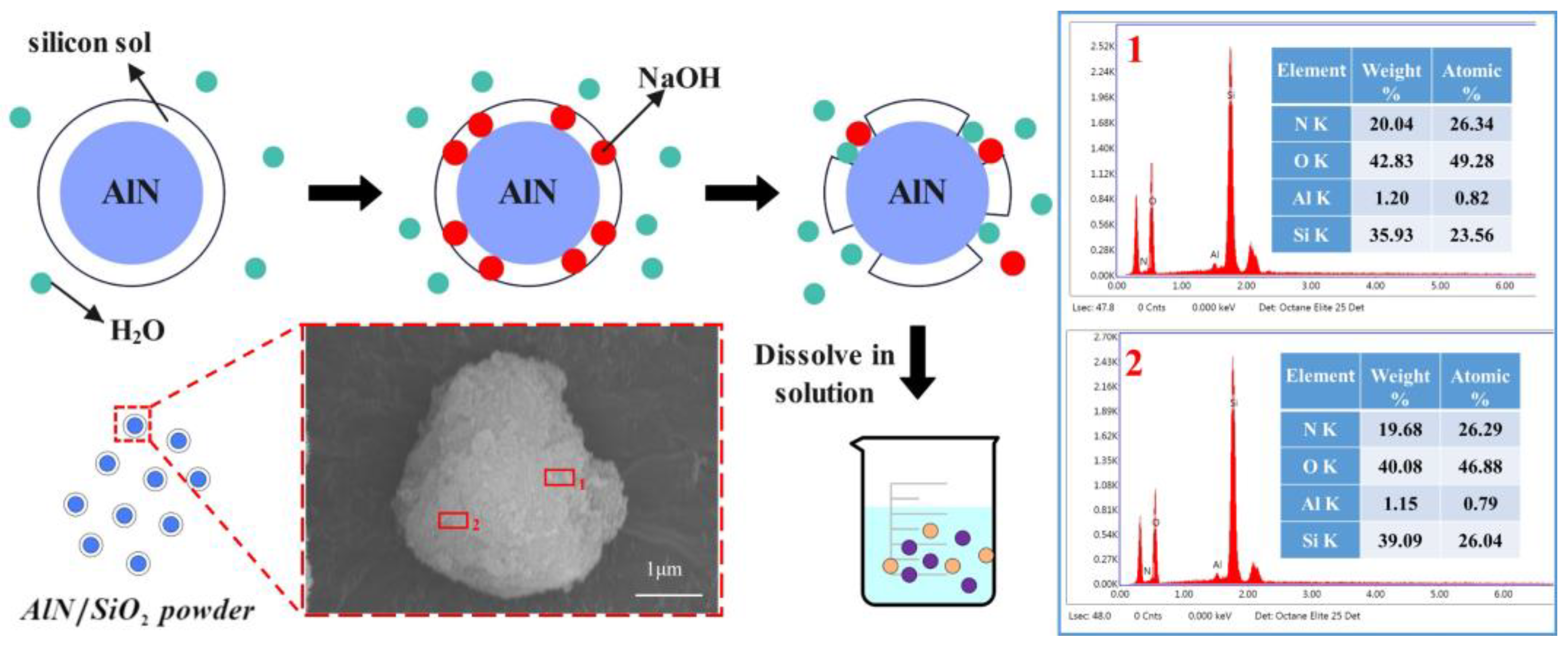
2.1. Self-Sharpening Mechanism
2.2. Preparation of Polishing Disk
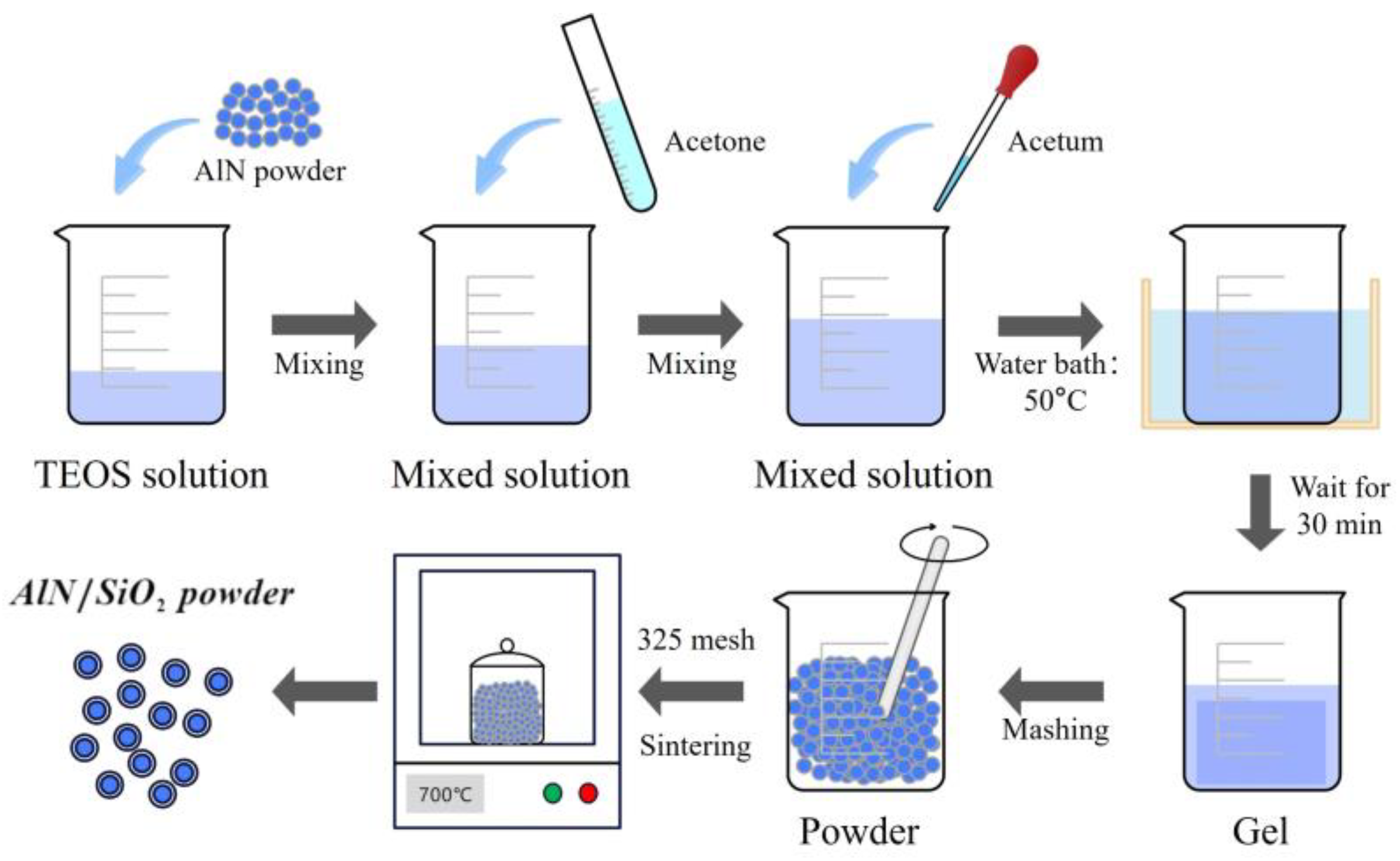
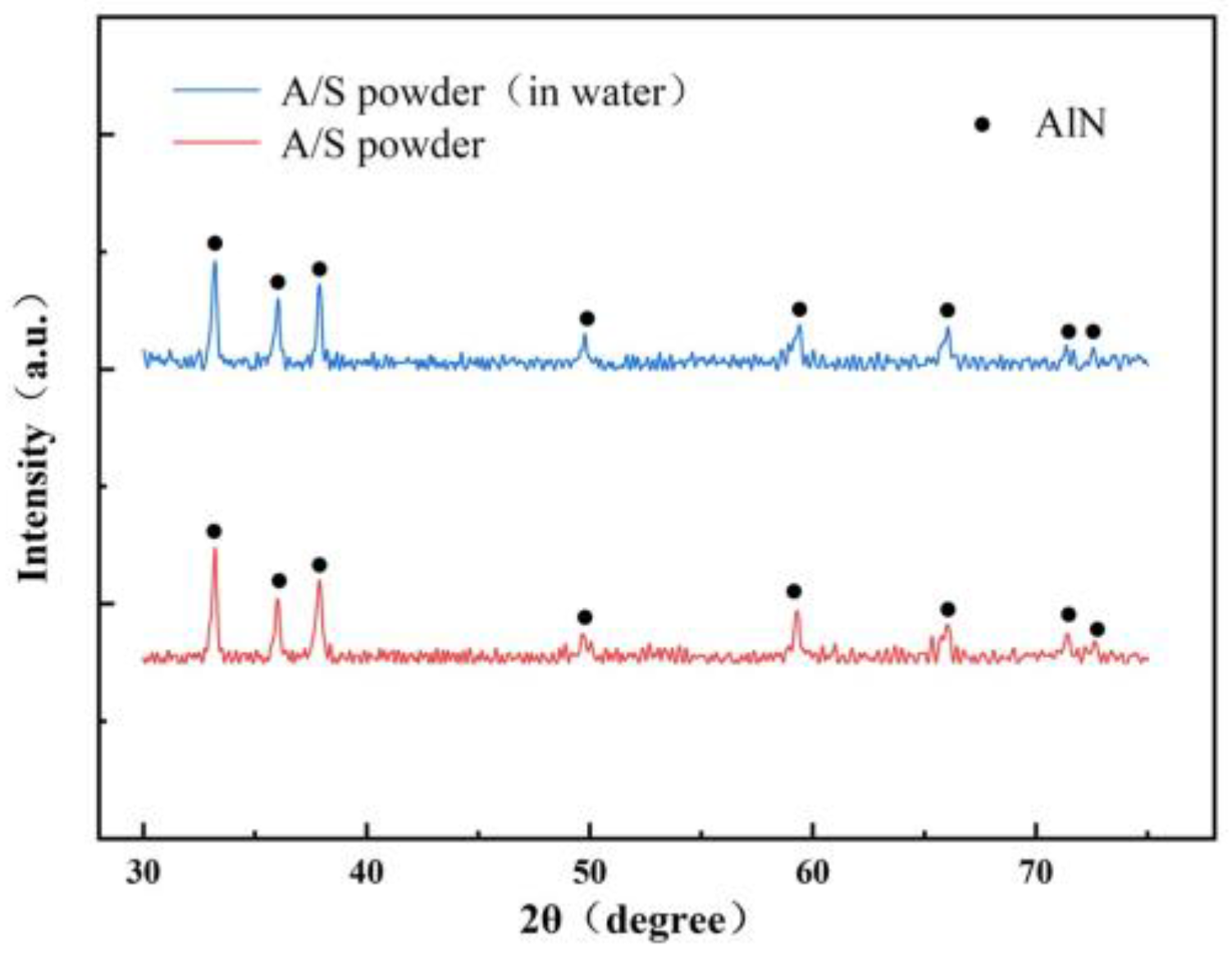
2.2.1. Preparation of A/S Powder
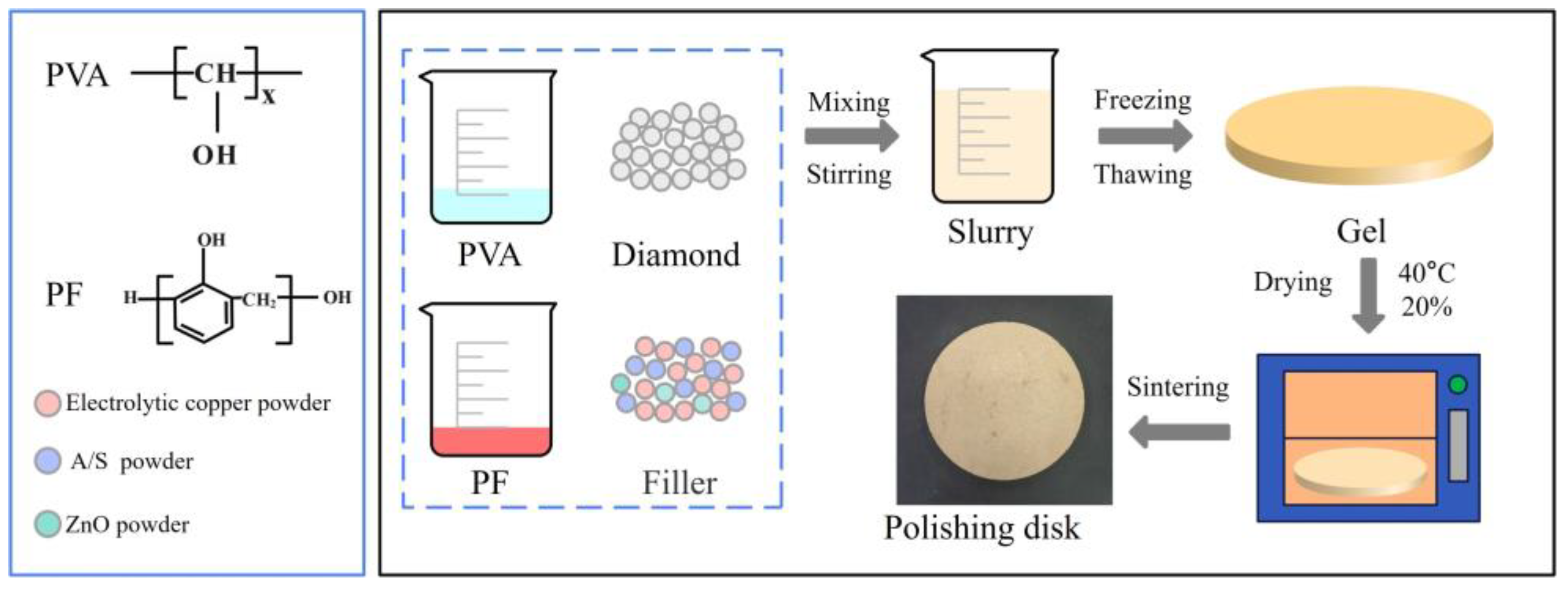
2.2.2. Preparation of the Polishing Disk
3. Equipment and Experimental Set-Up
3.1. Equipment
3.2. Setup of Experimental Set-Up
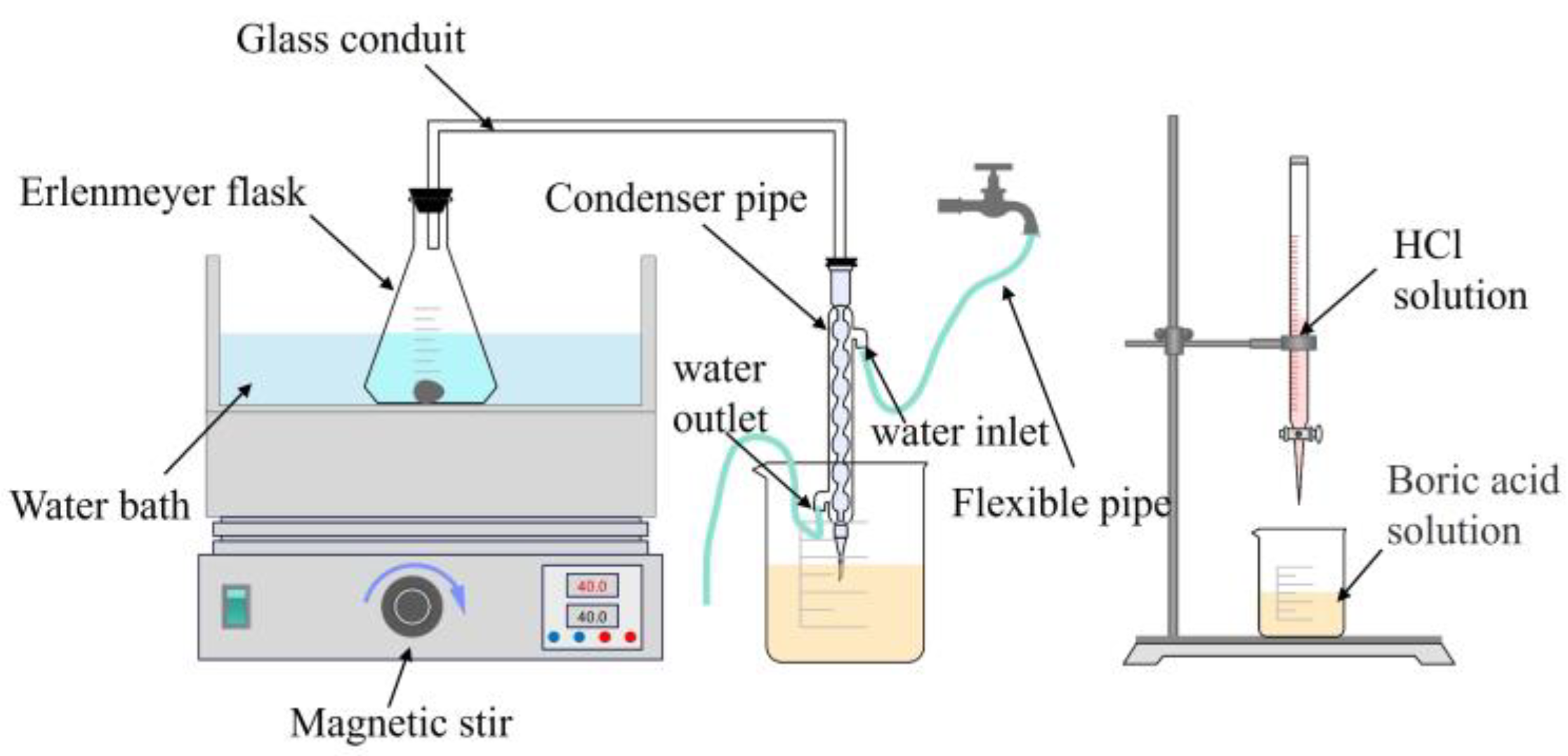
3.2.1. Setup of Dissolution Experiment
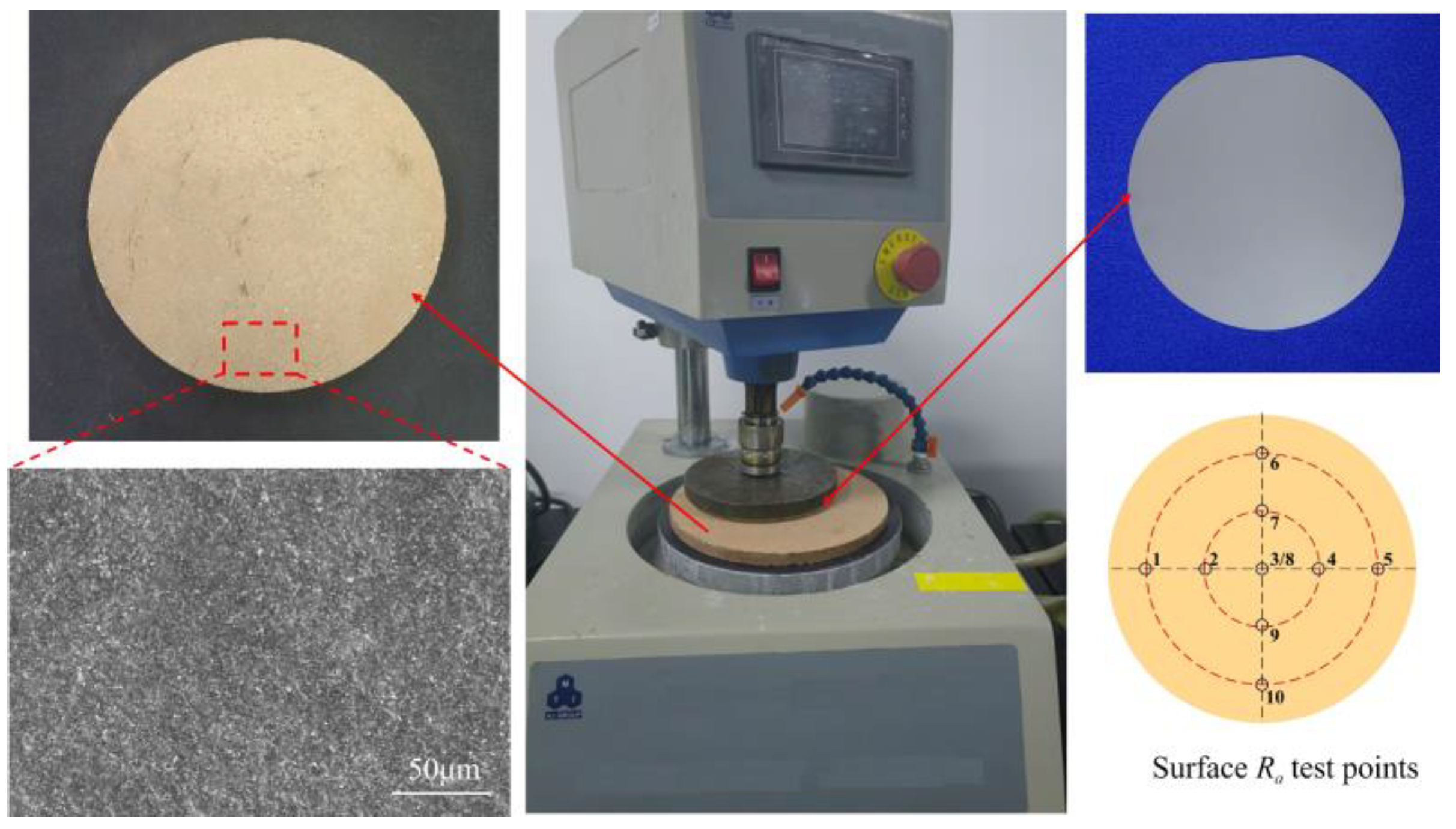
3.2.2. Setup of the Polishing Experiment
4. Results and Discussion
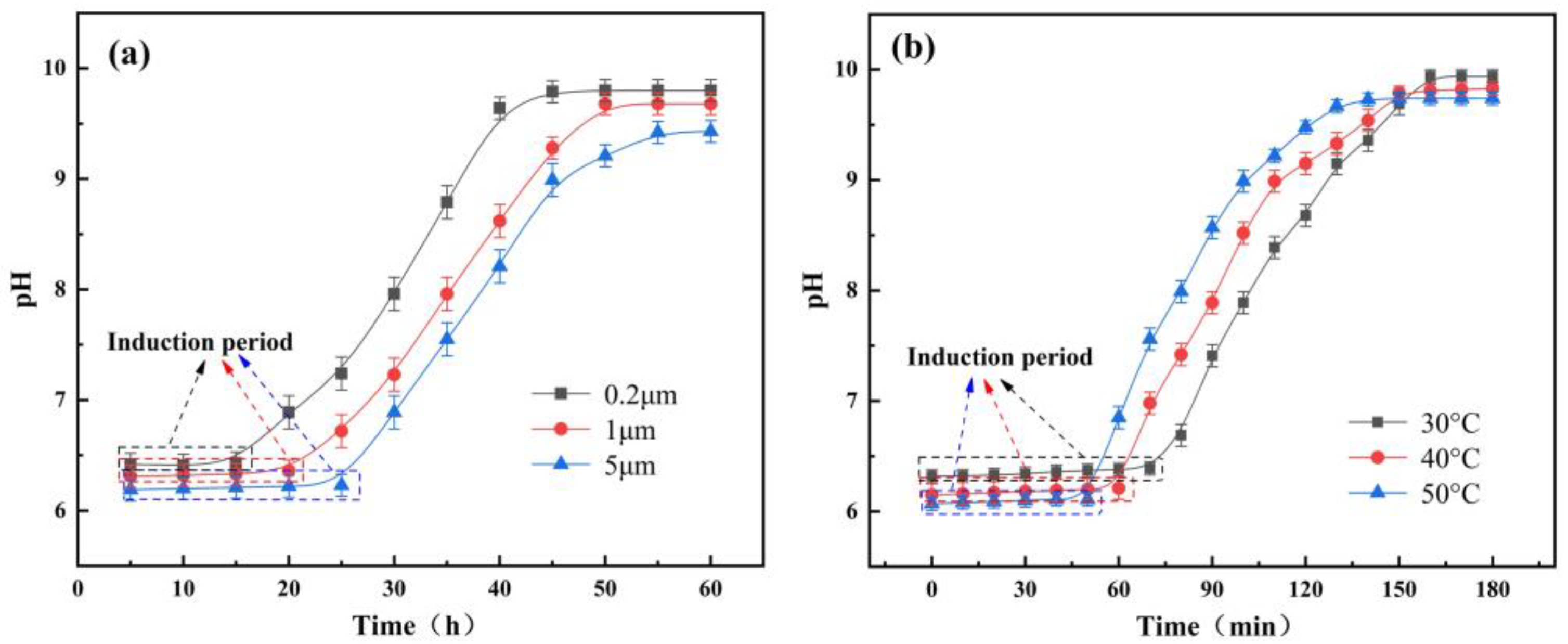
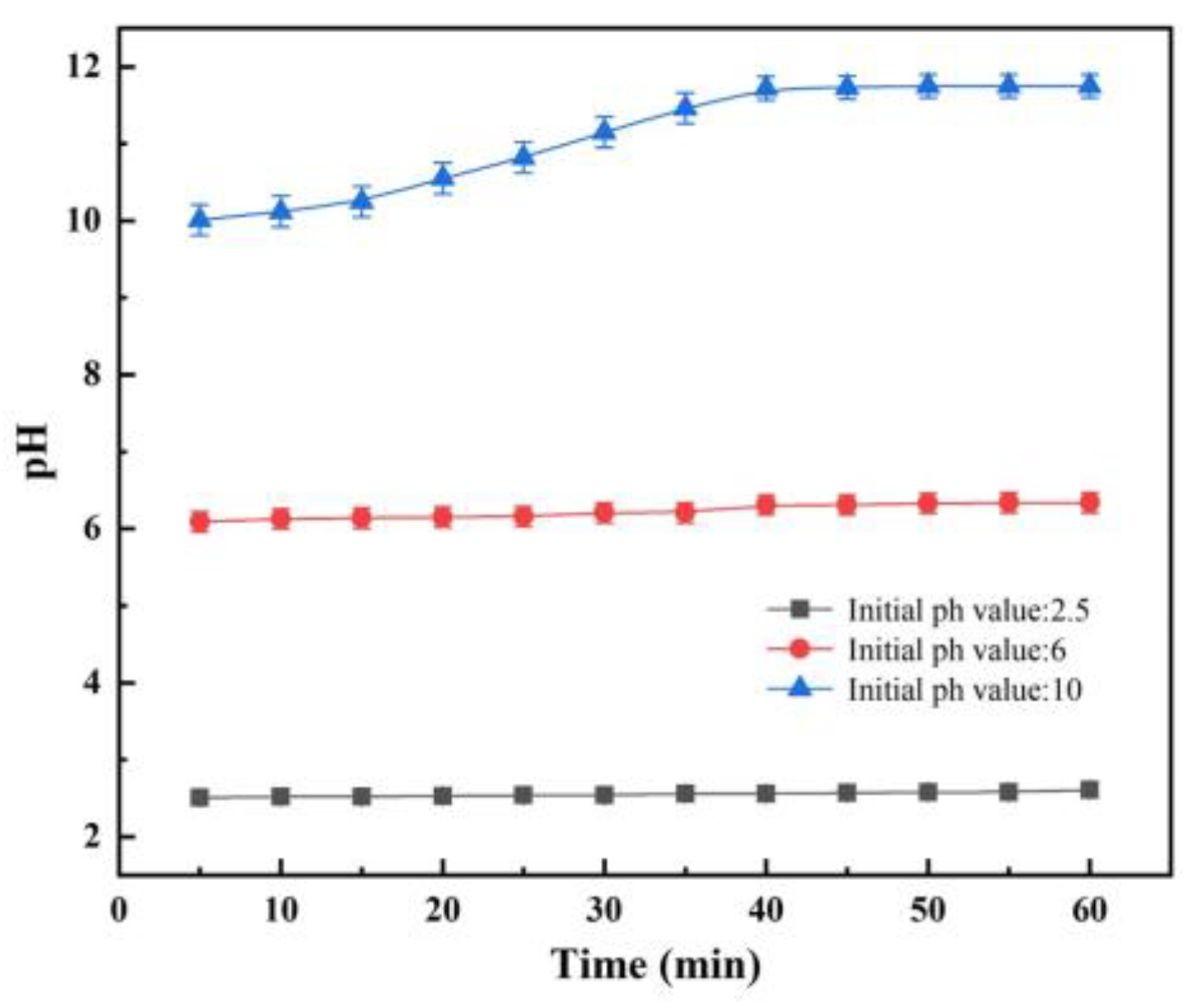
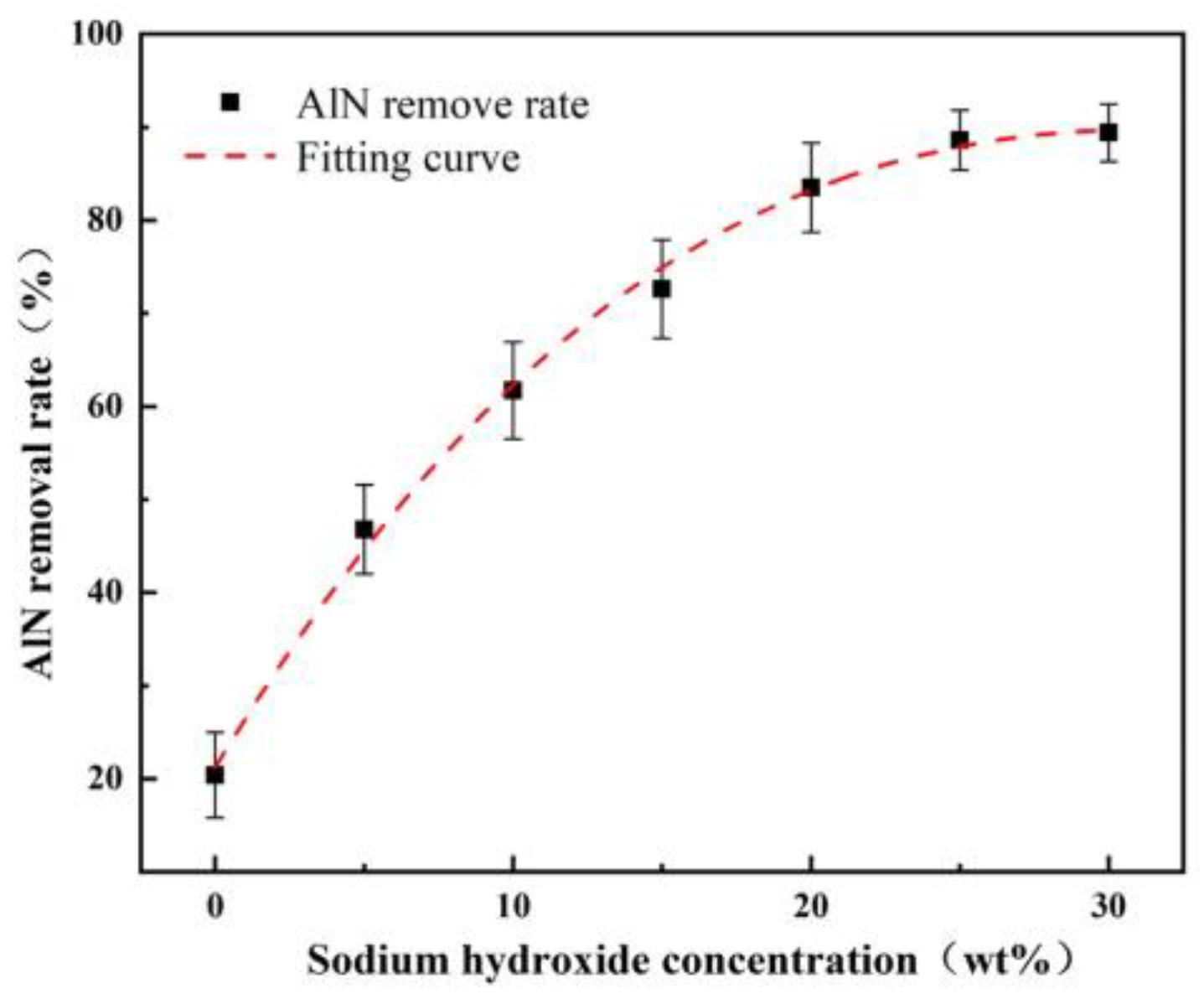
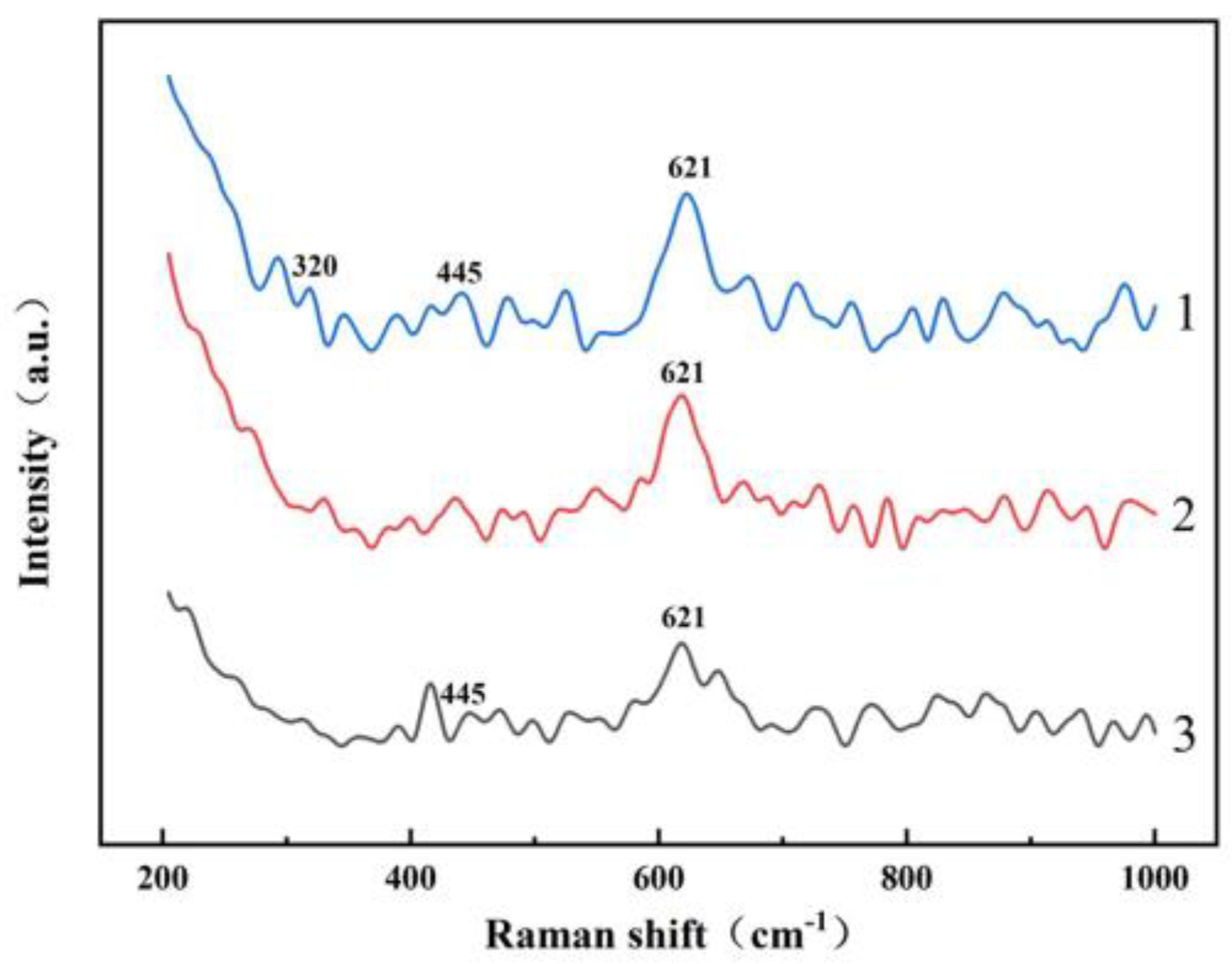
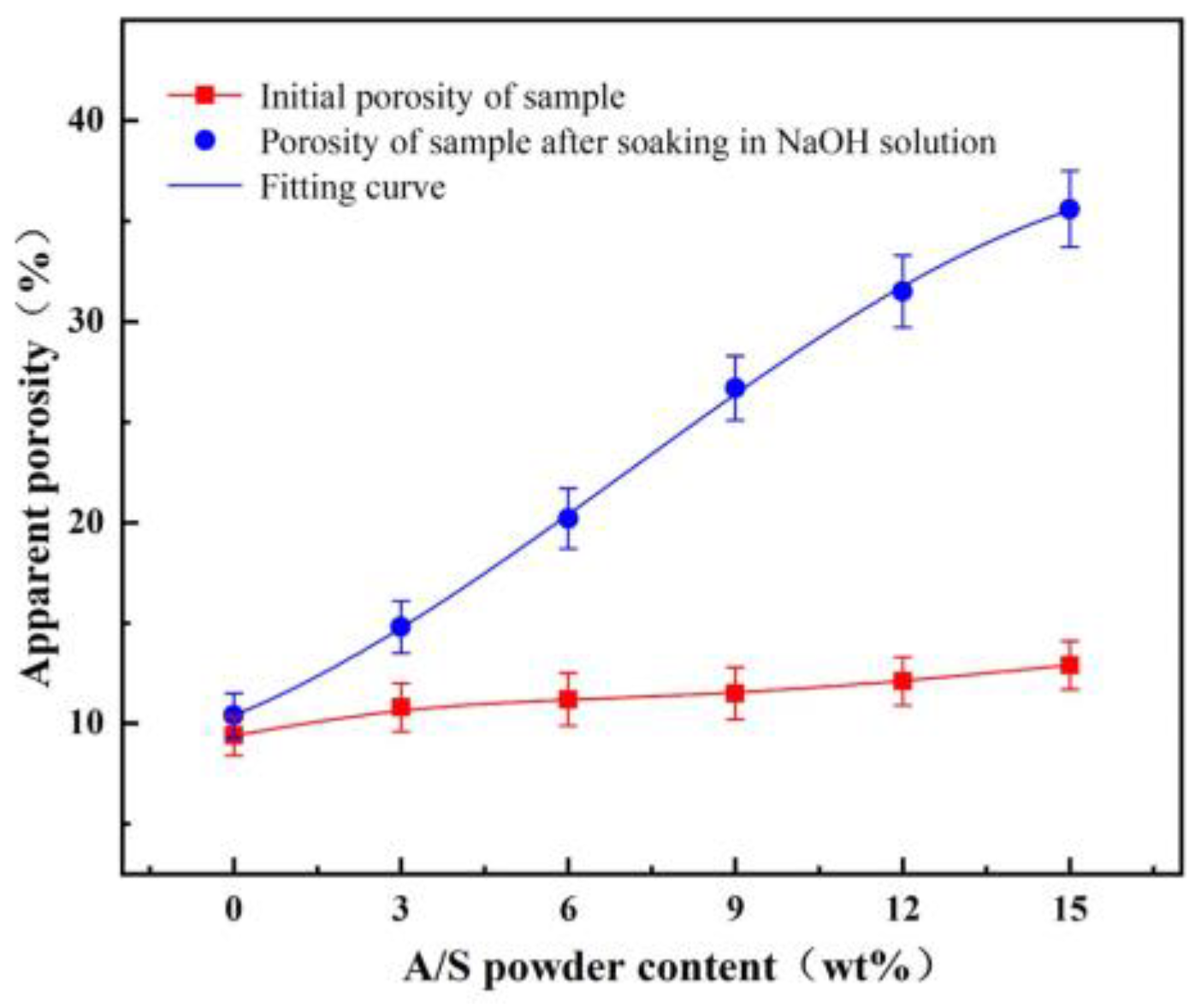
4.1. Dissolution of A/S Powder
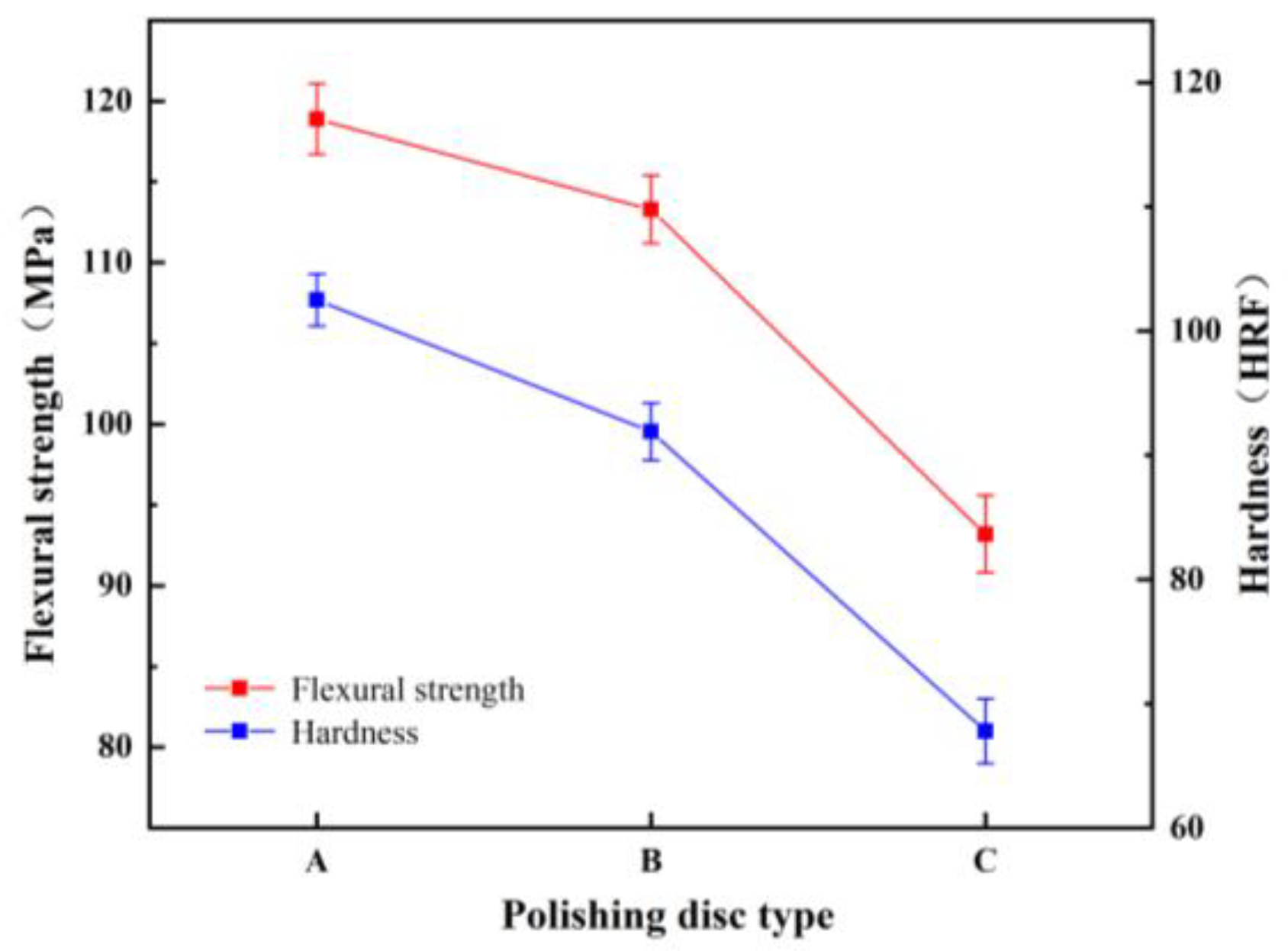
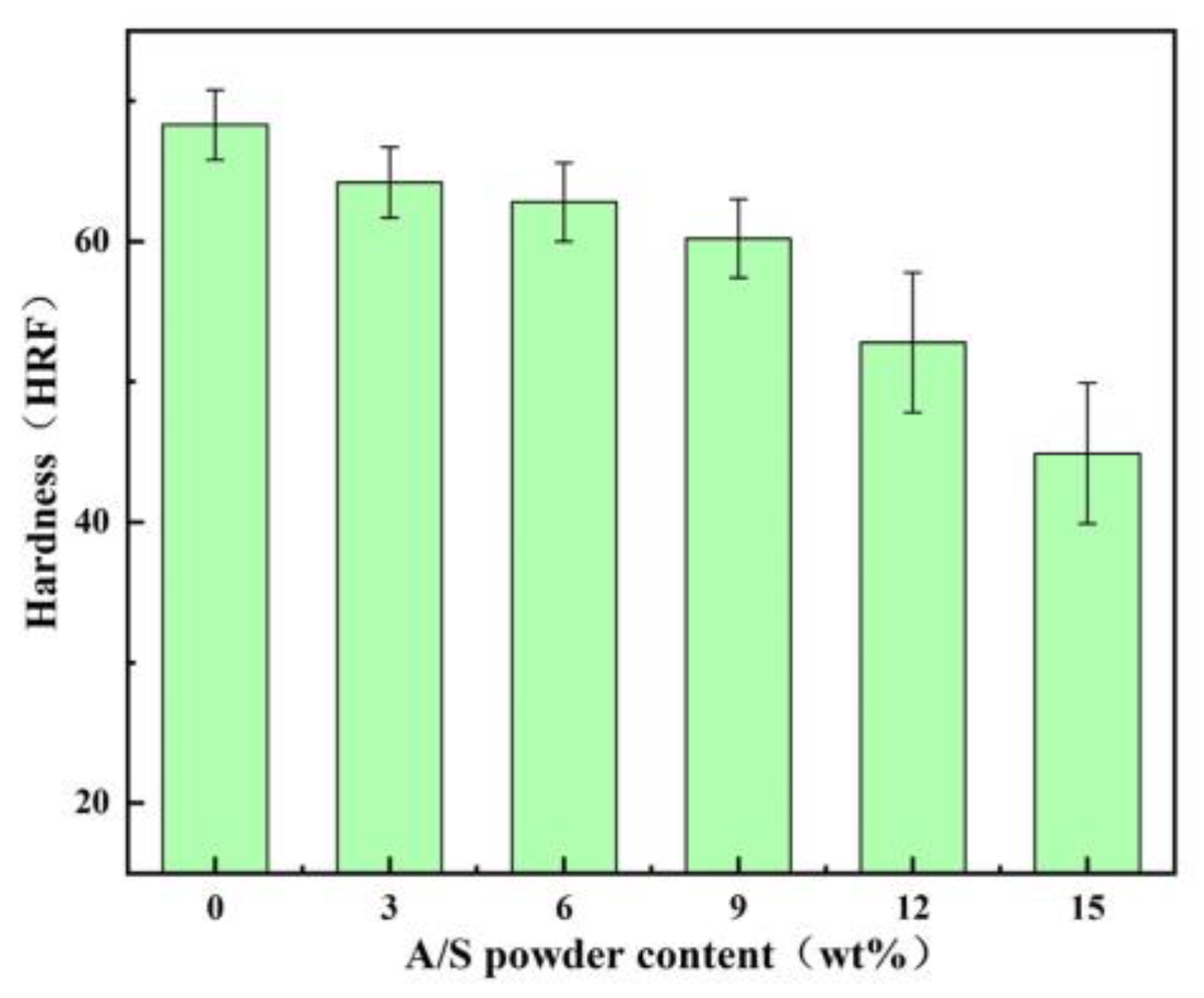
4.2. Effect of A/S Particle Size on the Performance of Polishing Disks
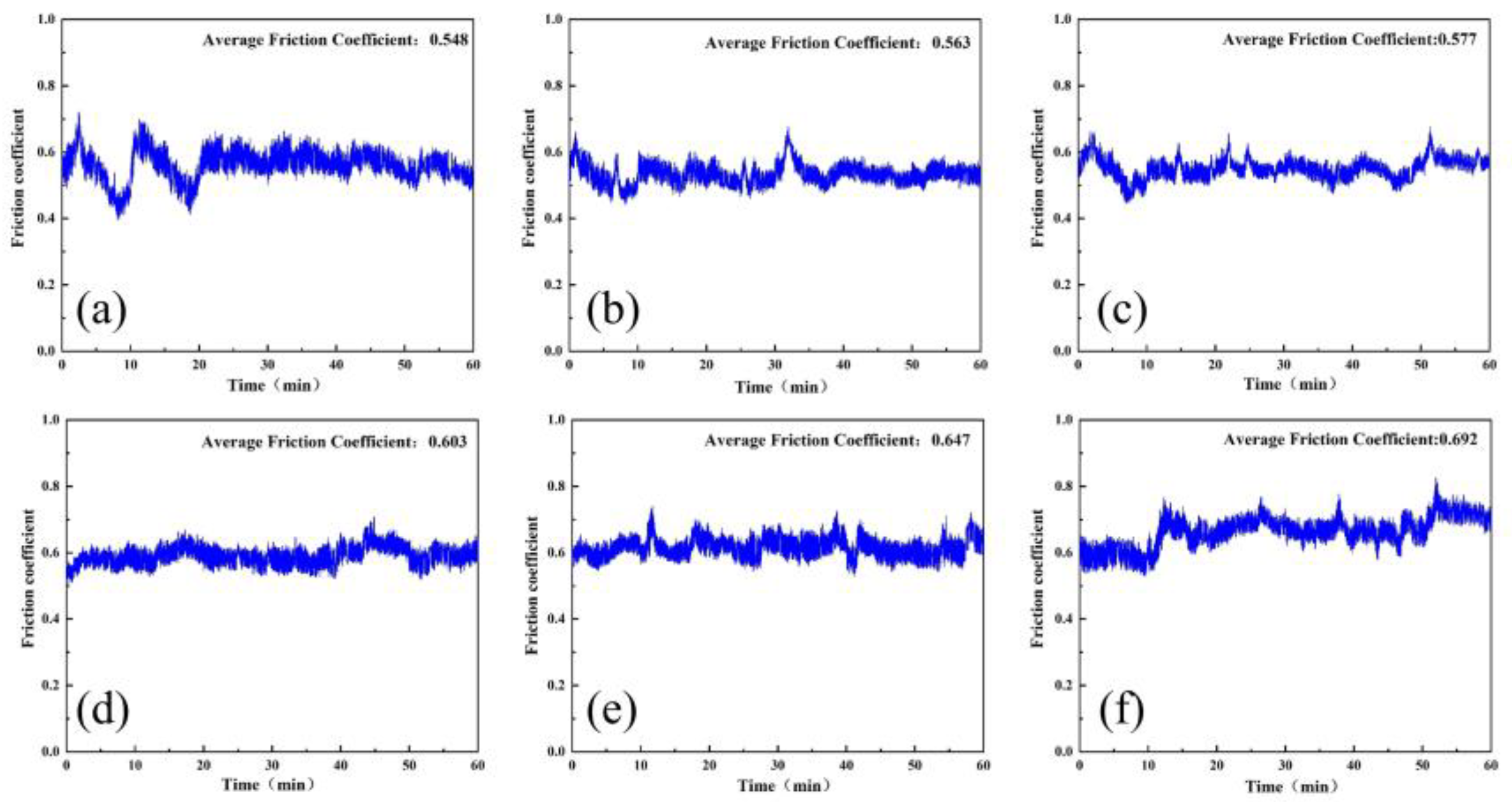
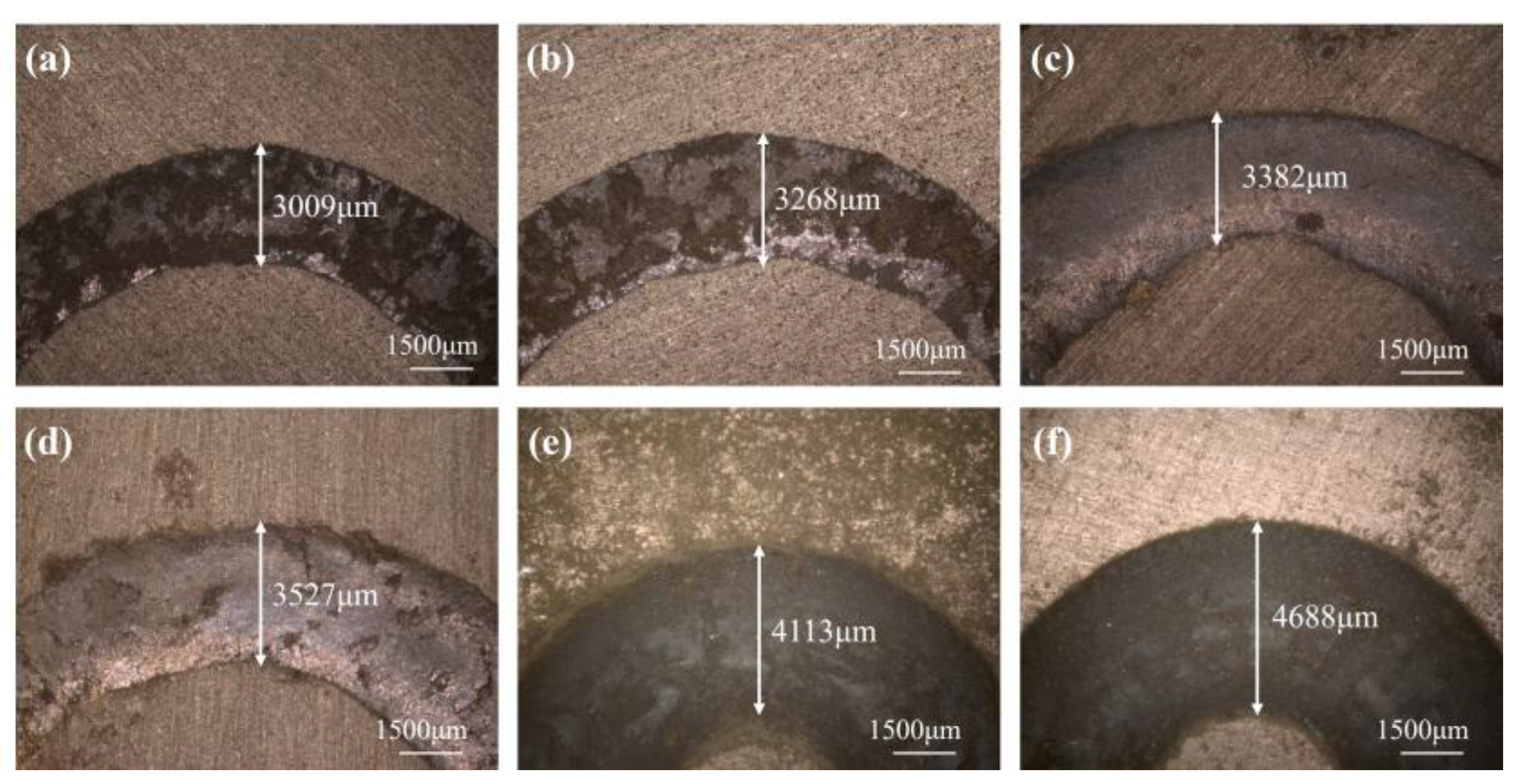
4.3. Friction Wear Test
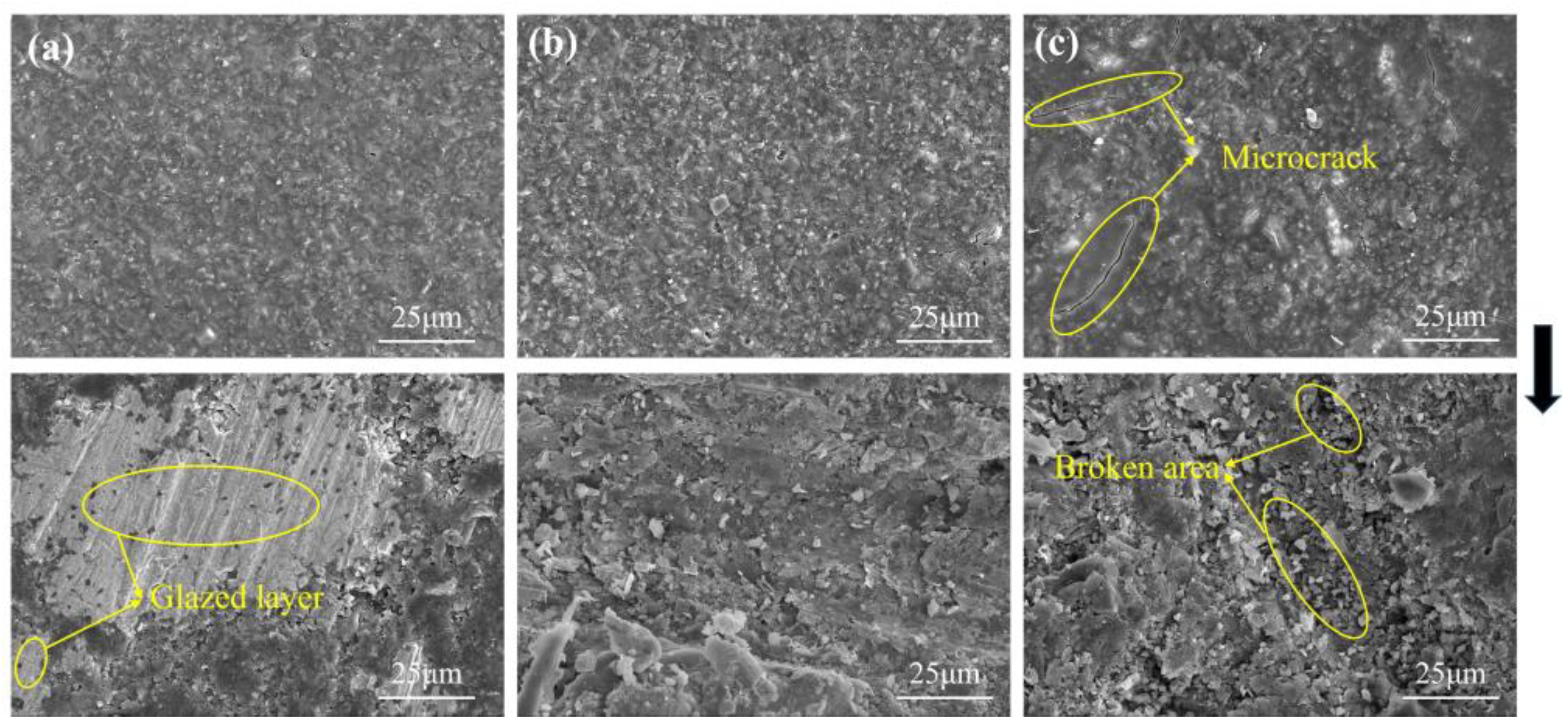
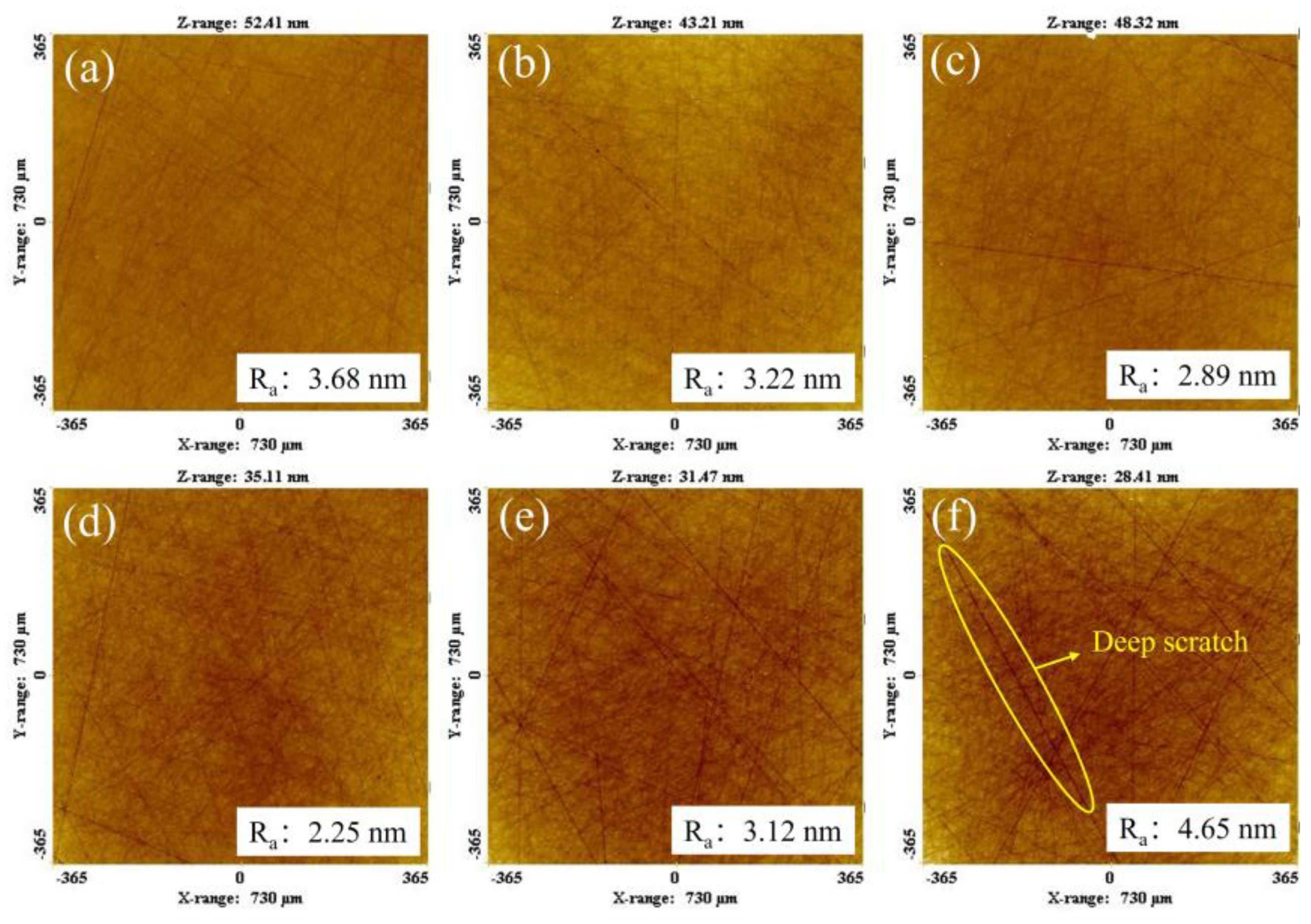
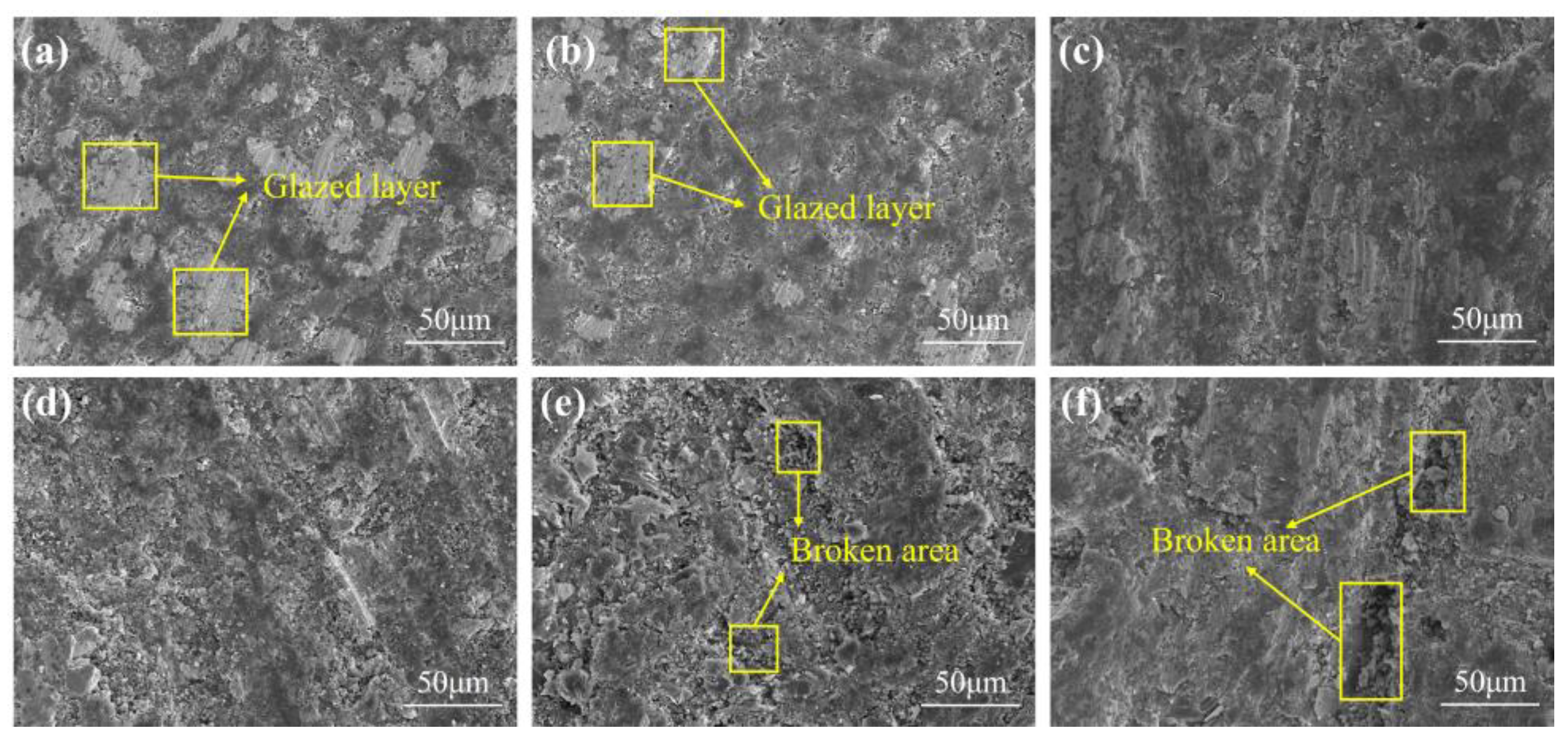
4.4. Polishing Experiment
5. Conclusions
- (1)
- The larger the AlN particle size inside the A/S powder, the longer the induction period required to start hydrolysis. Increasing the water bath temperature and adding NaOH can effectively shorten the induction period of AlN. The NaOH solution is added to the polishing disk, and the A/S powder dissolves to form new pores to increase the chip space and enhance self-sharpness.
