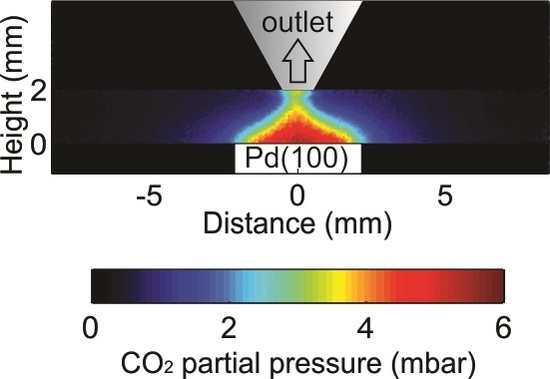
Visualization of Gas Distribution in a Model AP-XPS Reactor by PLIF: CO Oxidation over a Pd(100) Catalyst
Abstract
:1. Introduction
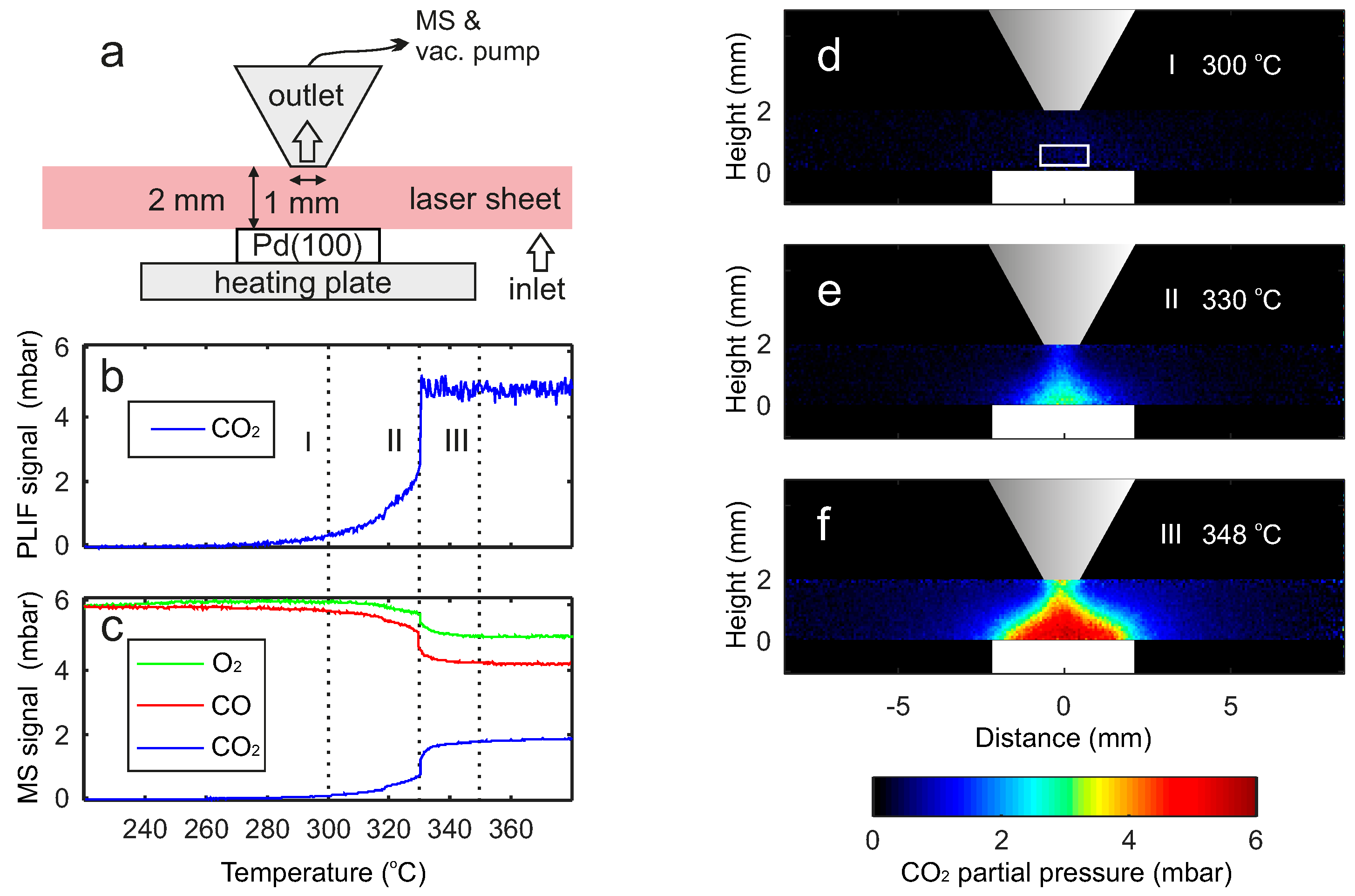
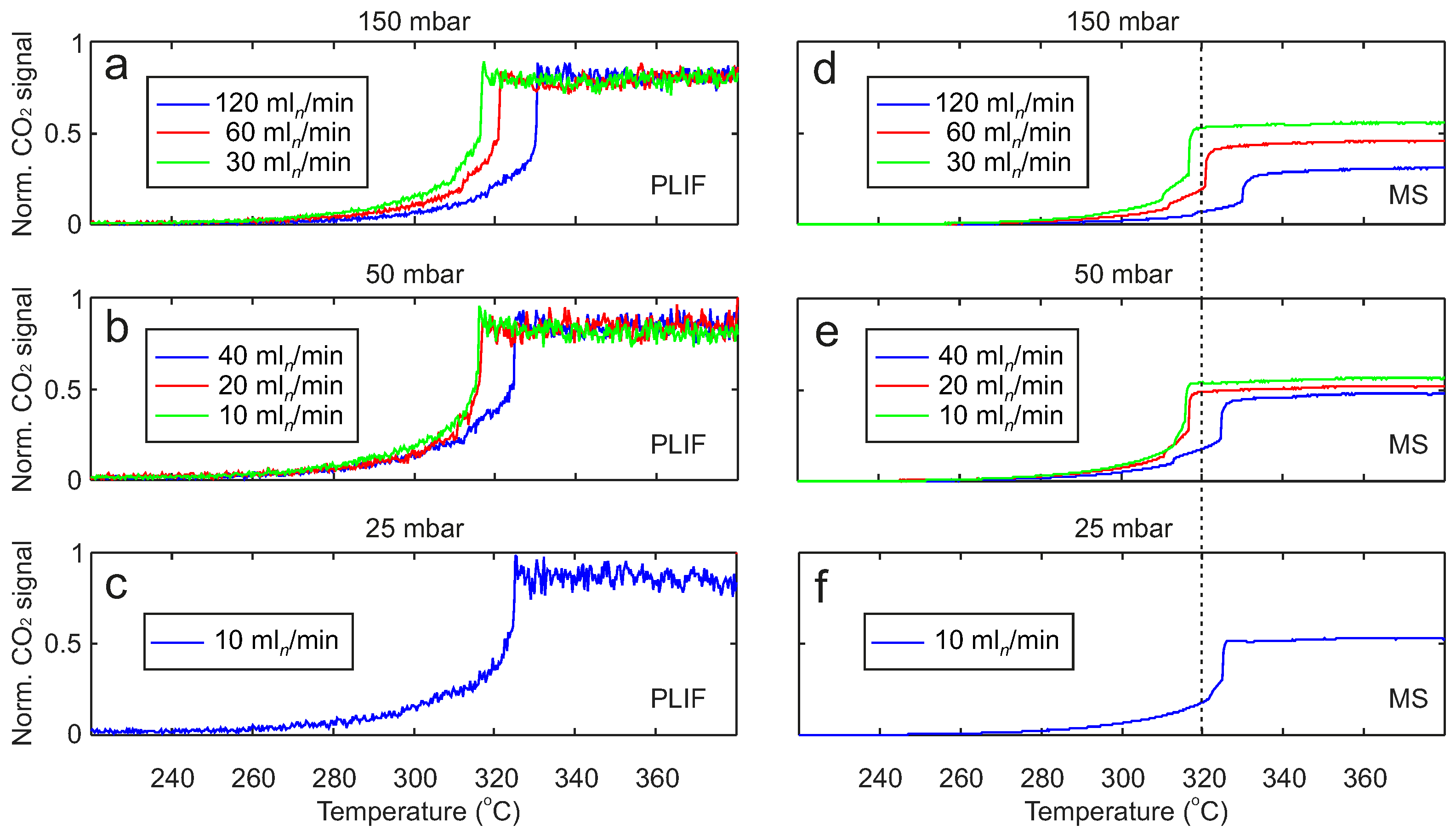
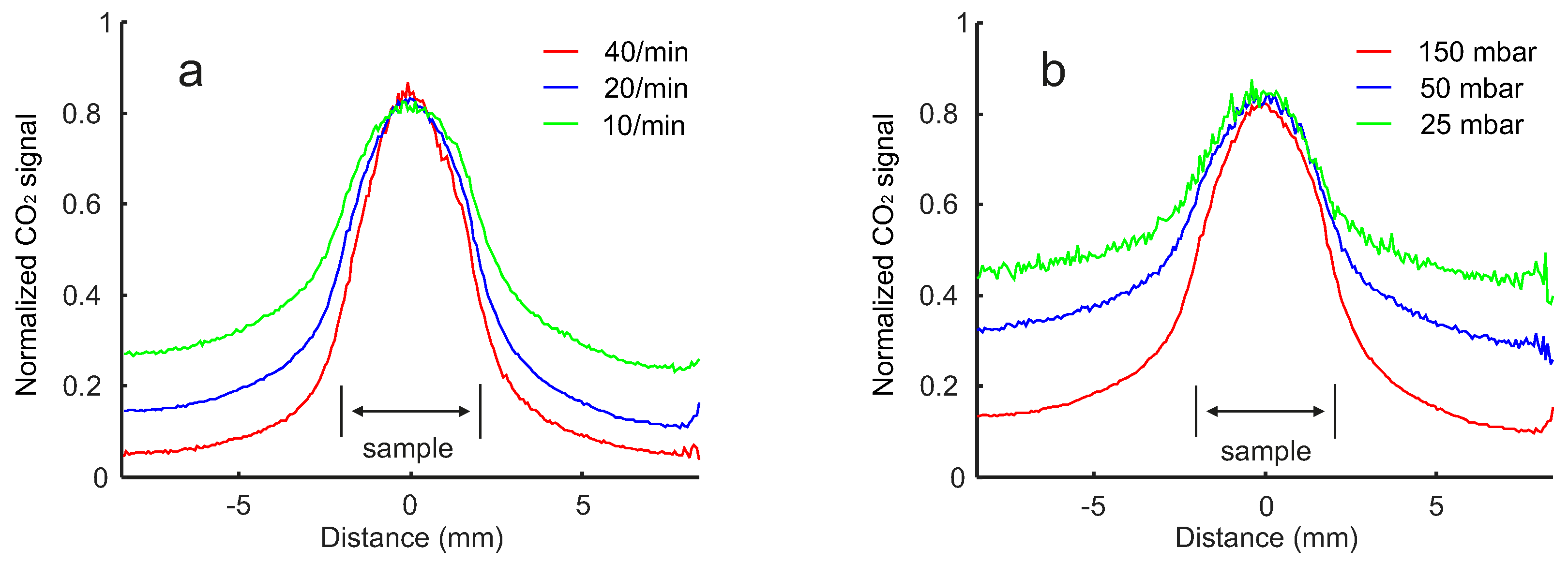
2. Results
2.1. Comparison between PLIF and MS
2.2. Pressure and Flow Rate Dependence
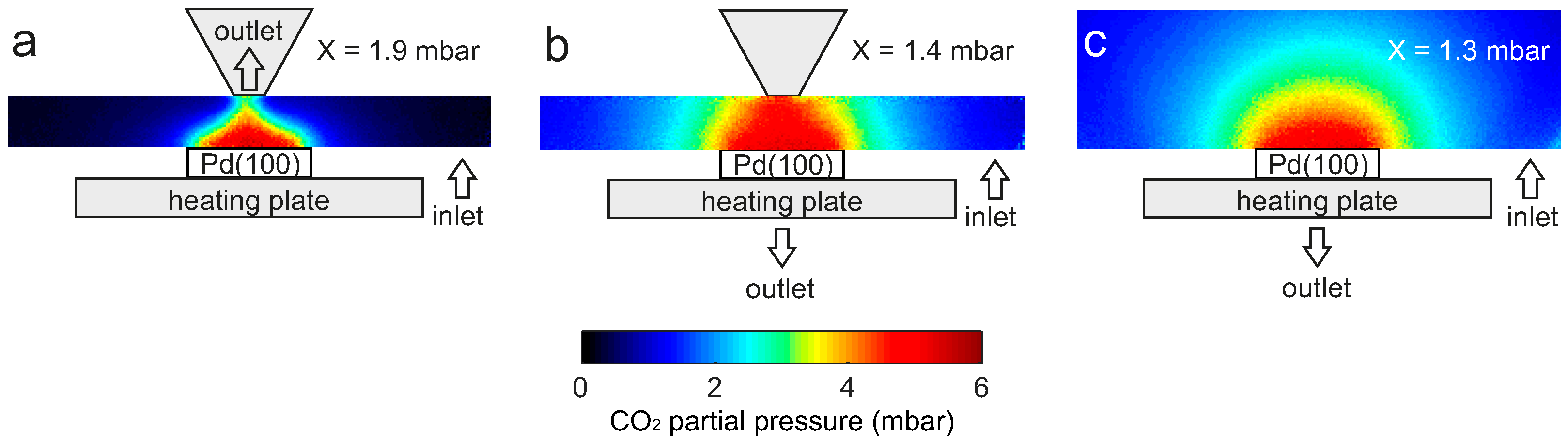
2.3. Flow Configuration Dependence
3. Discussion
4. Materials and Methods
4.1. Sample and Reactor
4.2. PLIF
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bowker, M. The Basis and Applications of Heterogeneous Catalysis; Oxford University Press Inc.: New York, NY, USA, 1998. [Google Scholar]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Hüfner, S. Photoelectron Spectroscopy; Springer: Berlin, Germany, 1995. [Google Scholar]
- Starr, D.E.; Liu, Z.; Havecker, M.; Knop-Gericke, A.; Bluhm, H. Investigation of solid/vapor interfaces using ambient pressure X-ray photoelectron spectroscopy. Chem. Soc. Rev. 2013, 42, 5833–5857. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; McClure, S.M.; Cai, Y.; Gath, K.K.; Wang, Y.; Chen, M.S.; Guo, Q.L.; Goodman, D.W. CO oxidation trends on Pt-group metals from ultrahigh vacuum to near atmospheric pressures: A combined in situ PM-IRAS and reaction kinetics study. Surf. Sci. 2009, 603, 65–70. [Google Scholar] [CrossRef]
