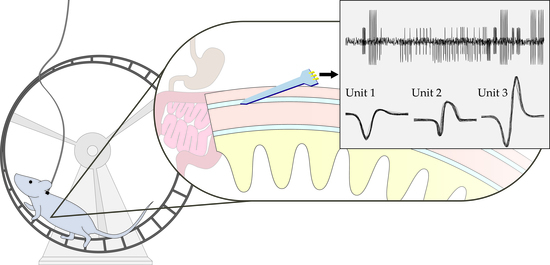
Opportunities and Challenges for Single-Unit Recordings from Enteric Neurons in Awake Animals
Abstract
:1. Introduction
2. Classical Methods for Enteric Electrophysiology
2.1. Neural Recordings in Excised Tissue
2.2. Challenges of Anesthetized Recordings from Enteric Neurons
3. Challenges to Gastrointestinal Neuro-Electrophysiology in Conscious Animals
3.1. Structural Challenges in Neurogastroenterology
3.2. Disrupting Gastrointestinal Physiology
3.3. Signal Quality
4. Enteric Microelectrode Design Criteria
4.1. Intrinsic Material Properties
4.2. Extrinsic Design Parameters
4.3. Implant Procedure
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabella, G. The number of neurons in the small intestine of mice, guinea-pigs and sheep. Neuroscience 1987, 22, 737–752. [Google Scholar] [CrossRef]
- Furness, J.B.; Costa, M. Types of nerves in the enteric nervous system. Neuroscience 1980, 5, 235–252. (In English) [Google Scholar] [CrossRef]
- Wood, J.D. Enteric nervous system: Reflexes, pattern generators and motility. Curr. Opin. Gastroenterol. 2008, 24, 149–158. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.P. Developmental disorders of the enteric nervous system. Gut 2000, 47, iv81–iv83. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. (In English) [Google Scholar] [CrossRef] [PubMed]
- Neckel, P.H.; Mattheus, U.; Hirt, B.; Just, L.; Mack, A.F. Large-scale tissue clearing (pact): Technical evaluation and new perspectives in immunofluorescence, histology, and ultrastructure. Sci. Rep. 2016, 6, 34331. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.M.; Foong, J.P.; Bornstein, J.C.; Li, Z.L.; Berghe, P.V.; Boesmans, W. Enteric nervous system assembly: Functional integration within the developing gut. Dev. Biol. 2016, 417, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Manns, I.D.; Sakmann, B.; Brecht, M. Whole-cell recordings in freely moving rats. Neuron 2006, 51, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Dombeck, D.A.; Khabbaz, A.N.; Collman, F.; Adelman, T.L.; Tank, D.W. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 2007, 56, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Ambache, N. Separation of the longitudinal muscle of the rabbit’s ileum as a broad sheet. J. Physiol. 1954, 125, 53–55. (In English) [Google Scholar] [PubMed]
- Nishi, S.; North, R.A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J. Physiol. 1973, 231, 471–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirst, G.D.S.; Holman, M.E.; Spence, I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J. Physiol. 1974, 236, 303–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.; Brookes, S.J.; Steeled, P.A.; Gibbins, I.; Burcher, E.; Kandiah, C.J. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 1996, 75, 949–967. [Google Scholar] [CrossRef]
- Wood, J.D. Application of classification schemes to the enteric nervous system. J. Auton. Nervous Syst. 1994, 48, 17–29. [Google Scholar] [CrossRef]
- Carbone, S.E.; Jovanovska, V.; Nurgali, K.; Brookes, S.J. Human enteric neurons: Morphological, electrophysiological, and neurochemical identification. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1812–1816. (In English) [Google Scholar] [CrossRef] [PubMed]
- Osorio, N.; Delmas, P. Patch clamp recording from enteric neurons in situ. Nat. Protoc. 2010, 6, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hibberd, T.J.; Travis, L.; Wiklendt, L.; Costa, M.; Hu, H.; Brookes, S.J.; Wattchow, D.A.; Dinning, P.G.; Keating, D.J.; et al. Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. J. Neurosci. 2018, 38, 5507–5522. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Hennig, G.W.; Dickson, E.; Smith, T.K. Synchronization of enteric neuronal firing during the murine colonic mmc. J. Physiol. 2005, 564, 829–847. [Google Scholar] [CrossRef] [PubMed]
- Fried, D.E.; Gulbransen, B.D. In situ Ca2+ imaging of the enteric nervous system. J. Vis. Exp. JoVE 2015, 52506. [Google Scholar] [CrossRef]