- (2)
- With the increase in A/S powder content, the average coefficient of friction gradually increases, and the wear of the polishing disk becomes more serious. The instantaneous friction coefficient of the polishing disk is most stable and the wear is moderate when the A/S powder content is 9 wt%.
- (3)
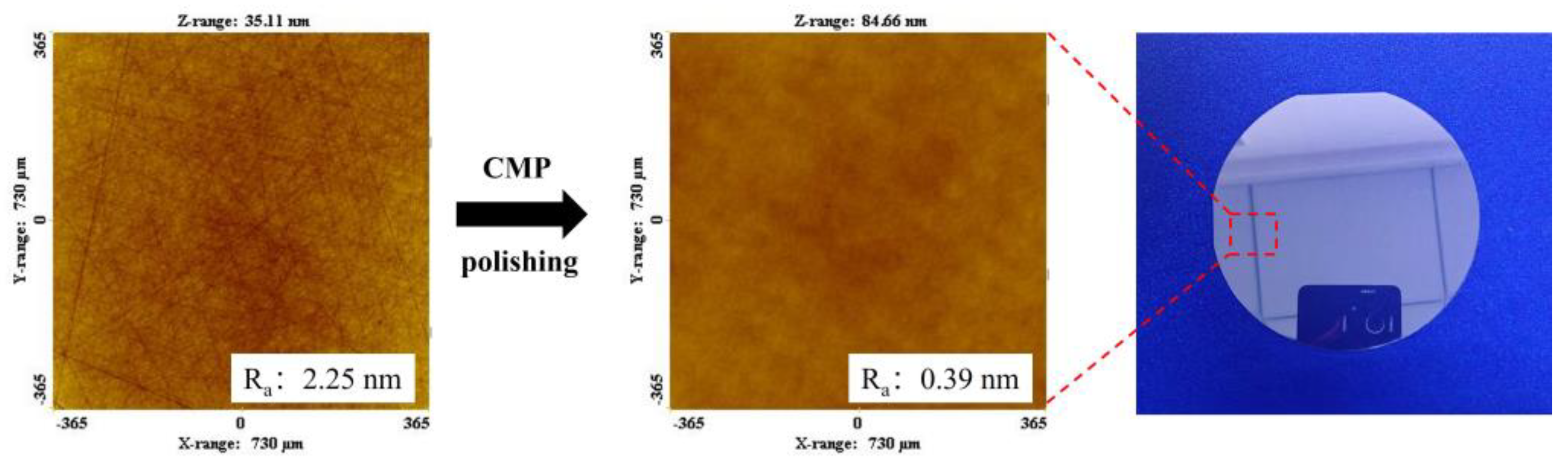
- The polishing disks with different A/S contents are prepared to rough-polish the 4H-SiC wafer workpieces. With the increase in A/S powder content, the surface roughness of the workpieces decreased first and then increased, and the material removal increased first and then decreased. The best 4H-SiC wafer surface is polished by a 9 wt% A/S powder content gel polishing disk, with a surface roughness of 2.25 nm and fewer surface scratches. The polishing disk had a strong self-sharpening effect, and there is no obvious glazed layer on the surface to hinder processing. Ultra-smooth 4H-SiC wafer surfaces with a Ra less than 0.4 nm are obtained after CMP fine polishing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kai, C.H.; Wang, R.; Yang, D.R.; Pi, X.D. Wide band gap semiconductor epitaxy based on SIC substrate. J. Synth. Cryst. 2021, 50, 1780–1795. [Google Scholar]
- Sun, H.Y.; Lien, S.C.; Qiu, Z.R.; Wang, H.C.; Mei, T.; Liu, C.W.; Feng, Z.C. Temperature dependence of Raman scattering in bulk 4H-SiC with different carrier concentration. Opt. Express. 2013, 21, 26475–26482. [Google Scholar] [CrossRef]
