Basic Properties of ZnO, Ga2O3, and MgO—Quantitative IR Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. CO2 Adsorption on ZnO
2.2. CO2 Adsorption on Ga2O3
2.3. CO2 Adsorption on MgO
2.4. Dehydroxylation of ZnO, Ga2O3, and MgO
2.5. Determination of Total Concentration of Basic Sites in Desorption Experiments
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pines, H.; Haag, W.O. Communications-stereoselectivity in the carbanion-catalyzed isomerization of 1-Butene. J. Org. Chem 1958, 23, 328–329. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Generation of basic sites and application to organic synthesis. Appl. Catal. A Gen. 2001, 222, 247–259. [Google Scholar] [CrossRef]
- Hideshi, H. Solid base catalysts: Fundamentals and their applications in organic reactions. Appl. Catal. A Gen. 2015, 504, 103–109. [Google Scholar] [CrossRef]
- Ono, Y.; Baba, T. Selective reactions over solid base catalysts. Catal. Today 1997, 38, 321–337. [Google Scholar] [CrossRef]
- Bing, W.; Wei, M. Recent advances for solid basic catalysts: Structure design and catalytic performance. J. Solid State Chem. 2019, 269, 184–194. [Google Scholar] [CrossRef]
- Derouane, E.G.; Védrine, J.C.; Ramos Pinto, R.; Borges, P.M.; Costa, L.; Lemos, M.A.N.D.A.; Lemos, F.; Ramôa Ribeiro, F. The acidity of zeolites: Concepts, measurements and relation to catalysis: A review on experimental and theoretical methods for the study of zeolite acidity. Catal. Rev. Sci. Eng. 2013, 55, 454–515. [Google Scholar] [CrossRef]
- Knözinger, H. Infrared spectroscopy for the characterization of surface acidity and basicity. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Kuterasiński, Ł.; Gackowski, M.; Podobiński, J.; Rutkowska-Zbik, D.; Datka, J. Nitrogen as a probe molecule for the IR studies of the heterogeneity of OH groups in zeolites. Molecules 2021, 26, 6261. [Google Scholar] [CrossRef] [PubMed]
- Ramis, G.; Busca, G.; Lorenzelli, V. Low-temperature CO2 adsorption on metal oxides: Spectroscopic characterization of some weakly adsorbed species. Mater. Chem. Phys. 1991, 29, 425–435. [Google Scholar] [CrossRef]
- Li, C.; Sakata, Y.; Arai, T.; Domen, K.; Maruya, K.; Onishi, T. Carbon Monoxide and carbon dioxide adsorption on cerium oxide studied by Fourier-transform infrared spectroscopy. Part 1.—Formation of carbonate species on dehydroxylated CeO2, at room temperature. J. Chem. Soc. Faraday Trans. I 1989, 85, 929–943. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Freund, H.J.; Roberts, M.W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 1996, 25, 225–273. [Google Scholar] [CrossRef]
- Mino, L.; Spoto, G.; Ferrari, A.M. CO2 capture by TiO2 anatase surfaces: A combined DFT and FTIR study. J. Phys. Chem. C 2014, 118, 25016–25026. [Google Scholar] [CrossRef]
- Cornu, D.; Guesmi, D.; Krafft, J.-M.; Lauron-Pernot, H. Lewis acido-basic interaction between CO2 and MgO surface: DFT and DRIFT approaches. J. Phys. Chem. C 2012, 116, 6645–6654. [Google Scholar] [CrossRef]
- Li, C.; Sakata, Y.; Arai, T.; Domen, K.; Maruya, K.; Onishi, T. Adsorption of carbon monoxide and carbon dioxide on cerium oxide studied by Fourier-transform infrared spectroscopy. Part 2.—Formation of formate species on partially reduced CeO2 at room temperature. J. Chem. Soc. Faraday Trans. I 1989, 85, 1451–1461. [Google Scholar] [CrossRef]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 adsorption on tetragonal and monoclinic zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Bachiller-Baeza, B.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Interaction of carbon dioxide with the surface of ZrO2 polymorphs. Langmuir 1998, 14, 3556–3564. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Calatayud, M.; Krause, A.O.I. Structure and Stability of Formates and Carbonates on Monoclinic Zirconia: A Combined Study by Density Functional Theory and Infrared Spectroscopy. J. Phys. Chem. C 2008, 112, 16096–16102. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR Study of polycrystalline ceria properties in oxidized and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Pacchioni, G.; Ricart, J.M.; Illas, F. Ab Initio Cluster Model Calculations on the Chemisorption of CO2 and SO2 Probe Molecules on MgO and CaO (100) Surfaces. A Theoretical Measure of Oxide Basicity. J. Am. Chem. Soc. 1994, 116, 10152–10158. [Google Scholar] [CrossRef]