- Hibberd, T.J.; Travis, L.; Wiklendt, L.; Costa, M.; Brookes, S.J.H.; Hu, H.; Keating, D.J.; Spencer, N.J. Synaptic activation of putative sensory neurons by hexamethonium-sensitive nerve pathways in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G53–G64. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Brooks, E.M.; Mawe, G.M. Gastrointestinal motility monitor (gimm). J. Vis. Exp. 2010, e2435. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Dinning, P.G.; Brookes, S.J.; Costa, M. Insights into the mechanisms underlying colonic motor patterns. J. Physiol. 2016, 594, 4099–4116. (In English) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortoff, A. Configuration of intestinal slow waves obtained by monopolar recording techniques. Am. J. Physiol. 1967, 213, 157–162. (In English) [Google Scholar] [CrossRef] [PubMed]
- Bozler, E. The action potentials of the stomach. Am. J. Physiol. Leg. Content 1945, 144, 693–700. [Google Scholar] [CrossRef]
- Angeli, T.R.; Du, P.; Paskaranandavadivel, N.; Janssen, P.W.; Beyder, A.; Lentle, R.G.; Bissett, I.P.; Cheng, L.K.; O’grady, G. The bioelectrical basis and validity of gastrointestinal extracellular slow wave recordings. J. Physiol. 2013, 591, 4567–4579. (In English) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brierley, S.M.; Jones, R.C., III; Gebhart, G.F.; Blackshaw, L.A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004, 127, 166–178. (In English) [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.M.; O’Malley, D. Development of an ex vivo method for multi-unit recording of microbiota-colonic-neural signaling in real time. Front. Neurosci. 2018, 12, 112. (In English) [Google Scholar] [CrossRef] [PubMed]
- Castelucci, P.; Robbins, H.L.; Poole, D.P.; Furness, J.B. The distribution of purine P2X2 receptors in the guinea-pig enteric nervous system. Histochem. Cell Biol. 2002, 117, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Brown, A. (Eds.) The Enteric Nervous System; Blackwell Publishing: Malden, MA, USA, 2006; p. 288. [Google Scholar]
- Liu, M.-T.; Rothstein, J.D.; Gershon, M.D.; Kirchgessner, A.L. Glutamatergic enteric neurons. J. Neurosci. 1997, 17, 4764–4784. [Google Scholar] [CrossRef] [PubMed]
- Neunlist, M.; Michel, K.; Reiche, D.; Dobreva, G.; Huber, K.; Schemann, M. Glycine activates myenteric neurones in adult guinea-pigs. J. Physiol. 2001, 536, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Dilger, J.P. The effects of general anaesthetics on ligand-gated ion channels. Br. J. Anaesth. 2002, 89, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohtala, S.; Theilmann, W.; Suomi, T.; Wigren, H.K.; Porkka-Heiskanen, T.; Elo, L.L.; Rokka, A.; Rantamäki, T. Brief isoflurane anesthesia produces prominent phosphoproteomic changes in the adult mouse hippocampus. ACS Chem. Neurosci. 2016, 7, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.-D.; Thacker, M.; Castelucci, P.; Bagyanszki, M.; Epstein, M.L.; Furness, J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008, 334, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.S.; Lee, S.J.; Barlow-Anacker, A.J.; Druckenbrod, N.R.; Epstein, M.L.; Gosain, A. Appearance of cholinergic myenteric neurons during enteric nervous system development: Comparison of different chat fluorescent mouse reporter lines. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Flood, P.; Krasowski, M.D. Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology 2000, 92, 1418–1425. (In English) [Google Scholar] [CrossRef] [PubMed]
- Violet, B.J.M.; Downie, D.L.; Nakisa, R.C.; Lieb, W.R.; Franks, N.P. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology 1997, 86, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Oz, M.; Stewart, R.R.; Peoples, R.W.; Weight, F.F. Volatile general anaesthetic actions on recombinant nach alpha 7, 5-ht3 and chimeric nach alpha 7-5-ht3 receptors expressed in xenopus oocytes. Br. J. Pharmacol. 1997, 120, 353–355. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Harris, R.A. The anesthetic mechanism of urethane: The effects on neurotransmitter-gated ion channels. Anesth. Analg. 2002, 94, 313–318. (In English) [Google Scholar] [PubMed]