- Suproniuk, M.; Kaminski, P.; Kozlowski, R.; Pawlowski, M.; Wierzbowski, M. Current status of modelling the semi-insulating 4H-SiC transient photoconductivity for application to photoconductive switches. Opto-Electron. Rev. 2017, 25, 171–180. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, R.Z.; Zhang, X.Q.; Wang, M.H.; Gao, Y.; Wang, R.; Yang, D.R.; Pi, X.D. Effect of abrasive morphology and dispersion medium on grinding quality of 4H silicon carbide wafer. J. Synth. Cryst. 2023, 52, 48–55. [Google Scholar]
- Wang, J.H.; Deng, Q.F.; Yuan, J.L.; Wang, X.; Lyu, B.H.; Zhao, P.; Nguyen, D.N. Simulation and experimental verification of high-speed polishing electrode distribution based on dialectrophoresis effect. Surf. Technol. 2020, 49, 314–322. [Google Scholar]
- He, C.; Wang, Y.M.; Li, B.; Xu, W.; Hao, W.Y. Current status and trend of SiC wafer processing technology. Equip. Electron. Prod. 2016, 45, 1–6+54. [Google Scholar]
- Huang, S.G.; Lu, J.; Lin, Y.C.; Yu, Y.Q.; Xu, X.P.; Cui, C.C. Study on the enhancement of sol-gel properties by binary compounding technology for dry polishing hard and brittle materials. J. Sol-Gel Sci. Technol. 2020, 96, 314–326. [Google Scholar] [CrossRef]
- Yang, X.F.; Li, Y.S.; Yan, G.H.; Li, C.; Xu, Z.L. Study on cutting performance of ultrafine grain diamond coated cutter in high-speed milling of graphite. Manuf. Technol. Mach. Tool 2013, 6, 58–60. [Google Scholar]
