Catalytic Decomposition of N2O over Cu–Zn/ZnAl2O4 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
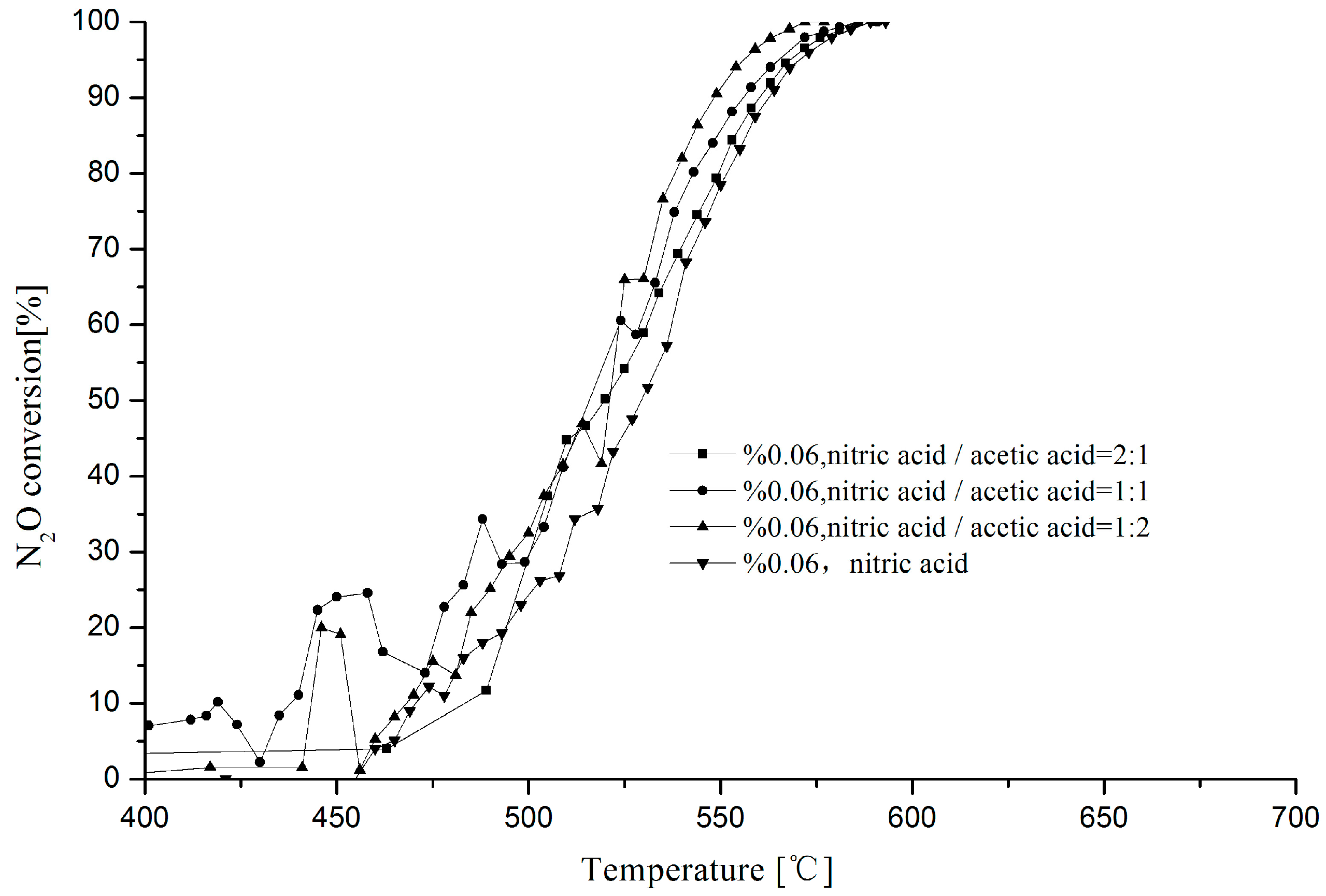
