Glu-Co-Assisted Iron-Based Metal–Organic Framework-Derived FeCo/N Co-Doped Carbon Material as Efficient Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries
Abstract
:1. Introduction
2. Results
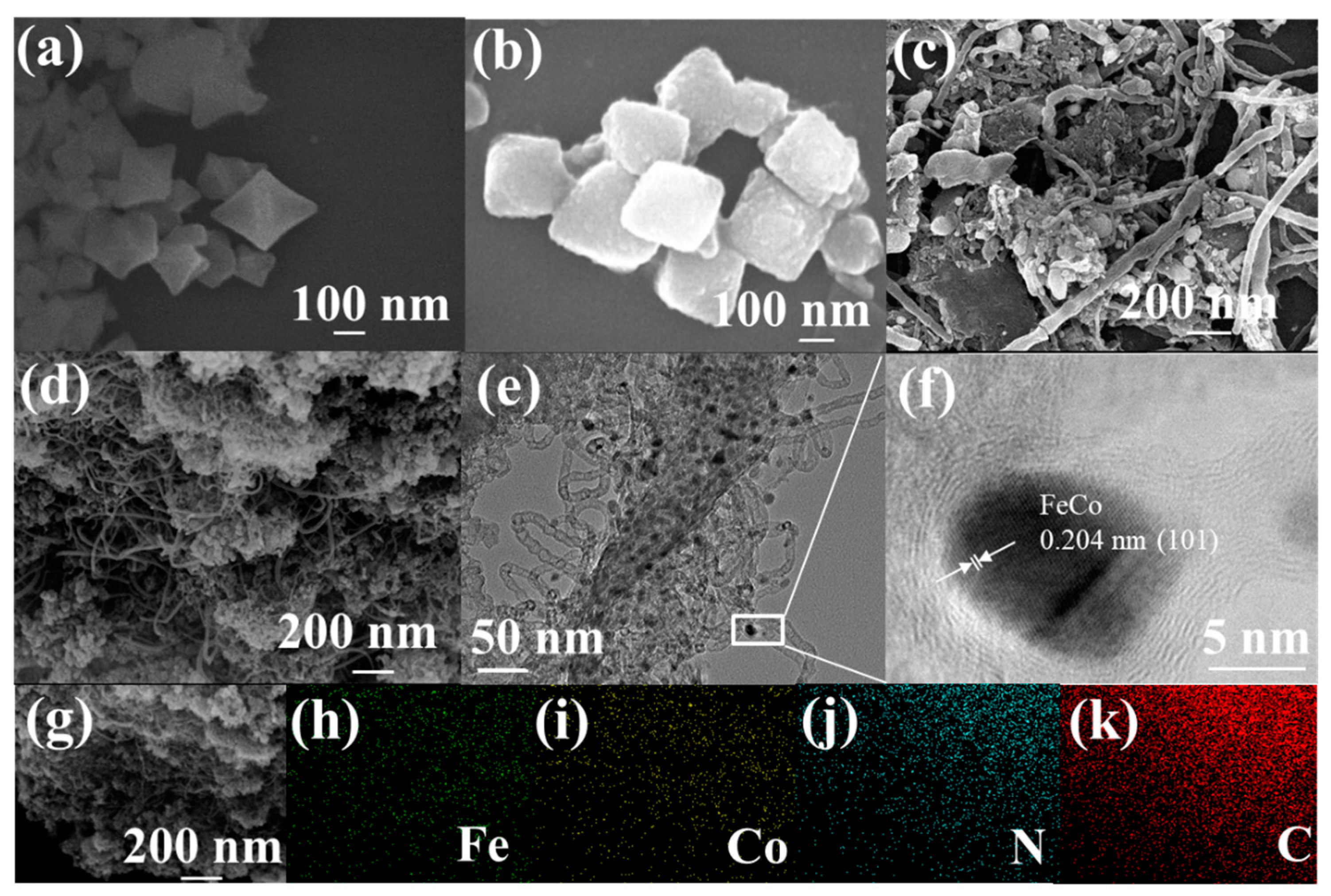
2.1. Structural Characterizations and Composition Analysis
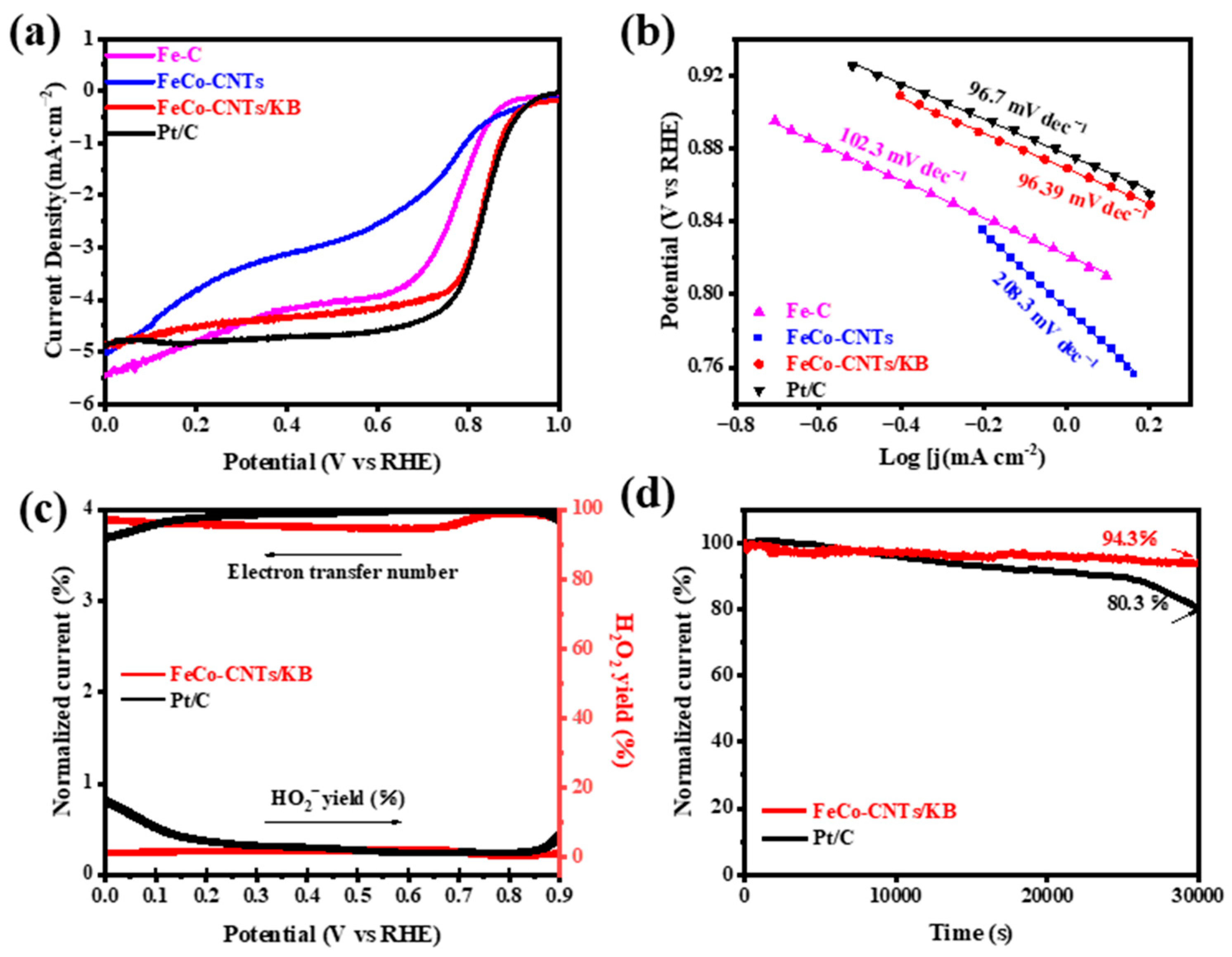
2.2. Electrocatalytic Properties
2.3. Aqueous Zn–Air Battery Performance
3. Experimental
3.1. Materials
3.2. Preparation of NH2-MIL-101(Fe)
3.3. Preparation of NH2-MIL-101(Fe)@glu-Co/KB
3.4. Synthesis of FeCo-CNTs/KB
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced Zinc-Air Batteries Based on High-Performance Hybrid Electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent Advances in Zinc–Air Batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.-N.; Cui, H.; Zhou, Z. Bifunctional Electrocatalysts for Rechargeable Zn-Air Batteries. Chin. J. Catal. 2019, 40, 1298–1310. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments—Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7, 1601275. [Google Scholar] [CrossRef]
- Chen, Y.; Yoo, S.; Zhang, W.; Kim, J.H.; Zhou, Y.; Pei, K.; Kane, N.; Zhao, B.; Murphy, R.; Choi, Y.; et al. Effective Promotion of Oxygen Reduction Reaction by in Situ Formation of Nanostructured Catalyst. ACS Catal. 2019, 9, 7137–7142. [Google Scholar] [CrossRef]
- Materials Design and Discovery Group; Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A Route to High Surface Area, Porosity and Inclusion of Large Molecules in Crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef]
- Gao, X.; Xu, T.; Jiang, Z.; Yu, H.; Wang, Y.; He, Y. Rational Construction and Remarkable Gas Adsorption Properties of a HKUST-1-like Tbo-Type MOF Based on a Tetraisophthalate Linker. Dalton Trans. 2019, 48, 16793–16799. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Sun, M.; Liu, H.; Qu, J.; Li, J. Earth-Rich Transition Metal Phosphide for Energy Conversion and Storage. Adv. Energy Mater. 2016, 6, 1600087. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon Nanocomposite Catalysts for Oxygen Reduction and Evolution Reactions: From Nitrogen Doping to Transition-Metal Addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Muthurasu, A.; Sampath, P.; Ko, T.H.; Lohani, P.C.; Pathak, I.; Acharya, D.; Chhetri, K.; Kim, D.H.; Kim, H.Y. Partial Selenium Surface Modulation of Metal Organic Framework Assisted Cobalt Sulfide Hollow Spheres for High Performance Bifunctional Oxygen Electrocatalysis and Rechargeable Zinc-Air Batteries. Appl. Catal. B Environ. 2023, 330, 122523. [Google Scholar] [CrossRef]