- Chandra, A.; Karra, P.; Bastawros, A.F.; Biswas, R.; Sherman, P.J.; Armini, S.; Lucca, D.A. Prediction of scratch generation in chemical mechanical planarization. CIRP Ann.-Manuf. Technol. 2008, 57, 559–562. [Google Scholar] [CrossRef]
- Egan, B.; Kim, H.J. Effect of Controlling Abrasive Size in Slurry for Tungsten Contact CMP Process. ECS J. Solid State Sci. Technol. 2019, 8, 3206–3211. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Xu, X.P. The effects of abrasive yielding on the polishing of SiC wafers using a semi-fixed flexible pad. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 170–177. [Google Scholar] [CrossRef]
- Hao, S.L.; Wang, X.; Cui, Y.F.; Wang, Y.M. Study on particle agglomeration and control methods in the preparation of nano-powders. J. Synth. Cryst. 2006, 2, 342–346. [Google Scholar]
- Wan, L.; Zhang, L.X.; Liu, X.P.; Song, D.D.; Hu, W.D.; Zhou, X.X. Microstructure and performance of LZAS vitrified bond /diamond grinding wheel by in-situ sol-gel method. Mater. Sci. Eng. Powder Metall. 2016, 21, 939–945. [Google Scholar]
- Chen, J.L.; Liu, Y.B.; Liu, Y.; Wang, X.F.; Tian, L.S.; Kong, S.F. Investigation on precision grinding of PcBN Inserts by vitrified diamond grinding wheels prepared by different processes. Powder Metall. Ind. 2023, 33, 38–43. [Google Scholar]
- Luo, Q.F.; Lu, J.; Li, Z.; Wang, J. Fabrication of a sol-gel polishing tool for green manufacturing of the seal stone. J. Sol.-Gel. Sci. Technol. 2020, 96, 576–588. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Q.F.; Song, Y.Y.; Hu, G.Q.; Xu, X.P. Preparation and application of ultrafine diamond abrasive tool with gel binder. J. Mech. Eng. 2015, 51, 205–212. [Google Scholar] [CrossRef]