- Rupprechter, G.; Weilach, C. Mind the gap! Spectroscopy of catalytically active phases. Nano Today 2007, 2, 20–29. [Google Scholar] [CrossRef]
- Hendriksen, B.L.M.; Bobaru, S.C.; Frenken, J.W.M. Oscillatory CO oxidation on Pd(100) studied with in situ scanning tunneling microscopy. Surf. Sci. 2004, 552, 229–242. [Google Scholar] [CrossRef]
- Gustafson, J.; Shipilin, M.; Zhang, C.; Stierle, A.; Hejral, U.; Ruett, U.; Gutowski, O.; Carlsson, P.-A.; Skoglundh, M.; Lundgren, E. High-energy surface X-ray diffraction for fast surface structure determination. Science 2014, 343, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Bañares, M.A. Operando methodology: Combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 2005, 100, 71–77. [Google Scholar] [CrossRef]
- Blomberg, S.; Zhou, J.; Gustafson, J.; Zetterberg, J.; Lundgren, E. 2D and 3D imaging of the gas phase close to an operating model catalyst by planar laser induced fluorescence. J. Phys. Condens. Matter 2016, 28, 453002. [Google Scholar] [CrossRef] [PubMed]
- Zellner, A.; Suntz, R.; Deutschmann, O. Two-dimensional spatial resolution of concentration profiles in catalytic reactors by planar laser-induced fluorescence: NO reduction over diesel oxidation catalysts. Angew. Chem. Int. Ed. 2015, 54, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.; Zetterberg, J.; Zhou, J.; Merte, L.R.; Gustafson, J.; Shipilin, M.; Trinchero, A.; Miccio, L.A.; Magaña, A.; Ilyn, M.; et al. Strain dependent light-off temperature in catalysis revealed by planar laser-induced fluorescence. ACS Catal. 2016, 110–114. [Google Scholar] [CrossRef]