2.1. Effect of Mixed Acid Ratio on N2O Decomposition
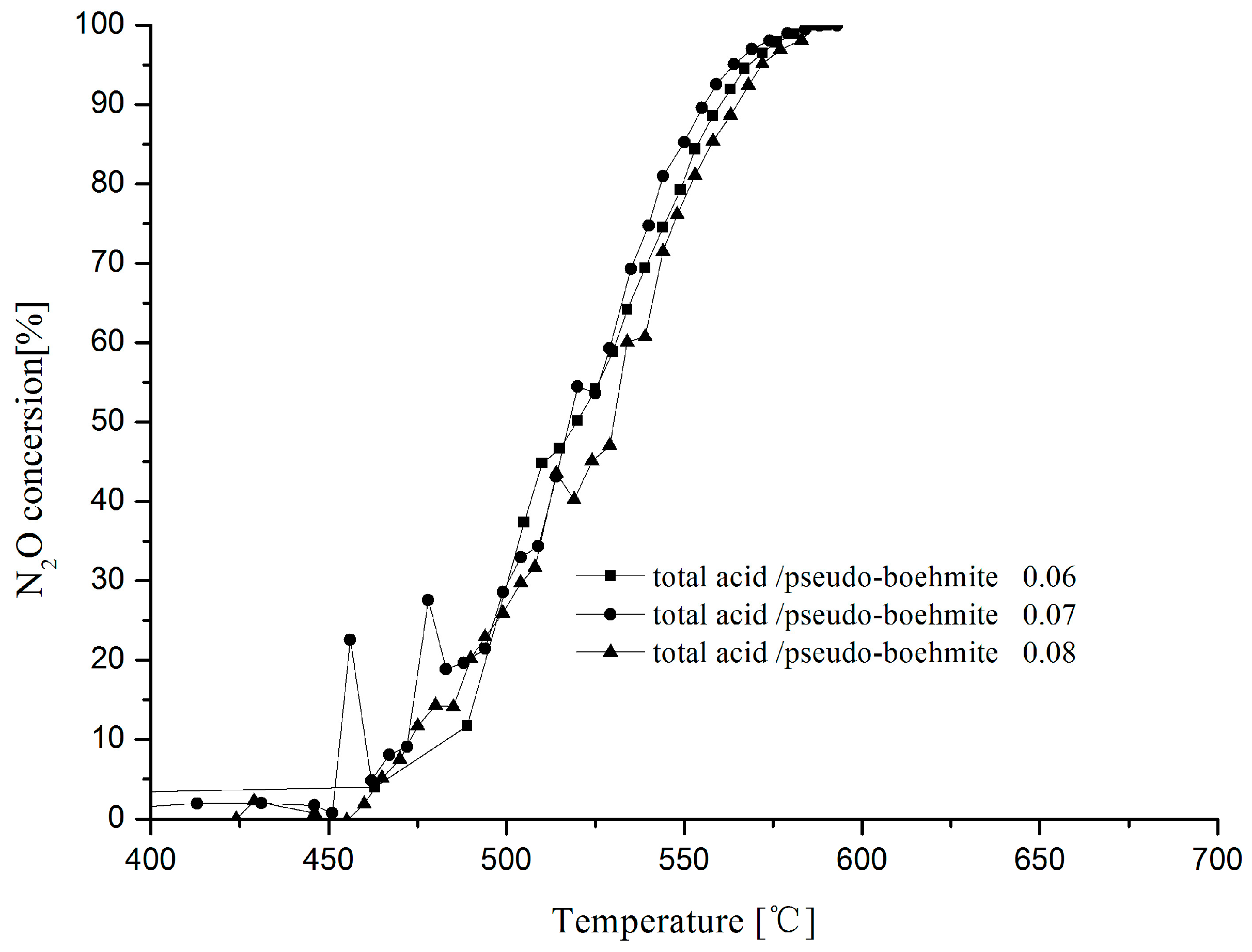
2.2. Effect of Total Acid and Pseudo-Boehmite