- Feng, K.P.; Lyu, B.H.; Wang, S.; Zhao, T.C.; Zhou, Z.Z. Preparation and performance analysis of SiC wafer diamond abrasive gel polishing disc. Mater. Rep. 2022, 36, 219–227. [Google Scholar]
- Zhao, L.; Feng, K.P.; Lyu, B.H.; Zhao, T.C.; Zhou, Z.Z. Fabrication and Characterization of Gel-Forming Cr2O3 Abrasive Tools for Sapphire Substrate Polishing. Appl. Sci. 2022, 12, 12949. [Google Scholar] [CrossRef]
- Chen, J.P.; Peng, Y.N.; Zhu, Y.W.; Sun, T.; Su, J.X.; Zuo, D.W.; Zhu, Y.W. Tribological effects of loose alumina abrasive assisted sapphire lapping by a fixed agglomerated diamond abrasive pad (FADAP). Mater. Sci. Semicond. Process. 2022, 143, 106556. [Google Scholar] [CrossRef]
- Ning, Y. The method of forced “self-sharpening” of bronze base artificial diamond wheel is discussed. Coal Technol. 2011, 30, 237–238. [Google Scholar]
- Ghosh, G.; Sidpara, A.; Bandyopadhyay, P.P. An investigation into the wear mechanism of zirconia-alumina polishing pad under different environments in shape adaptive grinding of WC-Co coating. Wear 2019, 428, 223–236. [Google Scholar] [CrossRef]
- Linke, B.S. Review on grinding tool wear with regard to sustainability. J. Manuf. Sci. Eng. Trans. ASME 2015, 137, 060801. [Google Scholar] [CrossRef]