- Xiang, Z.; Burnstock, G. P2X2 and PX3 purinoceptors in the rat enteric nervous system. Histochem. Cell Biol. 2004, 121, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Vanderwinden, J.-M.; Timmermans, J.-P.; Schiffmann, S.N. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003, 312, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Masaki, E.; Kawamura, M.; Kato, F. Reduction by sevoflurane of adenosine 5′-triphosphate-activated inward current of locus coeruleus neurons in pontine slices of rats. Brain Res. 2001, 921, 226–232. [Google Scholar] [CrossRef]
- Kitahara, S.; Yamashita, M.; Ikemoto, Y. Effects of ketamine and propofol on P2X receptors in dorsal root ganglion neurons of the rat. J. Jpn. Dent. Soc. Anesthesiol. 2003, 31, 11–16. [Google Scholar]
- Tomioka, A.; Ueno, S.; Kohama, K.; Goto, F.; Inoue, K. Propofol potentiates ATP-activated currents of recombinant P2X4 receptor channels expressed in human embryonic kidney 293 cells. Neurosci. Lett. 2000, 284, 167–170. (In English) [Google Scholar] [CrossRef]
- Nakanishi, M.; Mori, T.; Nishikawa, K.; Sawada, M.; Kuno, M.; Asada, A. The effects of general anesthetics on P2X7 and P2Y receptors in a rat microglial cell line. Anesth. Analg. 2007, 104, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Barann, M.; Göthert, M.; Fink, K.; Bönisch, H. Inhibition by anaesthetics of 14C-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 1993, 347, 125–132. [Google Scholar] [CrossRef]
- Emerit, M.B.; Riad, M.; Fattaccini, C.M.; Hamon, M. Characteristics of [14C]guanidinium accumulation in NG 108-15 cell exposed to serotonin 5-HT3 receptor ligands and substance P. J. Neurochem. 1993, 60, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Machu, T.K.; Harris, R.A. Alcohols and anesthetics enhance the function of 5-hydroxytryptamine3 receptors expressed in xenopus laevis oocytes. J. Pharmacol. Exp. Ther. 1994, 271, 898–905. (In English) [Google Scholar] [PubMed]
- MacDonald, J.F.; Bartlett, M.C.; Mody, I.; Pahapill, P.; Reynolds, J.N.; Salter, M.W.; Schneiderman, J.H.; Pennefather, P.S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J. Physiol. 1991, 432, 483–508. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dildy-Mayfield, J.E.; Eger, E.I., 2nd; Harris, R.A. Anesthetics produce subunit-selective actions on glutamate receptors. J. Pharmacol. Exp. Ther. 1996, 276, 1058–1065. (In English) [Google Scholar] [PubMed]
- Marszalec, W.; Narahashi, T. Use-dependent pentobarbital block of kainate and quisqualate currents. Brain Res. 1993, 608, 7–15. [Google Scholar] [CrossRef]
- Krantis, A.; Shabnavard, L.; Nichols, K.; De Blas, A.L.; Staines, W. Localization of gabaa receptor immunoreactivity in no synthase positive myenteric neurones. J. Auton. Nervous Syst. 1995, 53, 157–165. [Google Scholar] [CrossRef]
- Krantis, A. Gaba in the mammalian enteric nervous system. Physiology 2000, 15, 284–290. [Google Scholar] [CrossRef]
- Lin, L.H.; Chen, L.L.; Zirrolli, J.A.; Harris, R.A. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acida receptors expressed by xenopus oocytes: Lack of involvement of intracellular calcium. J. Pharmacol. Exp. Ther. 1992, 263, 569–578. (In English) [Google Scholar] [PubMed]
- Wan, X.; Mathers, D.A.; Puil, E. Pentobarbital modulates intrinsic and gaba-receptor conductances in thalamocortical inhibition. Neuroscience 2003, 121, 947–958. (In English) [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Whiting, P.J.; Wafford, K.A. Barbiturate interactions at the human gabaa receptor: Dependence on receptor subunit combination. Br. J. Pharmacol. 1996, 117, 521–527. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hales, T.G.; Lambert, J.J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br. J. Pharmacol. 1991, 104, 619–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.V.; Brooks, P.A.; Harrison, N.L. Enhancement of gamma-aminobutyric acid-activated cl- currents in cultured rat hippocampal neurones by three volatile anaesthetics. J. Physiol. 1992, 449, 279–293. (In English) [Google Scholar] [CrossRef] [PubMed]