- Markovits, A.; Fahmi, A.; Minot, C. A theoretical study of CO2 adsorption on TiO2. J. Mol. Struct. THEOCHEM 1996, 371, 219–235. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Al-Saidi, W.A.; Jordan, K.D. CO2 adsorption on TiO2(101) anatase: A dispersion-corrected density functional theory study. J. Chem. Phys. 2011, 135, 124701. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. A Theoretical Study of CO2 Anions on Anatase (101) Surface. J. Phys. Chem. C 2010, 114, 21474–21481. [Google Scholar] [CrossRef]
- Tosoni, S.; Spinnato, D.; Pacchioni, G. DFT study of CO2 activation on doped and ultrathin MgO films. J. Phys. Chem. C 2015, 119, 27594–27602. [Google Scholar] [CrossRef]
- Thomasson, P.; Tyagi, O.S.; Knozinger, H. Characterisation of the basicity of modified MgO-catalysts. Appl. Catal. A Gen. 1999, 181, 181–188. [Google Scholar] [CrossRef]
- Huang, M.; Kaliaguine, S. Zeolite basicity characterized by pyrrole chemisorption: An infrared study. J. Chem. Soc. Faraday Trans. 1992, 88, 751–758. [Google Scholar] [CrossRef]
- Auroux, A. Calorimetry and Thermal Methods in Catalysis, 1st ed.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 154, ISBN 978-3642119538. [Google Scholar]
- Köck, E.-M.; Kogler, M.; Bielz, T.; Klötzer, B.; Penner, S. In Situ FT-IR Spectroscopic Study of CO2 and CO Adsorption on Y2O3, ZrO2, and Yttria-Stabilized ZrO2. J. Phys. Chem. C 2013, 117, 17666–17673. [Google Scholar] [CrossRef]
- Morterra, C.; Orio, L. Mater. Chem. Phys. Surface characterization of zirconium oxide. II. The interaction with carbon dioxide at ambient temperature. Mater. Chem. Phys. 1990, 24, 247–268. [Google Scholar] [CrossRef]
- Collins, S.E.; Baltanas, M.A.; Bonivardi, A.L. Infrared spectroscopic study of the carbon dioxide adsorption on the surface of Ga2O3 polymorphs. J. Phys. Chem. B 2006, 110, 5498–5507. [Google Scholar] [CrossRef]
- Wachs, I. Infrared Spectroscopy of Supported Metal oxides. Colloids Surf. A Physicochem. Eng. Acpects 1995, 1, 143–149. [Google Scholar] [CrossRef]
- Podobiński, J.; Śliwa, M.; Datka, J. Determination of Concentration of Basic Sites on Oxides by IR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023. submitted. [Google Scholar]
- Śliwa, M.; Socha, R.P. Modification of CuO–ZrO2–ZnO Mixed Oxide Catalyst with Mn, Ga, Ni: Impact on Physicochemical Properties and Hydrogen Production via Low Temperature Steam Reforming of Ethanol. Catal. Lett. 2022, 152, 3747–3760. [Google Scholar] [CrossRef]
- Hamryszak, Ł.; Madej-Lachowska, M.; Grzesik, M.; Śliwa, M. Cu/Zn/Zr/Ga Catalyst for Utilisation of Carbon Dioxide to Methanol—Kinetic Equations. Catalysts 2022, 12, 757. [Google Scholar] [CrossRef]
- Tang, Q.L.; Luo, Q.H. Adsorption of CO2 at ZnO: A Surface Structure Effect From DFT-U Calculations. J. Phys. Chem. C 2013, 117, 22954–22966. [Google Scholar] [CrossRef]
- Carli, R.; Le Van Mao, R.; Bianchi, C.; Ragaini, V. Hydrogen Sorption Sites in the Gallium Containing Hybrid Catalysts Used for the Aromatization of Light Alkanes. Catal. Lett. 1993, 21, 265–274. [Google Scholar] [CrossRef]
- Takahara, I.; Saito, M.; Inaba, M.; Murata, K. Effects of Pre-Treatment of a Silica-Supported Gallium Oxide Catalyst with H2 on Its Catalytic Performance for Dehydrogenation of Propane. Catal. Lett. 2004, 96, 29–32. [Google Scholar] [CrossRef]