- Zetterberg, J.; Blomberg, S.; Gustafson, J.; Evertsson, J.; Zhou, J.; Adams, E.C.; Carlsson, P.-A.; Aldén, M.; Lundgren, E. Spatially and temporally resolved gas distributions around heterogeneous catalysts using infrared planar laser-induced fluorescence. Nat. Commun. 2015, 6, 7076. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.; Brackmann, C.; Gustafson, J.; Aldén, M.; Lundgren, E.; Zetterberg, J. Real-time gas-phase imaging over a Pd(110) catalyst during CO oxidation by means of planar laser-induced fluorescence. ACS Catal. 2015, 5, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, R.; Yoshida, M.; Monya, Y.; Kousa, Y.; Suzuki, K.; Abe, H.; Mun, B.S.; Mase, K.; Amemiya, K.; Kondoh, H. In situ ambient pressure XPS study of CO oxidation reaction on Pd(111) surfaces. J. Phys. Chem. C 2012, 116, 18691–18697. [Google Scholar] [CrossRef]
- Blomberg, S.; Hoffmann, M.J.; Gustafson, J.; Martin, N.M.; Fernandes, V.R.; Borg, A.; Liu, Z.; Chang, R.; Matera, S.; Reuter, K.; et al. In-situ X-ray photoelectron spectroscopy of model catalysts: At the edge of the gap. Phys. Rev. Lett. 2013, 110, 117601. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, R.; Yoshida, M.; Monya, Y.; Suzuki, K.; Mun, B.S.; Amemiya, K.; Mase, K.; Kondoh, H. Active surface oxygen for catalytic CO oxidation on Pd(100) proceeding under near ambient pressure conditions. J. Phys. Chem. Lett. 2012, 3, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, R.; Yoshida, M.; Monya, Y.; Suzuki, K.; Amemiya, K.; Mase, K.; Mun, B.S.; Kondoh, H. In situ photoemission observation of catalytic CO oxidation reaction on Pd(110) under near-ambient pressure conditions: Evidence for the Langmuir–Hinshelwood mechanism. J. Phys. Chem. C 2013, 117, 20617–20624. [Google Scholar] [CrossRef]
- Matera, S.; Reuter, K. Transport limitations and bistability for in situ CO oxidation at RuO2(110): First-principles based multiscale modeling. Phys. Rev. B 2010, 82, 085446. [Google Scholar] [CrossRef]
- Matera, S.; Blomberg, S.; Hoffmann, M.J.; Zetterberg, J.; Gustafson, J.; Lundgren, E.; Reuter, K. Evidence for the active phase of heterogeneous catalysts through in situ reaction product imaging and multiscale modeling. ACS Catal. 2015, 5, 4514–4518. [Google Scholar] [CrossRef]
- Blomberg, S.; Zetterberg, J.; Gustafson, J.; Zhou, J.; Brackmann, C.; Lundgren, E. Comparison of AP-XPS and PLIF measurements during CO oxidation over Pd single crystals. Top. Catal. 2016, 59, 478–486. [Google Scholar] [CrossRef]
- Bluhm, H.; Hävecker, M.; Knop-Gericke, A.; Kiskinova, M.; Schlögl, R.; Salmeron, M. In situ X-ray photoelectron spectroscopy studies of gas-solid interfaces at near-ambient conditions. MRS Bull. 2007, 32, 1022–1030. [Google Scholar] [CrossRef]
