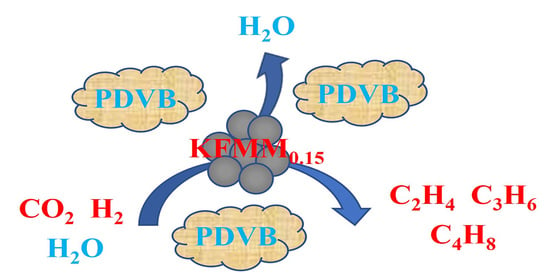
Developing Multifunctional Fe-Based Catalysts for the Direct Hydrogenation of CO2 in Power Plant Flue Gas to Light Olefins
Abstract
:1. Introduction
2. Results and Discussion
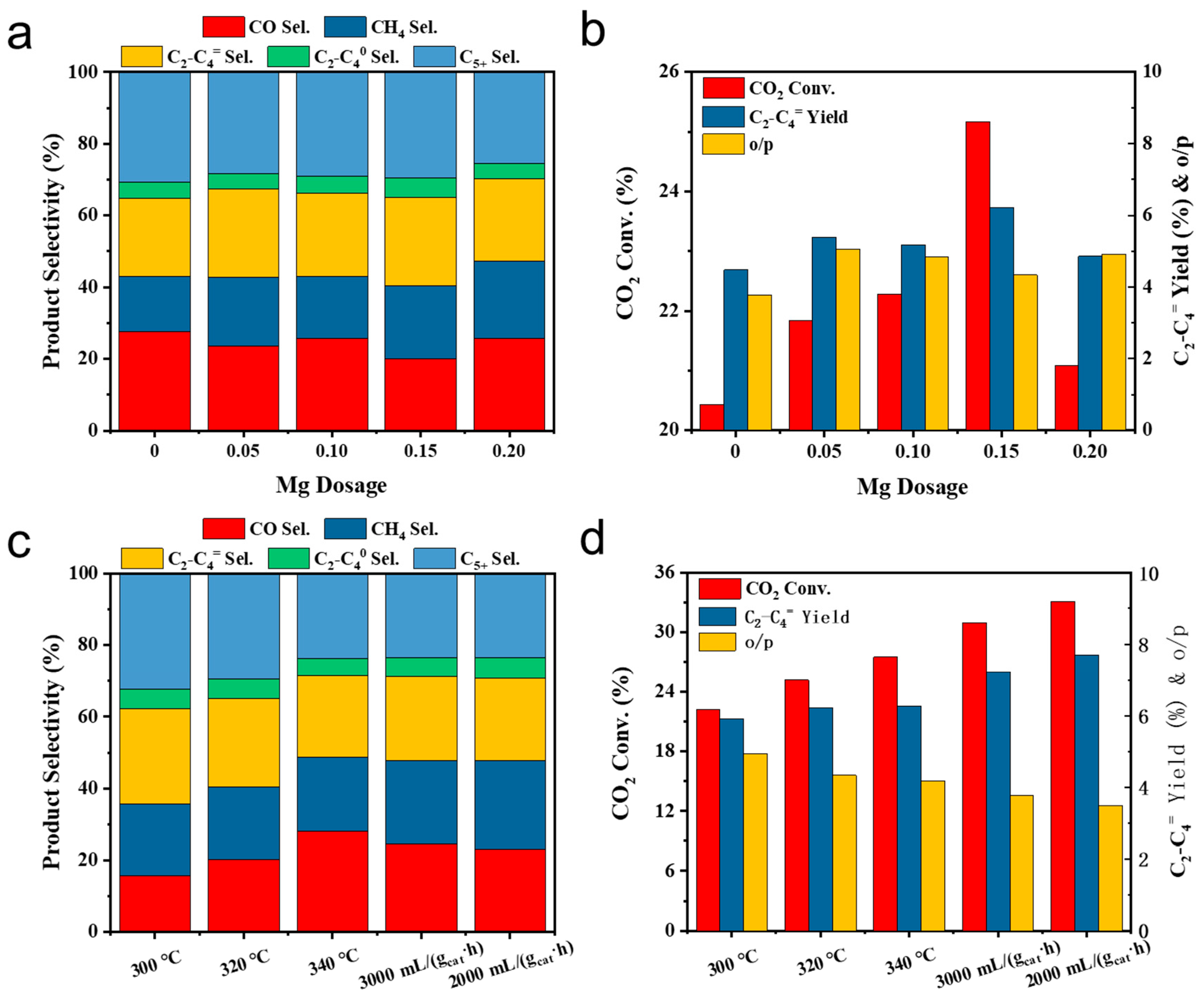
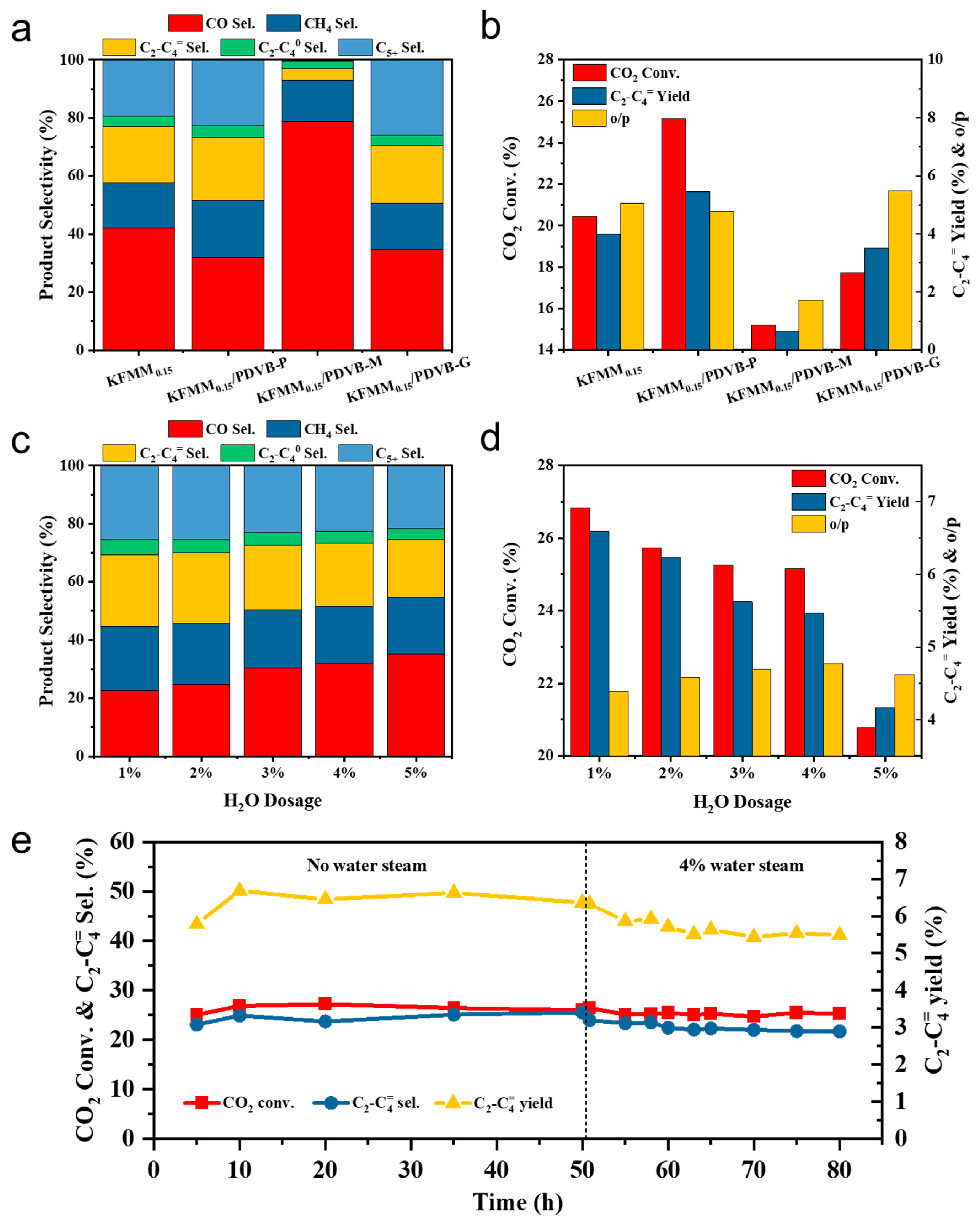
2.1. CO2 Hydrogenation Performance under Simulant Power Plant Flue Gas Conditions
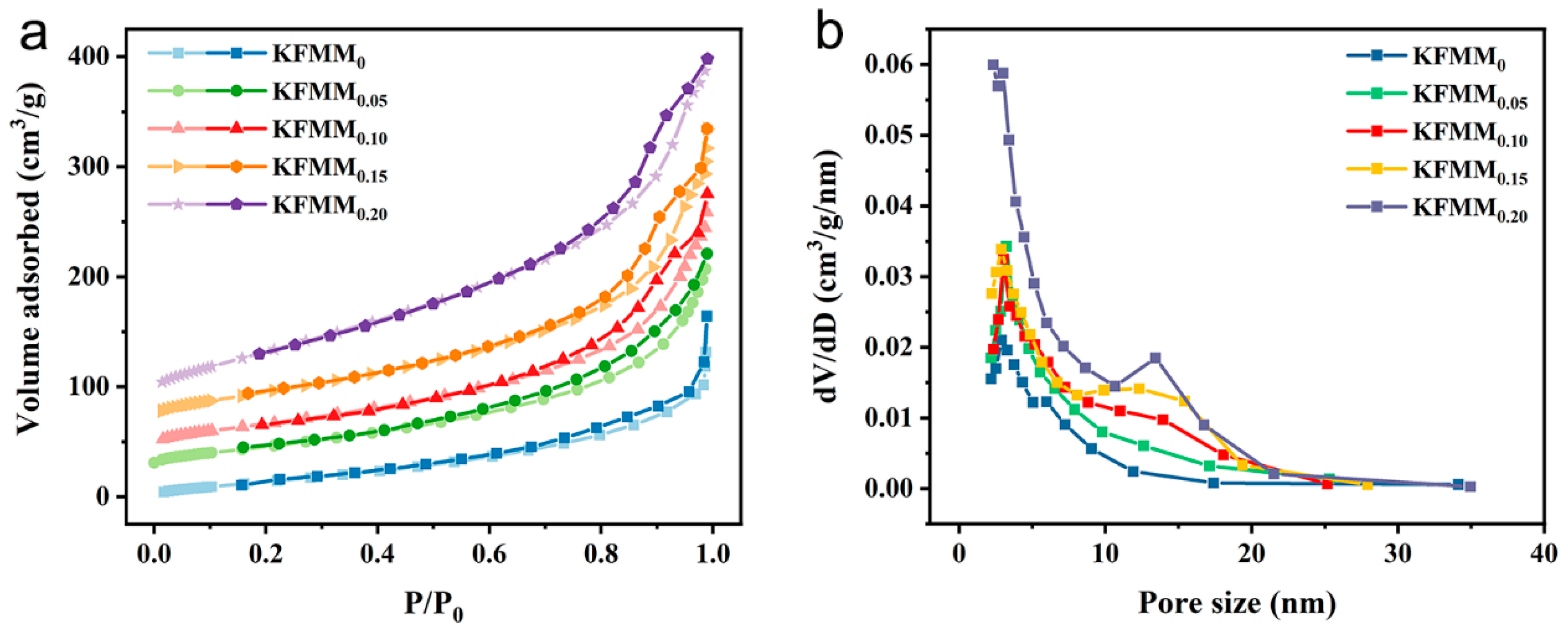
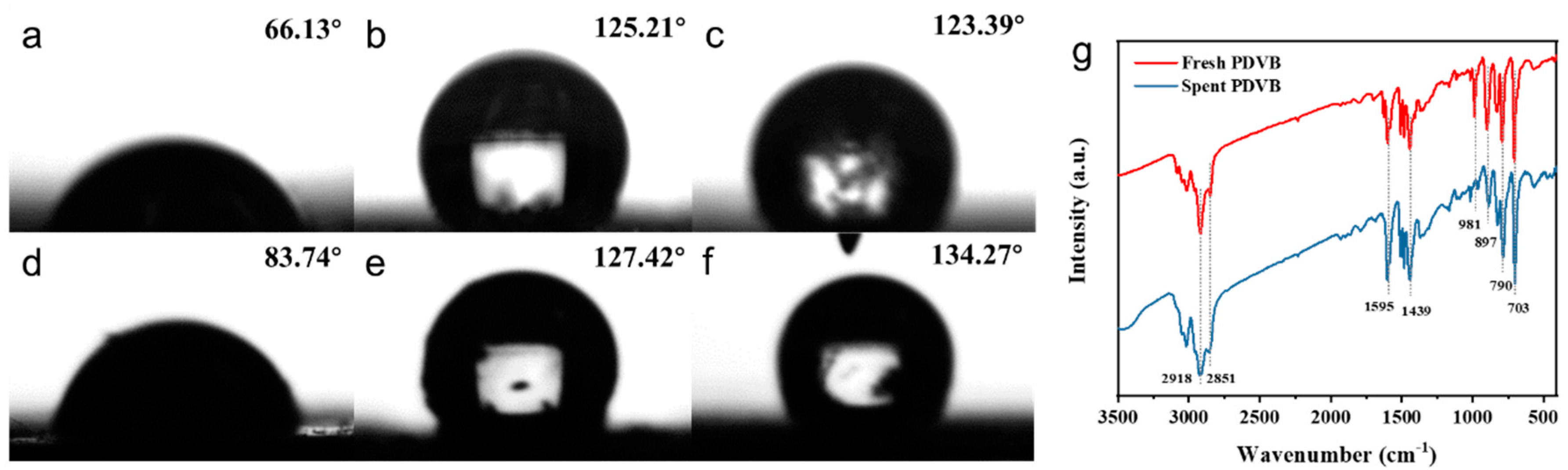