- Meitzner, G.D.; Iglesia, E.; Baumgartner, J.E.; Huang, E.S. The Chemical State of Gallium in Working Alkane Dehydrocyclodimerization Catalysts. In situ Gallium K-Edge X-Ray Absorption Spectroscopy. J. Catal. 1993, 140, 209–225. [Google Scholar] [CrossRef]
- Moreno, J.A.; Poncelet, G. Isomerization of n-Butane over Sulfated Al- and Ga-Promoted Zirconium Oxide Catalysts. Influence of Promoter and Preparation Method. J. Catal. 2001, 203, 453–465. [Google Scholar] [CrossRef]
- Collins, S.E.; Chiavassa, D.L.; Bonivardi, A.L.; Baltanas, L.A. Hydrogen Spillover in Ga2O3-Pd/SiO2 Catalysts for Methanol Synthesis from CO/H2. Catal. Lett. 2005, 103, 83–88. [Google Scholar] [CrossRef]
- Maeda, N.; Urakawa, A.; Baiker, A. Support Effects and Chemical Gradients along the Catalyst Bed in NOx Storage-Reduction Studied by Space- and Time-Resolved In Situ DRIFTS. J. Phys. Chem. C 2009, 113, 16724–16735. [Google Scholar] [CrossRef]
- Lin, C.H.; Ito, T.; Wang, J.; Lunsford, J.H. Oxidative Dimerization of Methane over Magnesium and Calcium Oxide Catalysts Promoted with Group IA Ions: The Role of [M+O-] Centers. J. Am. Chem. Soc. 1987, 109, 4808–4810. [Google Scholar] [CrossRef]
- Guzman, J.; Gates, B.C.; Bruce, C. A Mononuclear Gold Complex catalyst Supported on MgO: Spectroscopic Characterization during Ethylene Hydrogenation Catalysis. J. Catal. 2004, 226, 111–119. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.G.; Rodrigues, A.E. Adsorption of Carbon Dioxide on Chemically Modified on High Surface Area Carbon-Based Adsorbent at High Temperature. Adsorption 2001, 7, 41–50. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Equilibria and Kinetics of CO2 Adsorption on Hydrotalcite Adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Adsorption-enhanced steam–methane reforming. Chem. Eng. 2000, 55, 3929–3940. [Google Scholar] [CrossRef]
- Gankanda, A.; Cwiertny, D.M.; Grassian, V.H. Surface Phases and the Impact on Nanoparticle Dissolution. J. Phys. Chem. C 2016, 120, 19195–19203. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, A.; Wang, Y.; Loeffler, E.; Woell, C.; Muehler, M. The Identification of Hydroxyl Groups on ZnO Nanoparticles by Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tanabe, K. Infrared Study of Carbon Dioxide Adsorbed on Magnesium and Calcium Oxides. Bull. Chem. Soc. Jpn. 1973, 46, 1616–1619. [Google Scholar] [CrossRef]
- Yanagisawa, Y.K.; Takaoka, K.; Yamabe, S. Interaction of CO2 with Magnesium Oxide Surfaces: A TPD, FTIR, and Cluster-Model Calculation Study. J. Phys. Chem. 1995, 99, 3704–3710. [Google Scholar] [CrossRef]
- Jensen, M.B.; Pettersson, L.G.M.; Swang, O.; Olsbye, U. CO2 Sorption on MgO and CaO Surfaces: A Comparative Quantum Chemical Cluster Study. J. Phys. Chem. B 2005, 109, 16774–16781. [Google Scholar] [CrossRef]
- Podobiński, J.; Zimowska, M.; Samson, K.; Śliwa, M.; Datka, J. Ethoxy Groups on ZrO2, CuO, CuO/ZrO2 Al2O3, Ga2O3, and NiO. Formation and Reactivity. Molecules 2023, 28, 3463. [Google Scholar] [CrossRef]





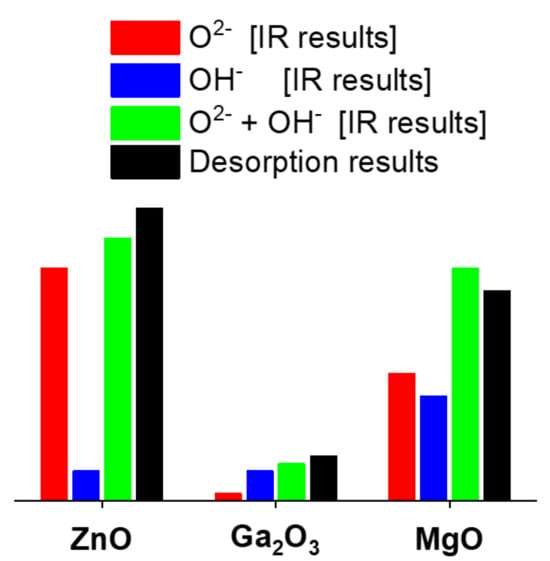
| Sample | Activ. Temp./K | IR Data | Desorption Data | ||
|---|---|---|---|---|---|
| O2− | OH− | O2 + OH− | |||
| ZnO | 720 | 31 | 4 | 35 | 39 |
| 820 | 28 | 2 | 30 | 35 | |
| Ga2O3 | 720 | 1 | 4 | 5 | 6 |
| 900 | 0 | 3 | 3 | 3 | |
| MgO | 720 | 17 | 14 | 31 | 28 |
| 900 | 27 | 1 | 28 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podobiński, J.; Datka, J. Basic Properties of ZnO, Ga2O3, and MgO—Quantitative IR Studies. Catalysts 2024, 14, 106. https://doi.org/10.3390/catal14020106
Podobiński J, Datka J. Basic Properties of ZnO, Ga2O3, and MgO—Quantitative IR Studies. Catalysts. 2024; 14(2):106. https://doi.org/10.3390/catal14020106
Chicago/Turabian StylePodobiński, Jerzy, and Jerzy Datka. 2024. "Basic Properties of ZnO, Ga2O3, and MgO—Quantitative IR Studies" Catalysts 14, no. 2: 106. https://doi.org/10.3390/catal14020106