- Downie, D.L.; Hall, A.C.; Lieb, W.R.; Franks, N.P. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in xenopus oocytes. Br. J. Pharmacol. 1996, 118, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Behm, B.; Stollman, N. Postoperative ileus: Etiologies and interventions. Clin. Gastroenterol. Hepatol. 2003, 1, 71–80. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wells, C.I.; O’Grady, G.; Bissett, I.P. Acute colonic pseudo-obstruction: A systematic review of aetiology and mechanisms. World J. Gastroenterol. 2017, 23, 5634–5644. [Google Scholar] [CrossRef] [PubMed]
- Durongphongtorn, S.; McDonell, W.N.; Kerr, C.L.; Neto, F.J.; Mirakhur, K.K. Comparison of hemodynamic, clinicopathologic, and gastrointestinal motility effects and recovery characteristics of anesthesia with isoflurane and halothane in horses undergoing arthroscopic surgery. Am. J. Vet. Res. 2006, 67, 32–42. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tournadre, J.P.; Allaouchiche, B.; Malbert, C.H.; Chassard, D. Metabolic acidosis and respiratory acidosis impair gastro-pyloric motility in anesthetized pigs. Anesth. Analg. 2000, 90, 74–79. (In English) [Google Scholar] [CrossRef] [PubMed]
- Grant, I.S.; Nimmo, W.S.; Clements, J.A. Lack of effect of ketamine analgesia on gastric-emptying in man. Br. J. Anaesth. 1981, 53, 1321–1323. (In English) [Google Scholar] [CrossRef] [PubMed]
- Fass, J.; Bares, R.; Hermsdorf, V.; Schumpelick, V. Effects of intravenous ketamine on gastrointestinal motility in the dog. Intensive Care Med. 1995, 21, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Klotz, M.; Laures, K.; Clasohm, J.; Bischof, M.; Schäfer, K.H. The mesenterially perfused rat small intestine: A versatile approach for pharmacological testings. Ann. Anat. Anatomischer Anzeiger 2014, 196, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, J.; Unger, J.K.; Kochs, B.; Silny, J.; Rossaint, R. Effects of a single dose of ketamine on duodenal motility activity in pigs. Can. Vet. J. 2005, 46, 147–152. [Google Scholar] [PubMed]
- Yuasa, H.; Watanabe, J. Influence of urethane anesthesia and abdominal surgery on gastrointestinal motility in rats. Biol. Pharm. Bull. 1994, 17, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Meli, A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia 1986, 42, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Grundy, D. The effect of surgical anaesthesia on antral motility in the ferret. Exp. Physiol. 1990, 75, 701–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qualls-Creekmore, E.; Tong, M.; Holmes, G.M. Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22, 181–185. (In English) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freye, E.; Sundermann, S.; Wilder-Smith, O.H.G. No inhibition of gastro-intestinal propulsion after propofol-or propofol/ketamine-N2O/O2 anaesthesia: A comparison of gastro-caecal transit after isoflurane anaesthesia. Acta Anaesthesiol. Scand. 1998, 42, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-L.; Ang, S.B.; Dambisya, Y.M.; Adaikan, G.P.; Lau, L.C. The effect of propofol on human gastric and colonic muscle contractions. Anesth. Analg. 1999, 89, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.L.; Bartholomeusz, F.D.; Kirkwood, I.D.; Chatterton, B.E. Liquid gastric emptying in the pig: Effect of concentration of inhaled isoflurane. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2002, 43, 968–971. (In English) [Google Scholar]
- Torjman, M.C.; Joseph, J.I.; Munsick, C.; Morishita, M.; Grunwald, Z. Effects of isoflurane on gastrointestinal motility after brief exposure in rats. Int. J. Pharm. 2005, 294, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ailiani, A.C.; Neuberger, T.; Brasseur, J.G.; Banco, G.; Wang, Y.; Smith, N.B.; Webb, A.G. Quantifying the effects of inactin vs isoflurane anesthesia on gastrointestinal motility in rats using dynamic magnetic resonance imaging and spatio-temporal maps. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2014, 26, 1477–1486. (In English) [Google Scholar] [CrossRef] [PubMed]