- Kahk, J.M.; Villar-Garcia, I.J.; Grechy, L.; Bruce, P.J.K.; Vincent, P.E.; Eriksson, S.K.; Rensmo, H.; Hahlin, M.; Åhlund, J.; Edwards, M.O.M.; et al. A study of the pressure profiles near the first pumping aperture in a high pressure photoelectron spectrometer. J. Electron Spectrosc. Relat. Phenom. 2015, 205, 57–65. [Google Scholar] [CrossRef]
- Matera, S.; Reuter, K. When atomic-scale resolution is not enough: Spatial effects on in situ model catalyst studies. J. Catal. 2012, 295, 261–268. [Google Scholar] [CrossRef]
- Matera, S.; Maestri, M.; Cuoci, A.; Reuter, K. Predictive-quality surface reaction chemistry in real reactor models: Integrating first-principles kinetic monte carlo simulations into computational fluid dynamics. ACS Catal. 2014, 4, 4081–4092. [Google Scholar] [CrossRef]
- Schnadt, J.; Knudsen, J.; Andersen, J.N.; Siegbahn, H.; Pietzsch, A.; Hennies, F.; Johansson, N.; Martensson, N.; Ohrwall, G.; Bahr, S.; et al. The new ambient-pressure X-ray photoelectron spectroscopy instrument at MAX-lab. J. Synchrotron Radiat. 2012, 19, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Maibach, J.; Xu, C.; Eriksson, S.K.; Åhlund, J.; Gustafsson, T.; Siegbahn, H.; Rensmo, H.; Edström, K.; Hahlin, M. A high pressure X-ray photoelectron spectroscopy experimental method for characterization of solid-liquid interfaces demonstrated with a Li-ion battery system. Rev. Sci. Instrum. 