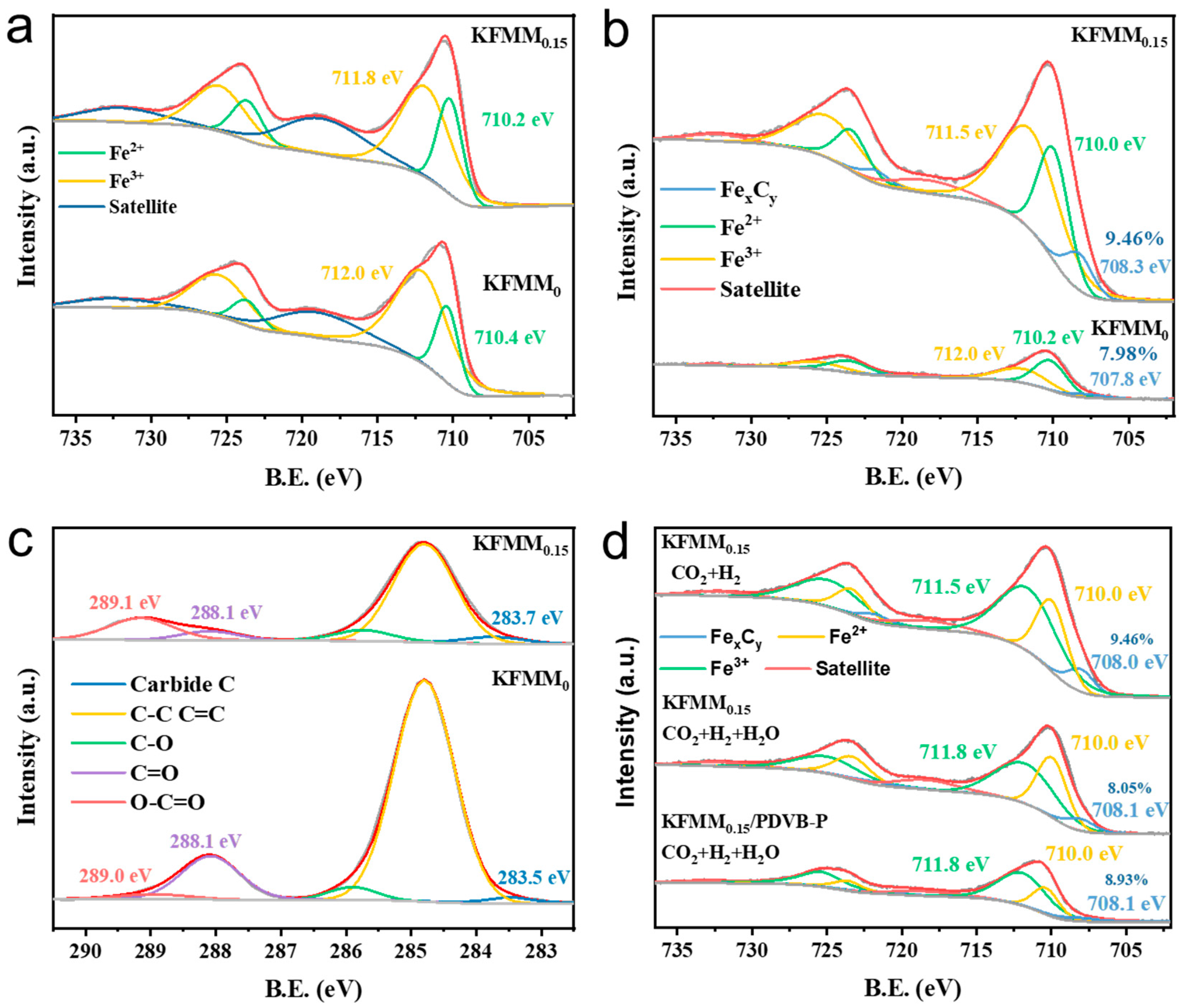
2.2. Structural Characterization
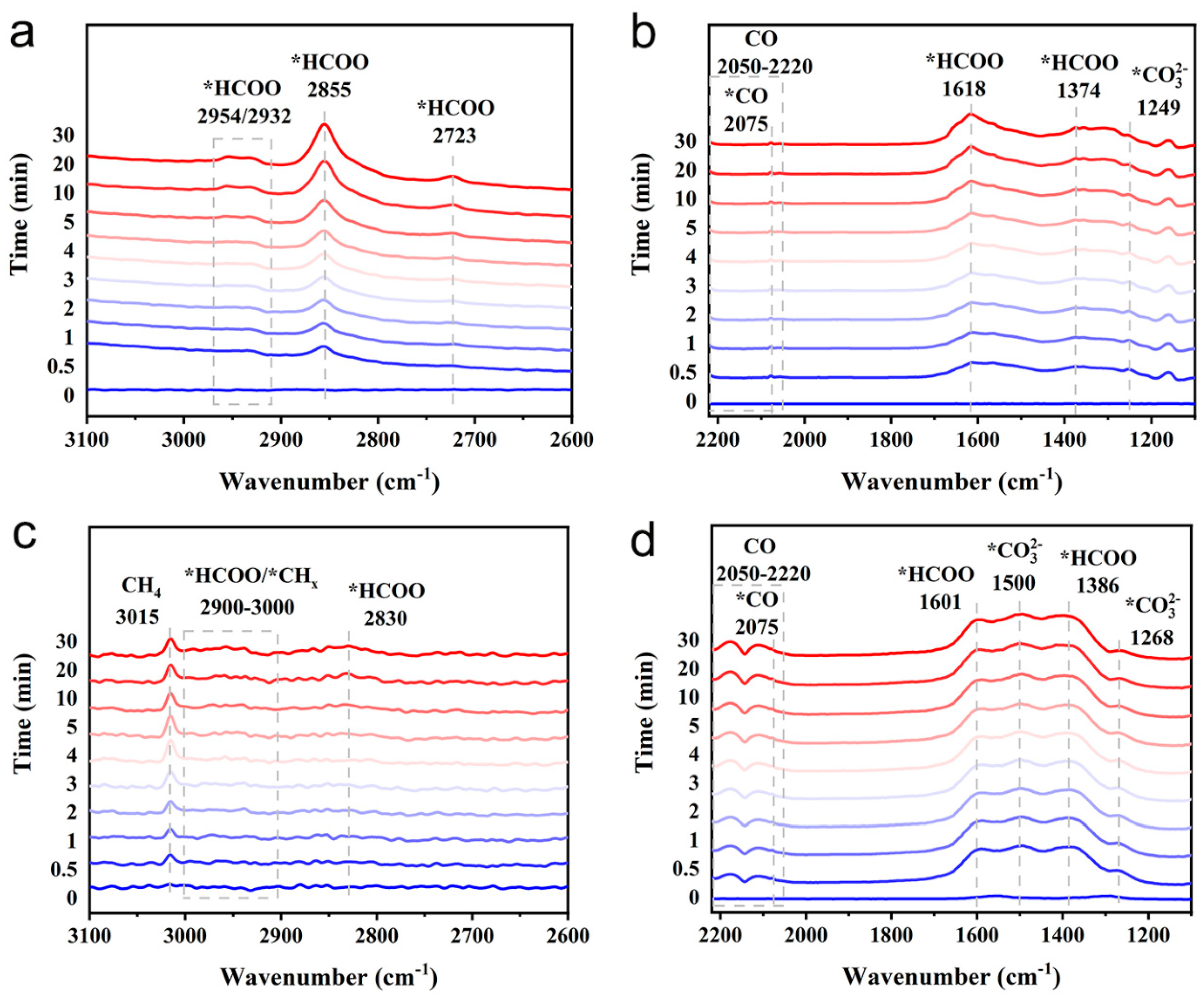
2.3. Reaction Mechanism Study
3. Experimental Section
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Oceanic & Atmospheric Administration. Carbon Cycle Greenhouse Gases—Trends in Atmospheric Carbon Dioxide; Global Monitoring Laboratory Earth System Research Laboratories: Boulder, CO, USA, 2024. Available online: https://gml.noaa.gov/ccgg/trends/mlo.html (accessed on 5 February 2024).
- IPCC. AR4 Climate Change 2007: Impacts, Adaptation, and Vulnerability—IPCC; IPCC: Geneva, Switzerland, 2007; Available online: https://www.ipcc.ch/report/ar4/wg2/ (accessed on 5 February 2024).