Ratio for N2O Decomposition
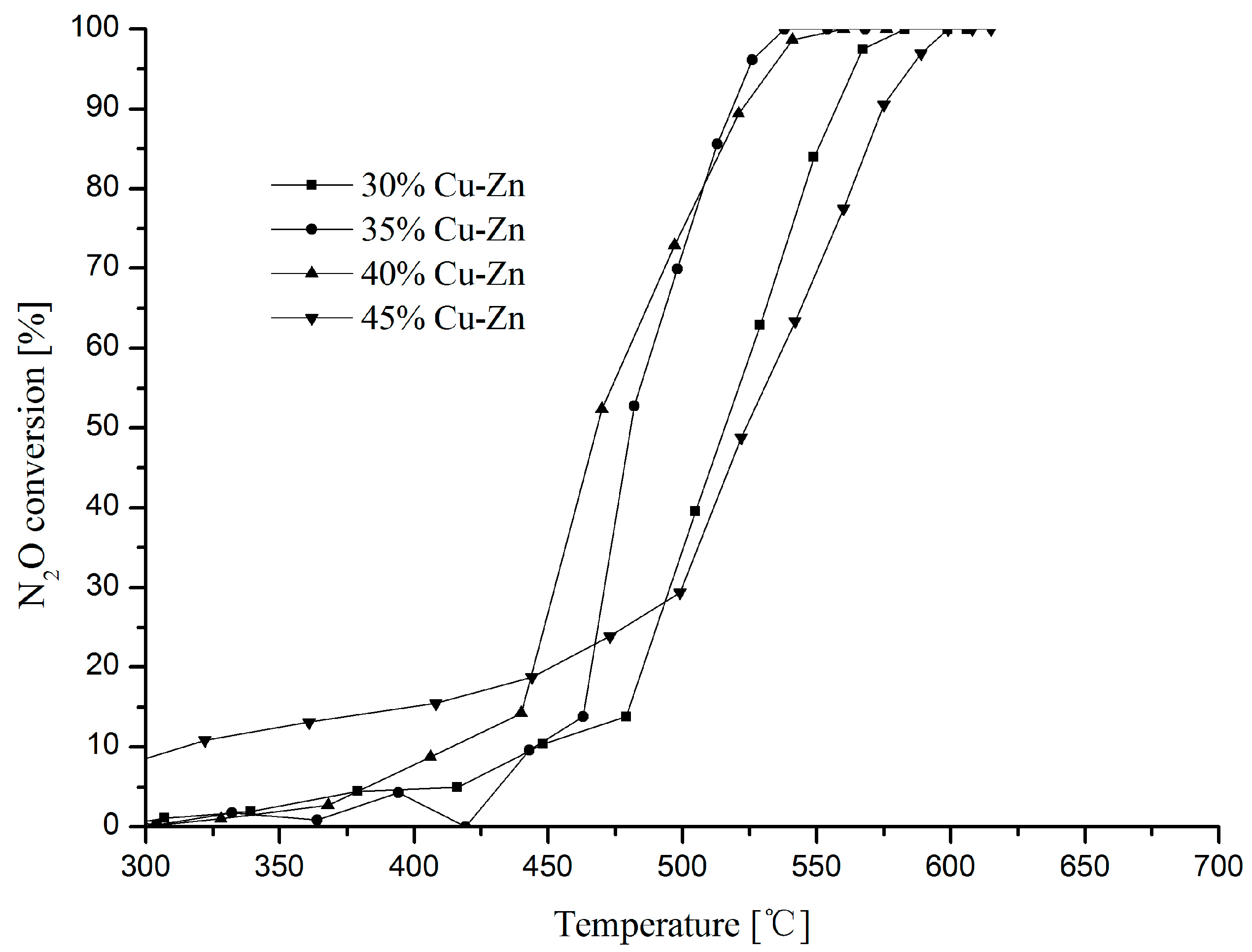
2.3. Effect of Cu-Zn Loading Amount on N2O Decomposition