- Boscan, P.; Cochran, S.; Monnet, E.; Webb, C.; Twedt, D. Effect of prolonged general anesthesia with sevoflurane and laparoscopic surgery on gastric and small bowel propulsive motility and ph in dogs. Vet. Anaesth. Analg. 2014, 41, 73–81. (In English) [Google Scholar] [CrossRef] [PubMed]
- Desmet, M.; Vander Cruyssen, P.; Pottel, H.; Carlier, S.; Devriendt, D.; Van Rooy, F.; De Corte, W. The influence of propofol and sevoflurane on intestinal motility during laparoscopic surgery. Acta Anaesthesiol. Scand. 2016, 60, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Schurizek, B.A.; Willacy, L.H.; Kraglund, K.; Juhl, B.; Andreasen, F. Effects of general anaesthesia with halothane on antroduodenal motility, ph and gastric emptying rate in man. Br. J. Anaesth. 1989, 62, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.S.F.N.; Pittinger, M.D.C.B.; Long, P.D.J.P. Effects of halothane on gastrointestinal motility. Anesthesiology 1961, 22, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W.; Healy, T.E.; Balfour, T.W.; Hardcastle, J.D. Effects of inhalation anaesthetic agents on the electrical and mechanical activity of the rat duodenum. Br. J. Anaesth. 1982, 54, 1223–1230. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; al-Kais, A.H.; Singh, S.A.; Arafat, K.H.; el-Sharkawy, T.Y. Multielectrode mapping of slow-wave activity in the isolated rabbit duodenum. J. Appl. physiol. 1993, 74, 1454–1461. (In English) [Google Scholar] [CrossRef] [PubMed]
- Du, P.; O’Grady, G.; Egbuji, J.U.; Lammers, W.J.; Budgett, D.; Nielsen, P.; Windsor, J.A.; Pullan, A.J.; Cheng, L.K. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: Methodology and validation. Ann. Biomed. Eng. 2009, 37, 839. [Google Scholar] [CrossRef] [PubMed]
- Angeli, T.R.; O’Grady, G.; Vather, R.; Bissett, I.P.; Cheng, L.K. Intra-operative high-resolution mapping of slow wave propagation in the human jejunum: Feasibility and initial results. Neurogastroenterol. Motil. 2018, 30, e13310. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Hadzipasic, M.; Ni, W.; Nagy, M.; Steenrod, N.; McGinley, M.J.; Kaushal, A.; Thomas, E.; McCormick, D.A.; Horwich, A.L. Reduced high-frequency motor neuron firing, emg fractionation, and gait variability in awake walking als mice. Proc. Natl. Acad. Sci. USA 2016, 113, E7600–E7609. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.W.; Chen, M.T.; Huang, H.C.; Hsiao, M.C.; Cheng, H. A method for unit recording in the lumbar spinal cord during locomotion of the conscious adult rat. J. Neurosci. Methods 2009, 182, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Aaron, G.; Jit, M. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 2006, 3, 189. [Google Scholar]
- Smith, T.K.; Oliver, G.R.; Hennig, G.W.; O’Shea, D.M.; Berghe, P.V.; Kang, S.H.; Spencer, N.J. A smooth muscle tone-dependent stretch-activated migrating motor pattern in isolated guinea-pig distal colon. J. Physiol. 2003, 551, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F.; et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Ruane, D.T.; Lavelle, E.C. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front. Immunol. 2011, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Bravo-Blas, A.; Scott, C.L.; Perdiguero, E.G.; Geissmann, F.; Henri, S.; Malissen, B.; Osborne, L.C.; Artis, D.; Mowat, A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014, 15, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, D.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, V.; Bain, C.C.; Mowat, A.M.; Milling, S.W. Intestinal macrophages and dendritic cells: What’s the difference? Trends Immunol. 2014, 35, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Müller, W.; Sparwasser, T.; Förster, R.; Pabst, O. Intestinal tolerance requires gut homing and expansion of Foxp3+ regulatory T cells in the lamina propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, L.; De Salvo, C.; Mercado, J.R.; Vecchi, M.; Pizarro, T.T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: Lessons learned from animal models and human genetics. Front. Immunol. 