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2013, 114, 1709–1742. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt Carbide Nanoprisms for Direct Production of Lower Olefins from Syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Yang, Q.; Kondratenko, V.A.; Petrov, S.A.; Doronkin, D.E.; Saraçi, E.; Lund, H.; Arinchtein, A.; Kraehnert, R.; Skrypnik, A.S.; Matvienko, A.A.; et al. Identifying Performance Descriptors in CO2 Hydrogenation over Iron-Based Catalysts Promoted with Alkali Metals. Angew. Chem. Int. Ed. 2022, 61, e202116517. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A Short Review of Recent Advances in CO2 Hydrogenation to Hydrocarbons over Heterogeneous Catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Produce Olefins and Aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent Advances in Direct Catalytic Hydrogenation of Carbon Dioxide to Valuable C2+ Hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Orege, J.I.; Wei, J.; Han, Y.; Yang, M.; Sun, X.; Zhang, J.; Amoo, C.C.; Ge, Q.; Sun, J. Highly Stable Sr and Na Co-Decorated Fe Catalyst for High-Valued Olefin Synthesis from CO2 Hydrogenation. Appl. Catal. B Environ. 2022, 316, 121640. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. 2020, 59, 21736–21744. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer–Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zhang, C.; Liu, C.; Wei, Y.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.; He, Y.; Qing, M.; et al. Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- Bukur, D.B.; Todic, B.; Elbashir, N. Role of Water-Gas-Shift Reaction in Fischer–Tropsch Synthesis on Iron Catalysts: A Review. Catal. Today 2016, 275, 66–75. [Google Scholar] [CrossRef]
- de Smit, E.; Cinquini, F.; Beale, A.M.; Safonova, O.V.; van Beek, W.; Sautet, P.; Weckhuysen, B.M. Stability and Reactivity of ϵ−χ−θ Iron Carbide Catalyst Phases in Fischer−Tropsch Synthesis: Controlling μC. J. Am. Chem. Soc. 2010, 132, 14928–14941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, B.; Jia, L.; Liu, W.; Gao, X.; Gao, J.; Meng, B.; Tan, Y.; He, Y.; Tu, W.; et al. Unraveling the Role of Fe5c2 in CH4 Formation during CO2 Hydrogenation over Hydrophobic Iron Catalysts. Appl. Catal. B Environ. 2023, 327, 122449. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Gao, J.; Wang, J.; Ma, G.; Wen, X.; Yang, Y.; Li, Y.; Ding, M. A Hydrophobic FeMn@Si Catalyst Increases Olefins from Syngas by Suppressing C1 By-Products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef]
- Javed, M.; Zhang, G.; Gao, W.; Cao, Y.; Dai, P.; Ji, X.; Lu, C.; Yang, R.; Xing, C.; Sun, J. From Hydrophilic to Hydrophobic: A Promising Approach to Tackle High CO2 Selectivity of Fe-Based Fischer-Tropsch Microcapsule Catalysts. Catal. Today 2019, 330, 39–45. [Google Scholar] [CrossRef]
- Anderson, K.; Peters, G. The Trouble with Negative Emissions. Science 2016, 354, 182–183. [Google Scholar] [CrossRef]
- Davis, S.J.; Socolow, R.H. Commitment Accounting of CO2 Emissions. Environ. Res. Lett. 2014, 9, 084018. [Google Scholar] [CrossRef]
- Voumik, L.C.; Islam, M.A.; Ray, S.; Mohamed Yusop, N.Y.; Ridzuan, A.R. CO2 Emissions from Renewable and Non-Renewable Electricity Generation Sources in the G7 Countries: Static and Dynamic Panel Assessment. Energies 2023, 16, 1044. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Environmental Impacts of Absorption-Based CO2 Capture Unit for Post-Combustion Treatment of Flue Gas from Coal-Fired Power Plant. Int. J. Greenh. Gas Control 2007, 1, 318–342. [Google Scholar] [CrossRef]