- Wang, Z.K.; Yang, Y.K.; Zhang, Z.; Pang, M.H.; Liang, M.C.; Ma, L.J.; Su, J.X. A Novel Technique for Dressing Fixed Abrasive Lapping Pad with Abrasive Water Jet. Int. J. Precis. Eng. Manuf. Technol. 2023, 10, 1351–1373. [Google Scholar] [CrossRef]
- Zuo, H.S.; Guan, C.L. Effect of pore-forming agent on mechanical properties of metal bonded diamond abrasives. Diam. Abras. Eng. 2009, 5, 82–85. [Google Scholar]
- Li, J.W.; Fang, W.J.; Wan, L.; Liu, X.P.; Hu, W.D.; Cao, D.; Han, K.; Li, Y.Y.; Yan, Y.G. Research on the bonding properties of vitrified bonds with porous diamonds and the grinding performance of porous diamond abrasive tools. Diam. Relat. Mater. 2022, 123, 108841. [Google Scholar] [CrossRef]
- Chen, J.P.; Zhu, Y.W.; Wang, J.B.; Peng, Y.N.; Yao, J.G.; Su, J.X.; Ming, S. Relationship between mechanical properties and processing performance of agglomerated diamond abrasive compared with single diamond abrasive. Diam. Relat. Mater. 2019, 100, 107595. [Google Scholar] [CrossRef]
- Chen, J.P.; Zhu, Y.W.; Peng, Y.N.; Guo, J.T.; Ding, C. Silica-assisted fixed agglomerated diamond abrasive polishing. J. Manuf. Process. 2020, 59, 595–603. [Google Scholar] [CrossRef]
- Young, C.D.; Duh, J.G. Corrosion of aluminum nitride substrates in acid, alkaline-solutions and water. J. Mater. Sci. 1995, 30, 185–195. [Google Scholar] [CrossRef]
- Zuo, Z.P. Study on Preparation of Aluminum-Based Alloy by Molten Salt Electrolysis of Aluminum Alloy Ash. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. [Google Scholar]