- Muthurasu, A.; Chae, S.-H.; Hoon Ko, T.; Chandra Lohani, P.; Yong Kim, H. Highly Ordered Nanoarrays Catalysts Embedded in Carbon Nanotubes as Highly Efficient and Robust Air Electrode for Flexible Solid-State Rechargeable Zinc-Air Batteries. J. Colloid Interface Sci. 2022, 616, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Muthurasu, A.; Dahal, B.; Mukhiya, T.; Chhetri, K.; Kim, H.Y. Fabrication of Nonmetal-Modulated Dual Metal–Organic Platform for Overall Water Splitting and Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 41704–41717. [Google Scholar] [CrossRef]
- Muthurasu, A.; Tiwari, A.P.; Chhetri, K.; Dahal, B.; Kim, H.Y. Construction of Iron Doped Cobalt- Vanadate- Cobalt Oxide with Metal-Organic Framework Oriented Nanoflakes for Portable Rechargeable Zinc-Air Batteries Powered Total Water Splitting. Nano Energy 2021, 88, 106238. [Google Scholar] [CrossRef]
- Du, Z.; Yu, P.; Wang, L.; Tian, C.; Liu, X.; Zhang, G.; Fu, H. Cubic Imidazolate Frameworks-Derived CoFe Alloy Nanoparticles-Embedded N-Doped Graphitic Carbon for Discharging Reaction of Zn-Air Battery. Sci. China Mater. 2020, 63, 327–338. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Han, M.; Dai, Z.-H.; Bao, J.-C.; Lan, Y.-Q. Nitrogen-Doped Fe/Fe3C@graphitic Layer/Carbon Nanotube Hybrids Derived from MOFs: Efficient Bifunctional Electrocatalysts for ORR and OER. Chem. Commun. 2015, 51, 2710–2713. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, X.; Li, L.; Qin, J.; Cao, M. Carbon Hybrid with 3D Nano-Forest Architecture in-Situ Catalytically Constructed by CoFe Alloy as Advanced Multifunctional Electrocatalysts for Zn-Air Batteries-Driven Water Splitting. J. Energy Chem. 2021, 53, 422–432. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Zeng, K.; Yan, J.; Sun, Z.; Rümmeli, M.H.; Yang, R. A Self-Jet Vapor-Phase Growth of 3D FeNi@NCNT Clusters as Efficient Oxygen Electrocatalysts for Zinc-Air Batteries. Small 2021, 17, 2006183. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Jeffery, A.A.; Min, J.; Jung, N.; Yoo, S.J. Emerging Carbon Shell-Encapsulated Metal Nanocatalysts for Fuel Cells and Water Electrolysis. Nanoscale 2021, 13, 15116–15141. [Google Scholar] [CrossRef]
- Cai, P.; Hong, Y.; Ci, S.; Wen, Z. In Situ Integration of CoFe Alloy Nanoparticles with Nitrogen-Doped Carbon Nanotubes as Advanced Bifunctional Cathode Catalysts for Zn–Air Batteries. Nanoscale 2016, 8, 20048–20055. [Google Scholar] [CrossRef]
- Khalid, M.; Bhardwaj, P.A.; Honorato, A.M.B.; Varela, H. Metallic Single-Atoms Confined in Carbon Nanomaterials for the Electrocatalysis of Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution Reactions. Catal. Sci. Technol. 2020, 10, 6420–6448. [Google Scholar] [CrossRef]