- Chapel, D.; Ernest, J.; Mariz, C. Recovery of CO2 from Flue Gases: Commercial Trends; Canadian Society of Chemical Engineers Annual Meeting: Saskatoon, SK, Canada, 1999. [Google Scholar]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.; Zaman, Q.; Ehsan, N.; Javied, S.; Schenk, P.M. Heavy Metal Bioremediation of Coal-Fired Flue Gas Using Microalgae under Different CO2 Concentrations. J. Environ. Manag. 2019, 241, 243–250. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Verweij, H.; Winston Ho, W.S. Membrane Processes for Carbon Capture from Coal-Fired Power Plant Flue Gas: A Modeling and Cost Study. J. Membr. Sci. 2012, 421–422, 299–310. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research Progress on CO2 Capture and Utilization Technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture from Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C Spheres as an Excellent Catalyst for Fischer−Tropsch Synthesis. J. Am. Chem. Soc. 2009, 132, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, P.; Zhang, X.; Zhang, G.; Li, R.; Li, W.; Senftle, T.P.; Liu, W.; Wang, J.; Wang, Y.; et al. Dynamic Structural Evolution of Iron Catalysts Involving Competitive Oxidation and Carburization during CO 2 Hydrogenation. Sci. Adv. 2022, 8, eabm3629. [Google Scholar] [CrossRef]
- Fang, W.; Wang, C.; Liu, Z.; Wang, L.; Liu, L.; Li, H.; Xu, S.; Zheng, A.; Qin, X.; Liu, L.; et al. Physical Mixing of a Catalyst and a Hydrophobic Polymer Promotes CO Hydrogenation through Dehydration. Science 2022, 377, 406–410. [Google Scholar] [CrossRef]
- Lu, F.; Chen, X.; Wang, W.; Zhang, Y. Adjusting the CO2 Hydrogenation Pathway via the Synergic Effects of Iron Carbides and Iron Oxides. Catal. Sci. Technol. 2021, 11, 7694–7703. [Google Scholar] [CrossRef]
- Martín, N.; Cirujano, F.G. Multifunctional Heterogeneous Catalysts for the Tandem CO2 Hydrogenation-Fischer Tropsch Synthesis of Gasoline. J. CO2 Util. 2022, 65, 102176. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.-X.; Fu, X.-P.; Jia, C.-J.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5c2 Catalyst. Angew. Chem. 2016, 55, 9902–9907. [Google Scholar] [CrossRef] [PubMed]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Light Olefin Synthesis from CO2 Hydrogenation over K-Promoted Fe–Co Bimetallic Catalysts. Catal. Today 2015, 251, 34–40. [Google Scholar] [CrossRef]
- Cho, J.M.; Kim, B.-G.; Han, G.Y.; Sun, J.; Jeong, H.-K.; Bae, J.W. Effects of Metal-Organic Framework-Derived Iron Carbide Phases for CO Hydrogenation Activity to Hydrocarbons. Fuel 2020, 281, 118779. [Google Scholar] [CrossRef]
- Heyl, D.; Rodemerck, U.; Bentrup, U. Mechanistic Study of Low-Temperature CO2 Hydrogenation over Modified Rh/Al2O3 Catalysts. ACS Catal. 2016, 6, 6275–6284. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Kwak, J.H.; Szanyi, J. Mechanism of CO2 Hydrogenation on Pd/al2O3 Catalysts: Kinetics and Transient DRIFTS-MS Studies. ACS Catal. 2015, 5, 6337–6349. [Google Scholar] [CrossRef]
- Tang, L.; Song, C.; Li, M.; Yang, X.; Hu, B. Study of K/Mn-MgO Supported Fe Catalysts with Fe(CO)5and Fe(NO3)3as Precursors for CO Hydrogenation to Light Alkenes. Chin. J. Chem. 2013, 31, 1263–1268. [Google Scholar] [CrossRef]
- de Araujo, J.C.S.; Sousa, C.B.A.; Oliveira, A.C.; Freire, F.N.A.; Ayala, A.P.; Oliveira, A.C. Dehydrogenation of Ethylbenzene with CO2 to Produce Styrene over Fe-Containing Ceramic Composites. Appl. Catal. A Gen. 2010, 377, 55–63. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Iwama, Y.; Tsubaki, N. Mechanistic Study of a New Low-Temperature Methanol Synthesis on Cu/MgO Catalysts. Appl. Catal. A Gen. 2005, 288, 126–133. [Google Scholar] [CrossRef]