- Lv, H.; Zhao, H.L.; Zuo, Z.P.; Li, R.B.; Liu, F.Q. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. JMRT 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Cao, X.Q.; Zhang, J.M.; Lin, C.H.; Xu, H.M. Research progress and prospect of aluminum nitride powder preparation technology. Machinery 2023, 50, 11–18. [Google Scholar]
- Bowen, P.; Highfield, J.G.; Mocellin, A.; Ring, T.A. Degradation of Aluminum Nitride Powder in an Aqueous Environment. J. Am. Ceram. Soc. 1990, 73, 724–728. [Google Scholar] [CrossRef]
- Krnel, K.; Kosmac, T. Protection of AlN powder against hydrolysis using aluminum dihydrogen phosphate. J. Eur. Ceram. Soc. 2001, 21, 2075–2079. [Google Scholar] [CrossRef]
- Krnel, K.; Drazi, G.; Kosmac, T. Degradation of AlN powder in aqueous environments. J. Mater. Res. 2004, 19, 1157–1163. [Google Scholar] [CrossRef]
- Kocjan, A.; Dakskobler, A.; Krnel, K.; Kosmac, T. The course of the hydrolysis and the reaction kinetics of AlN powder in diluted aqueous suspensions. J. Eur. Ceram. Soc. 2011, 31, 815–823. [Google Scholar] [CrossRef]
- Su, J.; Xu, R.; Wang, Y.; Li, J.; Liu, H. Study on Lapping Paste of 6H-SiC Single-Crystal Substrate in Tribochemical Mechanical Lapping. J. Inst. Eng. Ser. E 2020, 101, 141–148. [Google Scholar]
- Fu, Q.; You, J.L.; Wang, Y.Y.; Liu, X.F.; Yu, L.W. Ab initio study on microstructure of sodium aluminosilicate in aqueous solution by Raman spectroscopy and quantum chemistry. J. Light Scatt. 2014, 26, 265–271. [Google Scholar]