2013, 4, 280. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, L.; De Salvo, C.; Mercado, J.R.; Vecchi, M.; Pizarro, T.T. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the hnf4a region. Nat. Genet. 2009, 41, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Lenaerts, K.; Derikx, J.P.; Matthijsen, R.A.; de Bruïne, A.P.; van Bijnen, A.A.; van Dam, R.M.; Dejong, C.H.; Buurman, W.A. Human intestinal ischemia-reperfusion-induced inflammation characterized: Experiences from a new translational model. Am. J. Pathol. 2010, 176, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.M.; Moeser, A.J.; Blikslager, A.T. Animal models of ischemia-reperfusion-induced intestinal injury: Progress and promise for translational research. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G63–G75. [Google Scholar] [CrossRef] [PubMed]
- Veiga-Fernandes, H.; Mucida, D. Neuro-immune interactions at barrier surfaces. Cell 2016, 165, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Giron, M.C.; Qesari, M.; Porzionato, A.; Caputi, V.; Zoppellaro, C.; Banzato, S.; Grillo, A.R.; Spagnol, L.; De Caro, R.; et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 2013, 145, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Hammer, G.E.; Turer, E.E.; Taylor, K.E.; Fang, C.J.; Advincula, R.; Oshima, S.; Barrera, J.; Huang, E.J.; Hou, B.; Malynn, B.A.; et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 2011, 12, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Huang, H.I.; Benzatti, F.P.; Karlsson, A.B.; Zhang, J.J.; Youssef, N.; Ma, A.; Hale, L.P.; Hammer, G.E. Inflammatory Th1 and Th17 in the intestine are each driven by functionally specialized dendritic cells with distinct requirements for MyD88. Cell Rep. 2016, 17, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Rivollier, A.; He, J.; Kole, A.; Valatas, V.; Kelsall, B.L. Inflammation switches the differentiation program of ly6chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012, 209, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, C.C.; Scott, C.L.; Uronen-Hansson, H.; Gudjonsson, S.; Jansson, O.; Grip, O.; Guilliams, M.; Malissen, B.; Agace, W.W.; Mowat, A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal. Immunol. 2013, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Bashashati, M.; Hirota, S.A.; Gui, X.; Roberts, J.A.; MacDonald, J.A.; Muruve, D.A.; McKay, D.M.; Beck, P.L.; Mawe, G.M.; et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012, 18, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M.; Koh, S.D. Interstitial cells: Regulators of smooth muscle function. Physiol. Rev. 2014, 94, 859–907. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Martz, S.; Gil, V.; Wang, X.Y.; Jimenez, M.; Parsons, S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front. Neurosci. 2011, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Kito, Y.; Hwang, S.J.; Ward, S.M. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology 2016, 31, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Thuneberg, L.; Klüppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liu, L.W.; Huizinga, J.D. Canine colonic circular muscle generates action potentials without the pacemaker component. Can. J. Physiol. Pharmacol. 1994, 72, 70–81. (In English) [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Rakhilin, N.; Gordon, P.H.; Shen, X.; Kan, E.C. A real-time spike classification method based on dynamic time warping for extracellular enteric neural recording with large waveform variability. J. Neurosci. Methods 2016, 261, 97–109. (In English) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayguinov, O.; Hennig, G.W.; Sanders, K.M. Movement based artifacts may contaminate extracellular electrical recordings from gi muscles. Neurogastroenterol. Motil. 2011, 23, 1029-e498. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Ward, S.M.; Hennig, G.W. Problems with extracellular recording of electrical activity in gastrointestinal muscle. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.C.; Rubiano, A.; Santisteban, M.M.; Shenoy, V.; Qi, Y.; Pepine, C.J.; Raizada, M.K.; Simmons, C.S. Hypertension-linked mechanical changes of rat gut. Acta Biomater. 2016, 45, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stidham, R.W.; Xu, J.; Johnson, L.A.; Kim, K.; Moons, D.S.; McKenna, B.J.; Rubin, J.M.; Higgins, P.D. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with crohn’s disease. Gastroenterology 2011, 141, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.B.; Oberg, K.; Wolchok, J.C. Tensile properties of the rectal and sigmoid colon: A comparative analysis of human and porcine tissue. SpringerPlus 2015, 4, 142. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural Eng. 2017, 15, 031001. (In English) [Google Scholar] [CrossRef] [PubMed]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. (In English) [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, J.P.; Kipke, D.R. Neural probe design for reduced tissue encapsulation in cns. Biomaterials 2007, 28, 3594–3607. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, J.; Fu, T.M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, L.; Wei, X.; Zhao, Z.; Siegel, J.J.; Potnis, O.; Tuppen, C.A.; Lin, S.; Kazmi, S.; Fowler, R.A.; Holloway, S.; et al. Ultraflexible nanoelectronic probes form reliable, glial scar-free neural integration. Sci. Adv. 2017, 3, e1601966. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Luan, L.; Zhao, Z.; Li, X.; Zhu, H.; Potnis, O.; Xie, C. Nanofabricated ultraflexible electrode arrays for high-density intracortical recording. Adv. Sci. 2018, 5, 1700625. (In English) [Google Scholar] [CrossRef] [PubMed]
- Naples, G.G.; Mortimer, J.T.; Scheiner, A.; Sweeney, J.D. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 1988, 35, 905–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobo, A.M.; Boyajian, B.; Larson, C.; Schotten, K.; Pikov, V.; Meng, E. A parylene cuff electrode for peripheral nerve recording and drug delivery. In Proceeding of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 506–509. [Google Scholar]
- Chen, J.D.; Schirmer, B.D.; McCallum, R.W. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 266, G90–G98. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hayes, J.; Peters, L.J.; Zhang, M.; Chen, J.D.Z. Electrical stimulation of small intestine using intraluminal ring electrodes. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol.20 Biomedical Engineering Towards the Year 2000 and Beyond (Cat. No.98CH36286), Hong Kong, China, 1 November 1998; Volume 6, pp. 3230–3233. [Google Scholar]
- Lin, X.; Hayes, J.; Peters, L.J.; Chen, J.D. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann. Biomed. Eng. 2000, 28, 582–587. (In English) [Google Scholar] [CrossRef] [PubMed]
- Drake, K.L.; Wise, K.D.; Farraye, J.; Anderson, D.J.; BeMent, S.L. Performance of planar multisite microprobes in recording extracellular single-unit intracortical activity. IEEE Trans. Biomed. Eng. 1988, 35, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. (In English) [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J. Biomed. Mater. Res. Part A 2007, 82A, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Yoseph, B.P.; Klingensmith, N.J.; Liang, Z.; Breed, E.R.; Burd, E.M.; Mittal, R.; Dominguez, J.A.; Petrie, B.; Ford, M.L.; Coopersmith, C.M. Mechanisms of intestinal barrier dysfunction in sepsis. Shock (Augusta, Ga.) 2016, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef] [PubMed]