- Pei, Y.; Qi, Z.; Li, X.; Maligal-Ganesh, R.V.; Goh, T.W.; Xiao, C.; Wang, T.; Huang, W. Morphology Inherence from Hollow MOFs to Hollow Carbon Polyhedrons in Preparing Carbon-Based Electrocatalysts. J. Mater. Chem. A 2017, 5, 6186–6192. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Hu, W.; Huang, Y. A Metal-Organic Framework-Derived Fe–N–C Electrocatalyst with Highly Dispersed Fe–Nx towards Oxygen Reduction Reaction. Int. J. Hydrog. Energy 2019, 44, 27379–27389. [Google Scholar] [CrossRef]
- Niu, H.-J.; Chen, S.-S.; Feng, J.-J.; Zhang, L.; Wang, A.-J. Assembled Hollow Spheres with CoFe Alloyed Nanocrystals Encapsulated in N, P-Doped Carbon Nanovesicles: An Ultra-Stable Bifunctional Oxygen Catalyst for Rechargeable Zn-Air Battery. J. Power Sources 2020, 475, 228594. [Google Scholar] [CrossRef]
- Gallardo-Donaire, J.; Hermsen, M.; Wysocki, J.; Ernst, M.; Rominger, F.; Trapp, O.; Hashmi, A.S.K.; Schäfer, A.; Comba, P.; Schaub, T. Direct Asymmetric Ruthenium-Catalyzed Reductive Amination of Alkyl–Aryl Ketones with Ammonia and Hydrogen. J. Am. Chem. Soc. 2018, 140, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, H.; Chen, J.; Ma, F.; Zhen, L.; Wen, Z.; Xu, C. Transition Metal (Co, Ni, Fe, Cu) Single-Atom Catalysts Anchored on 3D Nitrogen-Doped Porous Carbon Nanosheets as Efficient Oxygen Reduction Electrocatalysts for Zn–Air Battery. Small 2022, 18, 2202476. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, L.; Liu, X.; Jia, J.; Guo, S. A New Method for Developing Defect-Rich Graphene Nanoribbons/Onion-like carbon@Co Nanoparticles Hybrid Materials as an Excellent Catalyst for Oxygen Reactions. Nanoscale 2017, 9, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Chen, Y.; Wei, B.; Chen, L.; Peng, L.; Xiang, R.; Li, J.; Wang, Z.; Wei, Z. Carbon-Based Catalysts by Structural Manipulation with Iron for Oxygen Reduction Reaction. J. Mater. Chem. A 2018, 6, 8405–8412. [Google Scholar] [CrossRef]
- Mahmood, A.; Tabassum, H.; Zhao, R.; Guo, W.; Aftab, W.; Liang, Z.; Sun, Z.; Zou, R. Fe2N/S/N Codecorated Hierarchical Porous Carbon Nanosheets for Trifunctional Electrocatalysis. Small 2018, 14, 1803500. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kou, Z.; Mu, S.; Liu, J.; He, D.; Amiinu, I.S.; Meng, W.; Zhou, K.; Luo, Z.; Chaemchuen, S.; et al. 2D Dual-Metal Zeolitic-Imidazolate-Framework-(ZIF)-Derived Bifunctional Air Electrodes with Ultrahigh Electrochemical Properties for Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2018, 28, 1705048. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Pu, Z.; Liu, X.; Owusu, K.A.; Monestel, H.G.R.; Boakye, F.O.; Zhang, H.; Mu, S. Multifunctional Mo–N/C@MoS2 Electrocatalysts for HER, OER, ORR, and Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1702300. [Google Scholar] [CrossRef]