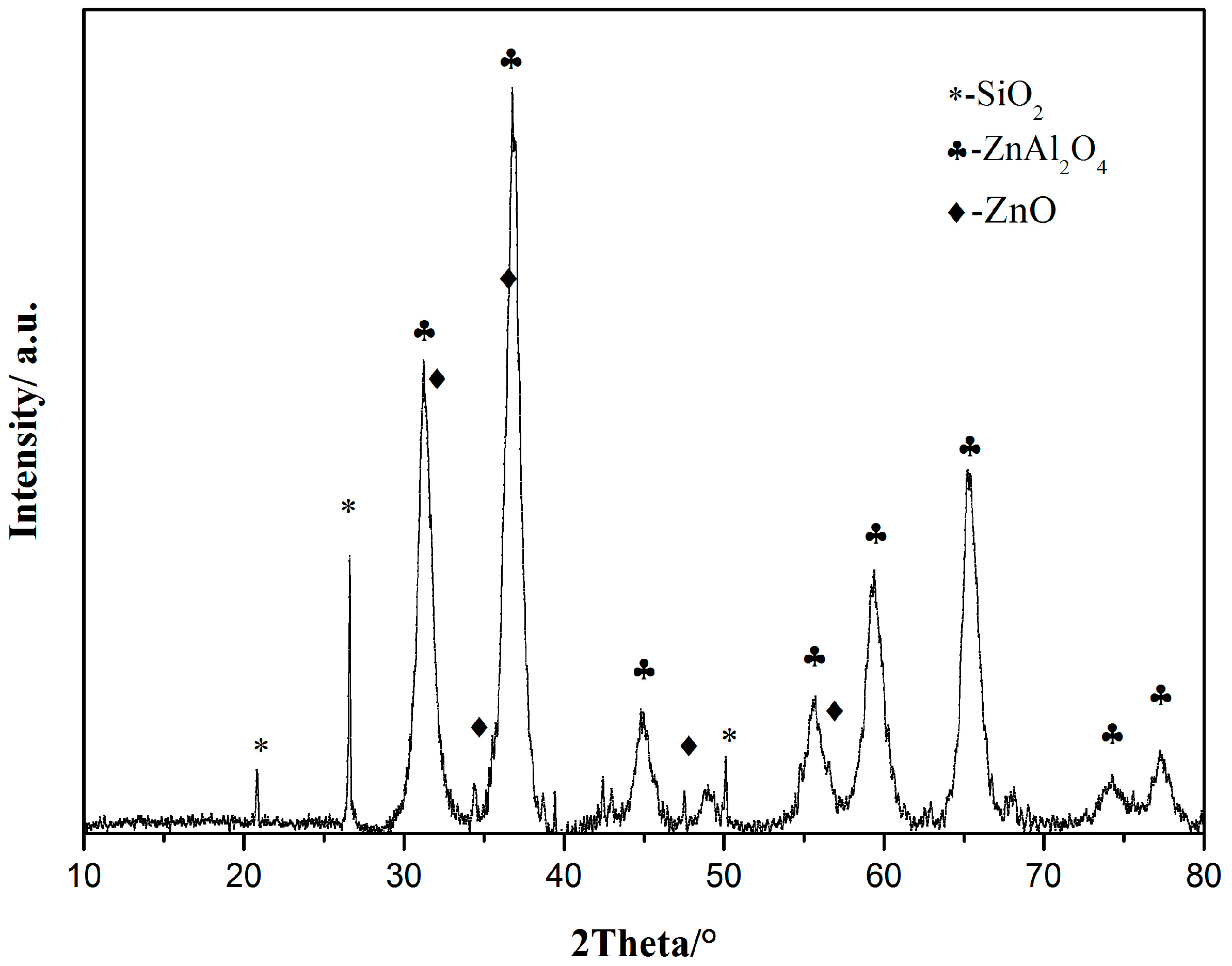
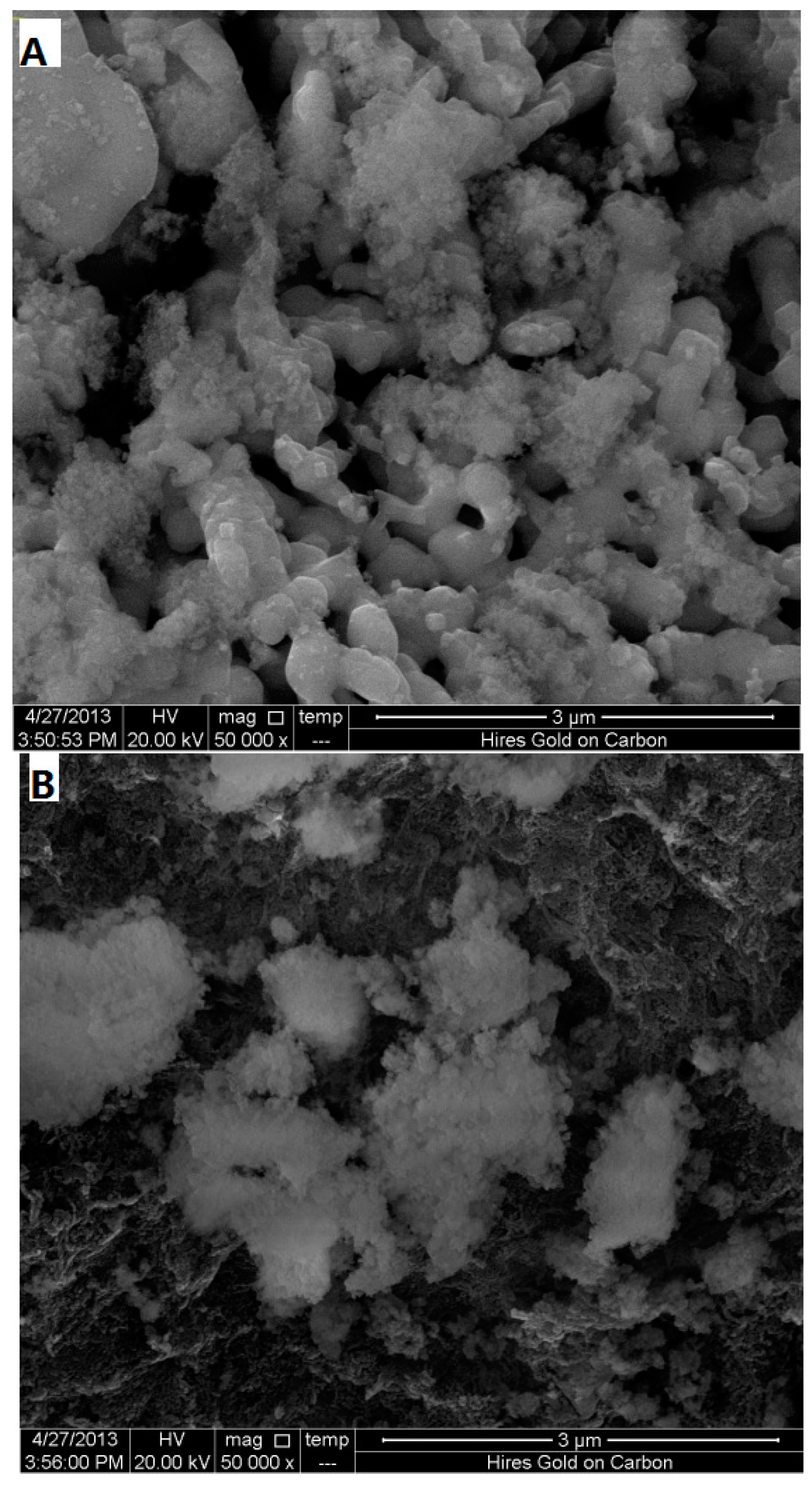
2.4. Characterization of the Catalyst
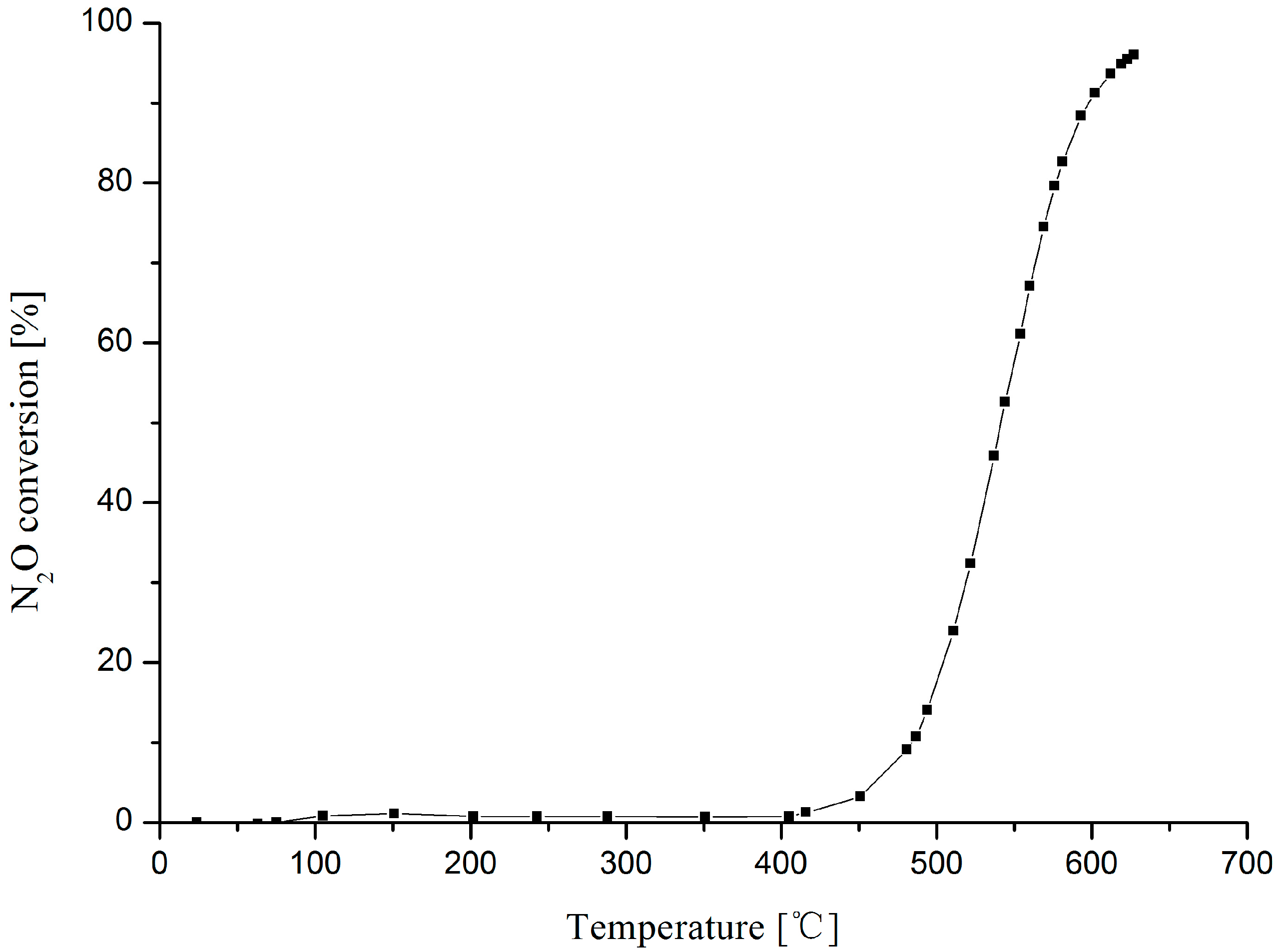