- Rakhilin, N.; Barth, B.; Choi, J.; Munoz, N.L.; Kulkarni, S.; Jones, J.S.; Small, D.M.; Cheng, Y.-T.; Cao, Y.; LaVinka, C.; et al. Simultaneous optical and electrical in vivo analysis of the enteric nervous system. Nat. Commun. 2016, 7, 11800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, B.B.; Henriquez, C.S.; Grill, W.M.; Shen, X. Electrical stimulation of gut motility guided by an in silico model. J. Neural Eng. 2017, 14, 066010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, B.B.; Shen, X. Computational motility models of neurogastroenterology and neuromodulation. Brain Res. 2018, 1693, 174–179. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D. Emerging tools to study enteric neuromuscular function. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G420–G426. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Smith, T.K. Calcium imaging of gut activity. Neurogastroenterol. Motil. 2004, 16, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Boesmans, W.; Hao, M.M.; Vanden Berghe, P. Optogenetic and chemogenetic techniques for neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, T.J.; Feng, J.; Luo, J.; Yang, P.; Samineni, V.K.; Gereau, R.W., 4th; Kelley, N.; Hu, H.; Spencer, N.J. Optogenetic induction of colonic motility in mice. Gastroenterology 2018, 155, 514–528. (In English) [Google Scholar] [CrossRef] [PubMed]


| Neuron Species | Approximate Percentage | Affected Receptors | Inhibiting Anesthetic Agents | Potentiating Anesthetic Agents |
|---|---|---|---|---|
| Cholinergic | ChAT-positive neurons:
| Neuronal nACh | Ketamine [36], pentobarbital [37], propofol [37], isoflurane [37,38], halothane [37,38], sevoflurane [37] | Urethane [39] |
| Purinergic | ATP-releasing neurons:
| P2X2 | Sevoflurane [42] | - |
| P2X3 | Pentobarbital [43] | - | ||
| P2X4 | - | Propofol [44] | ||
| P2X7 | - | Ketamine [45], propofol [45] | ||
| Serotinergic | 5-HT-positive neurons:
| 5-HT3 | Ketamine [46,47], pentobarbital [46], propofol [46] | Isoflurane [38,48], halothane [38,48] |
| Glutamatergic | NMDA-positive neurons: | NMDA | Ketamine [49], urethane [39], pentobarbital [50] | - |
| AMPA-positive neurons: | AMPA | Urethane [39], pentobarbital [51], propofol [50] | - | |
GABAA-positive neurons:
| GABAA | - | Ketamine [54], urethane [39], pentobarbital [55,56], propofol [54,57], isoflurane [54,58], halothane [54,58] | |
| Glycinergic | Glycine-responsive:
| Glycine | - | Urethane [39], propofol [57], isoflurane [59], sevoflurane [59], halothane [59] |
| Anesthetic Agent | Route of Administration | Gastric Emptying | Intestinal Transit |
|---|---|---|---|
| Ketamine | Injection | Unaffected [64,65] | Unaffected/slight decrease [64,65,66,67] |
| Urethane | Injection | Decrease [68,69,70,71] | Decrease [68,69] |
| Pentobarbital | Injection | Decrease [70] | Dose-dependent increase/decrease [66] |
| Propofol | Injection | Decrease [72,73] | Slight decrease [66,67] |
| Isoflurane | Inhalation | Decrease [74,75] | Decrease [62,76] |
| Sevoflurane | Inhalation | Decrease [77] | Decrease [77,78] |
| Halothane | Inhalation | Decrease [79] | Decrease [79,80,81] |
| Categories | Challenges |
|---|---|
| Structural | Large tissue displacements and no rigid structures on which to mount a device |
| Physiological | Ischemia and reperfusion injury Maintaining gastrointestinal homeostasis |
| Signal Quality | Electrical slow waves Smooth muscle action potentials Artifact due to tissue movement |
| Design Criteria | Features |
|---|---|
| Material Properties | Low Young’s modulus High elasticity |
| Design Parameters | Low cross-sectional area Tethered recording platform Multiple recording sites along the length of the shank |
| Implant Procedure | Implant along longitudinal axis Shallow insertion angle Undisturbed submucosa and epithelial layer |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barth, B.B.; Huang, H.-I.; Hammer, G.E.; Shen, X. Opportunities and Challenges for Single-Unit Recordings from Enteric Neurons in Awake Animals. Micromachines 2018, 9, 428. https://doi.org/10.3390/mi9090428
Barth BB, Huang H-I, Hammer GE, Shen X. Opportunities and Challenges for Single-Unit Recordings from Enteric Neurons in Awake Animals. Micromachines. 2018; 9(9):428. https://doi.org/10.3390/mi9090428
Chicago/Turabian StyleBarth, Bradley B., Hsin-I Huang, Gianna E. Hammer, and Xiling Shen. 2018. "Opportunities and Challenges for Single-Unit Recordings from Enteric Neurons in Awake Animals" Micromachines 9, no. 9: 428. https://doi.org/10.3390/mi9090428