- JB/T 7999-2013; Bonded Abrasive Products-Testing Method for the Volume Density. General Porosity and Water Absorption; Zhengzhou Institute of Abrasive Grinding Abrasive: Zhengzhou, China, 2013.
- Chen, J.P.; Peng, Y.N. Super hard and brittle material removal mechanism in fixed abrasive lapping: Theory and modelin. Tribol. Int. 2023, 184, 108493. [Google Scholar] [CrossRef]
- Chen, J.P.; Sun, T.; Su, J.X.; Li, J.; Zhou, P.; Peng, Y.N.; Zhu, Y.W. A novel agglomerated diamond abrasive with excellent micro-cutting and self-sharpening capabilities in fixed abrasive lapping processes. Wear 2021, 464, 203531. [Google Scholar] [CrossRef]



| Component | Granularity/μm | Solid Content/wt% |
|---|---|---|
| PVA/PF resin | 15 | |
| Diamond powder | 2.5 | 20 |
| A/S powder | 9 | |
| Electrolytic copper | 3 | 40 |
| ZnO powder | 1 | 5 |
| Graphite powder | 2 | 3 |
| Wetting agent | ≤1 | |
| Toughening agent | ≤1 | |
| Others | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Feng, K.; Zhao, L.; Lyu, B. Fabrication and Polishing Performance of Diamond Self-Sharpening Gel Polishing Disk. Micromachines 2024, 15, 56. https://doi.org/10.3390/mi15010056
Xu L, Feng K, Zhao L, Lyu B. Fabrication and Polishing Performance of Diamond Self-Sharpening Gel Polishing Disk. Micromachines. 2024; 15(1):56. https://doi.org/10.3390/mi15010056
Chicago/Turabian StyleXu, Lanxing, Kaiping Feng, Liang Zhao, and Binghai Lyu. 2024. "Fabrication and Polishing Performance of Diamond Self-Sharpening Gel Polishing Disk" Micromachines 15, no. 1: 56. https://doi.org/10.3390/mi15010056