- Borchert, H.; Jürgens, B.; Zielasek, V.; Rupprechter, G.; Giorgio, S.; Henry, C.; Bäumer, M. Pd Nanoparticles with Highly Defined Structure on MgO as Model Catalysts: An FTIR Study of the Interaction with CO, O2, and H2 under Ambient Conditions. J. Catal. 2007, 247, 145–154. [Google Scholar] [CrossRef]
- Xu, M.; Qin, X.; Xu, Y.; Zhang, X.; Zheng, L.; Liu, J.-X.; Wang, M.; Liu, X.; Ma, D. Boosting CO Hydrogenation towards c2+ Hydrocarbons over Interfacial TiO2−x/Ni Catalysts. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Braga, A.H.; Arpini, B.H.; Szanyi, J.; Gonçalves, R.V.; Zornio, B.F.; Miranda, C.R.; Rossi, L.M. Optimizing Active Sites for High CO Selectivity during CO2 Hydrogenation over Supported Nickel Catalysts. J. Am. Chem. Soc. 2021, 143, 4268–4280. [Google Scholar] [CrossRef]
- Topsøe, N.-Y.; Topsøe, H. FTIR Studies of Dynamic Surface Structural Changes in Cu-Based Methanol Synthesis Catalysts. J. Mol. Catal. A Chem. 1999, 141, 95–105. [Google Scholar] [CrossRef]
- Dandekar, A.; Vannice, M.A. Determination of the Dispersion and Surface Oxidation States of Supported Cu Catalysts. J. Catal. 1998, 178, 621–639. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, L.; Calizzi, M.; Moioli, E.; Züttel, A. In Situ Control of the Adsorption Species in CO2 Hydrogenation: Determination of Intermediates and Byproducts. J. Phys. Chem. C 2018, 122, 20888–20893. [Google Scholar] [CrossRef]
- Yanagisawa, Y.; Takaoka, K.; Yamabe, S.; Ito, T. Interaction of CO2 with Magnesium Oxide Surfaces: A TPD, FTIR, and Cluster-Model Calculation Study. J. Phys. Chem. 1995, 99, 3704–3710. [Google Scholar] [CrossRef]
- Yang, C.; Mu, R.; Wang, G.; Song, J.; Tian, H.; Zhao, Z.-J.; Gong, J. Hydroxyl-Mediated Ethanol Selectivity of CO2 Hydrogenation. Chem. Sci. 2019, 10, 3161–3167. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Santos, J.L.; Ivanova, S.; Odriozola, J.A.; Urakawa, A. Unravelling the Role of Oxygen Vacancies in the Mechanism of the Reverse Water–Gas Shift Reaction by Operando DRIFTS and Ultraviolet–Visible Spectroscopy. ACS Catal. 2018, 8, 7455–7467. [Google Scholar] [CrossRef]
- Das, T.; Deo, G. Synthesis, Characterization and in Situ DRIFTS during the CO2 Hydrogenation Reaction over Supported Cobalt Catalysts. J. Mol. Catal. A Chem. 2011, 350, 75–82. [Google Scholar] [CrossRef]



| Catalysts | Element Contents (%) | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | |||
|---|---|---|---|---|---|---|
| Fe | Mn | Mg | K | |||
| KFMM0 | 73.9 | 22.5 | - | 3.6 | 47.6 | 0.25 |
| KFMM0.05 | 71.3 | 21.7 | 3.5 | 3.6 | 70.6 | 0.30 |
| KFMM0.10 | 68.6 | 21.3 | 6.8 | 3.3 | 68.7 | 0.35 |
| KFMM0.15 | 66.1 | 20.7 | 9.7 | 3.5 | 81.8 | 0.40 |
| KFMM0.20 | 64.6 | 20.0 | 12.1 | 3.3 | 145.7 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Guo, S.; Yu, Z.; Cheng, Y.; Ming, J.; Song, X.; Cao, Q.; Zhu, X.; Wang, G.; Xu, D.; et al. Developing Multifunctional Fe-Based Catalysts for the Direct Hydrogenation of CO2 in Power Plant Flue Gas to Light Olefins. Catalysts 2024, 14, 204. https://doi.org/10.3390/catal14030204
Feng L, Guo S, Yu Z, Cheng Y, Ming J, Song X, Cao Q, Zhu X, Wang G, Xu D, et al. Developing Multifunctional Fe-Based Catalysts for the Direct Hydrogenation of CO2 in Power Plant Flue Gas to Light Olefins. Catalysts. 2024; 14(3):204. https://doi.org/10.3390/catal14030204
Chicago/Turabian StyleFeng, Likui, Shuai Guo, Zhiyong Yu, Yijie Cheng, Julan Ming, Xiaoning Song, Qiuyang Cao, Xiaofeng Zhu, Guanghui Wang, Di Xu, and et al. 2024. "Developing Multifunctional Fe-Based Catalysts for the Direct Hydrogenation of CO2 in Power Plant Flue Gas to Light Olefins" Catalysts 14, no. 3: 204. https://doi.org/10.3390/catal14030204