- Su, C.; Cheng, H.; Li, W.; Liu, Z.; Li, N.; Hou, Z.; Bai, F.; Zhang, H.; Ma, T. Atomic Modulation of FeCo–Nitrogen–Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc–Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Ju, J.; Zhang, W.; He, L.; Zhang, J.; Fu, C.; Sun, B. Space-Confined Synthesis of CoNi Nanoalloy in N-Doped Porous Carbon Frameworks as Efficient Oxygen Reduction Catalyst for Neutral and Alkaline Aluminum-Air Batteries. Energy Storage Mater. 2020, 27, 96–108. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Li, G.; Sha, P.; Yu, J.; Yu, L.; Dong, L. FeNi Incorporated N Doped Carbon Nanotubes from Glucosamine Hydrochloride as Highly Efficient Bifunctional Catalyst for Long Term Rechargeable Zinc-Air Batteries. Electrochim. Acta 2022, 428, 140938. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Li, Y.; Liang, D.; Hu, Y.; Li, C. In-Situ Enriching Active Sites on Co-Doped Fe-Co4N@N-C Nanosheet Array as Air Cathode for Flexible Rechargeable Zn-Air Batteries. Appl. Catal. B Environ. 2019, 256, 117893. [Google Scholar] [CrossRef]
- Duan, X.; Ren, S.; Pan, N.; Zhang, M.; Zheng, H. MOF-Derived Fe, Co@N–C Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries. J. Mater. Chem. A 2020, 8, 9355–9363. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, Y.-P.; Cao, Y.; Zhang, L.; Feng, J.-J.; Wang, A.-J. Aminouracil-Assisted Synthesis of CoFe Decorated Bougainvillea-like N-Doped Carbon Nanoflowers for Boosting Zn–Air Battery and Water Electrolysis. J. Power Sources 2022, 521, 230926. [Google Scholar] [CrossRef]
- Sun, R.-M.; Zhang, L.; Feng, J.-J.; Fang, K.-M.; Wang, A.-J. In Situ Produced Co9S8 Nanoclusters/Co/Mn-S, N Multi-Doped 3D Porous Carbon Derived from Eriochrome Black T as an Effective Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Batteries. J. Colloid Interface Sci. 2022, 608, 2100–2110. [Google Scholar] [CrossRef]
- Meng, H.-L.; Lin, S.-Y.; Feng, J.-J.; Zhang, L.; Wang, A.-J. Coordination Regulated Pyrolysis Synthesis of Ultrafine FeNi/(FeNi)9S8 Nanoclusters/Nitrogen, Sulfur-Codoped Graphitic Carbon Nanosheets as Efficient Bifunctional Oxygen Electrocatalysts. J. Colloid Interface Sci. 2022, 610, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, D.-J.; Yang, W.-G.; Ryu, K.-S. Comparison of Electrochemical Performance for Zinc Anode via Various Electrolytes and Conducting Agents in Zn-Air Secondary Batteries. Ionics 2017, 23, 1801–1809. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Brett, D.J.L.; Shearing, P.R.; Skipper, N.T.; Parkin, I.P.; Gadipelli, S. High-Performance Zinc–Air Batteries with Scalable Metal–Organic Frameworks and Platinum Carbon Black Bifunctional Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42696–42703. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhu, J.; Chen, C.; Xie, J.; Wang, M. Bi2S3/Ketjen Black as a Highly Efficient Bifunctional Catalyst for Long-Cycle Lithium-Oxygen Batteries. ChemElectroChem 2019, 6, 3885–3891. [Google Scholar] [CrossRef]
- Xun, S.; Xu, Y.; He, J.; Jiang, D.; Yang, R.; Li, D.; Chen, M. MOF-Derived Cobalt Oxides Nanoparticles Anchored on CoMoO4 as a Highly Active Electrocatalyst for Oxygen Evolution Reaction. J. Alloys Compd. 2019, 806, 1097–1104. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Lou, X.W. (David) Co–Fe Alloy/N-Doped Carbon Hollow Spheres Derived from Dual Metal–Organic Frameworks for Enhanced Electrocatalytic Oxygen Reduction. Small 2019, 15, 1805324. [Google Scholar] [CrossRef]
- Gao, S.; Fan, B.; Feng, R.; Ye, C.; Wei, X.; Liu, J.; Bu, X. N-Doped-Carbon-Coated Fe3O4 from Metal-Organic Framework as Efficient Electrocatalyst for ORR. Nano Energy 2017, 40, 462–470. [Google Scholar] [CrossRef]
- Lu, X.; Yang, P.; Wan, Y.; Zhang, H.; Xu, H.; Xiao, L.; Li, R.; Li, Y.; Zhang, J.; An, M. Active Site Engineering toward Atomically Dispersed M−N−C Catalysts for Oxygen Reduction Reaction. Coord. Chem. Rev. 2023, 495, 215400. [Google Scholar] [CrossRef]
- Poudel, M.B.; Vijayapradeep, S.; Sekar, K.; Kim, J.S.; Dong, J.Y. Pyridinic-N exclusively enriched CNT encapsulated NiFe interfacial alloy nanoparticles on knitted carbon fiber cloth for bifunctional oxygen catalysts and biaxially flexible zinc-air batteries. J. Mater. Chem. A 2024. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhang, M.; Li, S.; Liu, R.; Li, Z. Metal-Organic Frameworks-Derived Hollow-Structured Iron-Cobalt Bimetallic Phosphide Electrocatalysts for Efficient Oxygen Evolution Reaction. J. Alloys Compd. 2020, 821, 153463. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-Template-Assisted Synthesis of Hollow Fullerene-like MoS2 Nanocages as a Lithium Ion Battery Anode Material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Lv, S.; Li, Y.; Ren, J.; Huang, Y. Hollow NH2-MIL-101@TA Derived Electrocatalyst for Enhanced Oxygen Reduction Reaction and Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2021, 46, 38692–38700. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Zhang, L.; Wu, H.; Jin, Y.; Li, Y.; Shi, Y.; Zhu, T.; Mao, H.; Liu, J.; et al. Simultaneously Realizing Rapid Electron Transfer and Mass Transport in Jellyfish-Like Mott–Schottky Nanoreactors for Oxygen Reduction Reaction. Adv. Funct. Mater. 2020, 30, 1910482. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Xie, Y.; Sun, F.; Liang, Z.; Wang, L.; Fu, H. N-Doped Carbon Coating Enhances the Bifunctional Oxygen Reaction Activity of CoFe Nanoparticles for a Highly Stable Zn–Air Battery. J. Mater. Chem. A 2020, 8, 21189–21198. [Google Scholar] [CrossRef]