2015, 86, 044101. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Wang, H.; Uemura, Y.; Ikenaga, E.; Sekizawa, O.; Uruga, T.; Ohashi, H.; Senba, Y.; Yumoto, H.; Yamazaki, H.; et al. In situ study of an oxidation reaction on a Pt/C electrode by ambient pressure hard X-ray photoelectron spectroscopy. Appl. Phys. Lett. 2014, 105, 131602. [Google Scholar] [CrossRef]
- Zetterberg, J.; Blomberg, S.; Gustafson, J.; Sun, Z.W.; Li, Z.S.; Lundgren, E.; Aldén, M. An in situ set up for the detection of CO2 from catalytic CO oxidation by using planar laser-induced fluorescence. Rev. Sci. Instrum. 2012, 83, 053104. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Blomberg, S.; Gustafson, J.; Lundgren, E.; Zetterberg, J. Visualization of Gas Distribution in a Model AP-XPS Reactor by PLIF: CO Oxidation over a Pd(100) Catalyst. Catalysts 2017, 7, 29. https://doi.org/10.3390/catal7010029
Zhou J, Blomberg S, Gustafson J, Lundgren E, Zetterberg J. Visualization of Gas Distribution in a Model AP-XPS Reactor by PLIF: CO Oxidation over a Pd(100) Catalyst. Catalysts. 2017; 7(1):29. https://doi.org/10.3390/catal7010029
Chicago/Turabian StyleZhou, Jianfeng, Sara Blomberg, Johan Gustafson, Edvin Lundgren, and Johan Zetterberg. 2017. "Visualization of Gas Distribution in a Model AP-XPS Reactor by PLIF: CO Oxidation over a Pd(100) Catalyst" Catalysts 7, no. 1: 29. https://doi.org/10.3390/catal7010029