- Huang, L.-B.; Zhao, L.; Zhang, Y.; Luo, H.; Zhang, X.; Zhang, J.; Pan, H.; Hu, J.-S. Engineering Carbon-Shells of M@NC Bifunctional Oxygen Electrocatalyst towards Stable Aqueous Rechargeable Zn-Air Batteries. Chem. Eng. J. 2021, 418, 129409. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Huang, S.; Hu, B.; Wang, M.; Zhang, Z.; He, L.; Du, M. Photocatalytic Degradation of Oxytetracycline under Visible Light by Nanohybrids of CoFe Alloy Nanoparticles and Nitrogen-/Sulfur-Codoped Mesoporous Carbon. Chem. Eng. J. 2021, 420, 130516. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Yang, P.; Lu, X.; Ma, J.; Li, R.; Xiao, L.; Zhang, J.; An, M. Fe–N4 and Co–N4 Dual Sites for Boosting Oxygen Electroreduction in Zn–Air Batteries. J. Mater. Chem. A 2021, 9, 13678–13687. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Pan, G.; Miao, L.; Chen, S.; Song, Y. Polyaniline-Carbon Nanotubes@Zeolite Imidazolate Framework 67-Carbon Cloth Hierarchical Nanostructures for Supercapacitor Electrode. Electrochim. Acta 2017, 240, 16–23. [Google Scholar] [CrossRef]
- Taha, A.A.; Huang, L.; Ramakrishna, S.; Liu, Y. MOF [NH2-MIL-101(Fe)] as a Powerful and Reusable Fenton-like Catalyst. J. Water Process Eng. 2020, 33, 101004. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, P.; Yong, X.; Chen, D.; Chen, X.; Yan, F.; Pang, B.; Dong, H.; Yu, J.; Yu, L.; Dong, L. Glu-Co-Assisted Iron-Based Metal–Organic Framework-Derived FeCo/N Co-Doped Carbon Material as Efficient Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries. Catalysts 2024, 14, 205. https://doi.org/10.3390/catal14030205
Sha P, Yong X, Chen D, Chen X, Yan F, Pang B, Dong H, Yu J, Yu L, Dong L. Glu-Co-Assisted Iron-Based Metal–Organic Framework-Derived FeCo/N Co-Doped Carbon Material as Efficient Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries. Catalysts. 2024; 14(3):205. https://doi.org/10.3390/catal14030205
Chicago/Turabian StyleSha, Pengfei, Xiao Yong, Di Chen, Xing Chen, Fengying Yan, Beili Pang, Hongzhou Dong, Jianhua Yu, Liyan Yu, and Lifeng Dong. 2024. "Glu-Co-Assisted Iron-Based Metal–Organic Framework-Derived FeCo/N Co-Doped Carbon Material as Efficient Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries" Catalysts 14, no. 3: 205. https://doi.org/10.3390/catal14030205