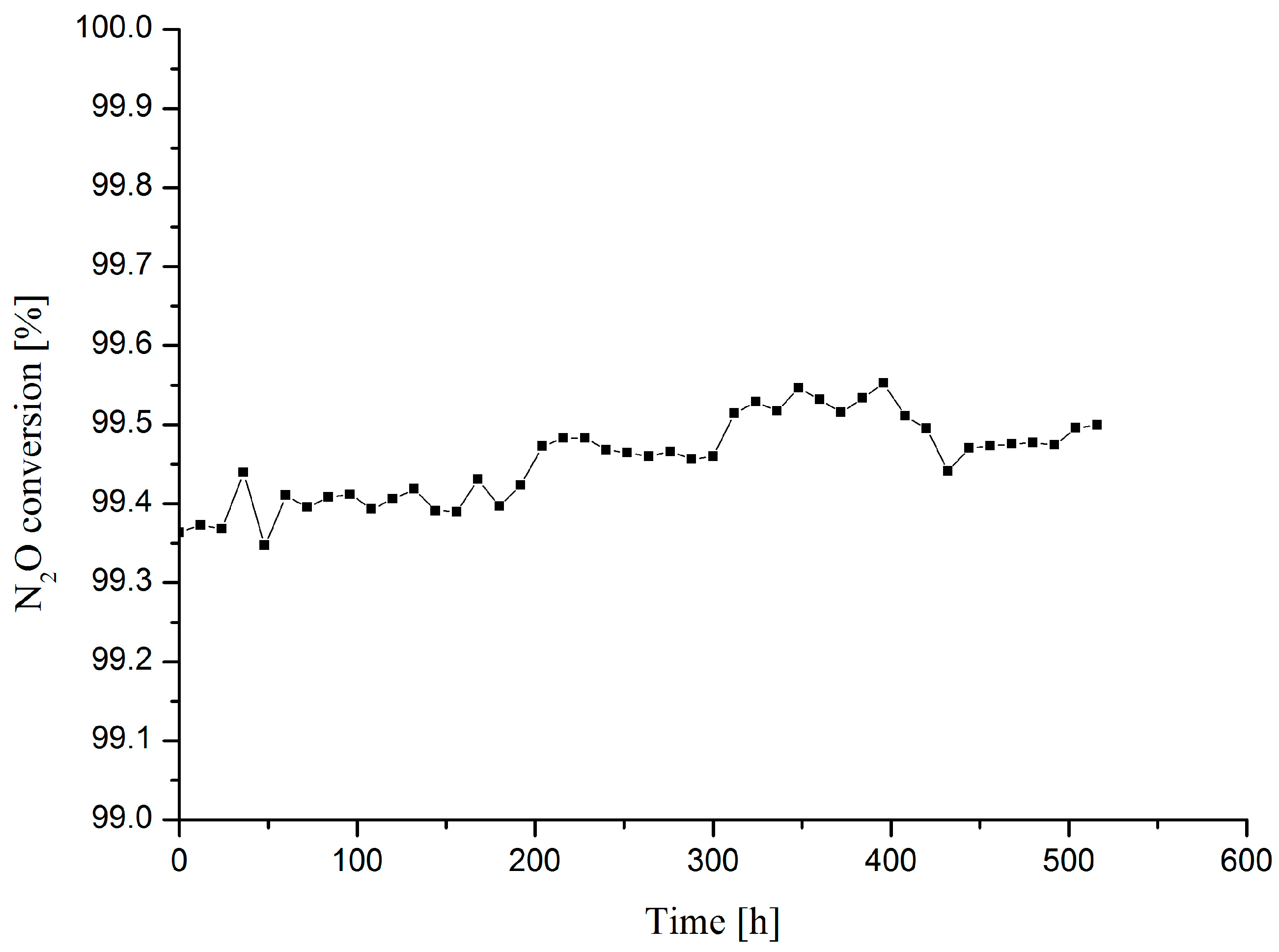
2.5. Catalytic Performance
3. Experimental
3.1. Supports Preparation
3.2. Preparation of Cu-Zn/ZnAl2O4 Catalysts
3.3. Catalysts Characterization
3.4. Activity Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WMO Greenhouse Gas Bulletin. 2014. Available online: http://www.wmo.int/pages/prog/arep/gaw/ghg/documents/GHG_Bulletin_10_Nov2014_EN.pdf (accessed on 1 January 2016).
- Zamudio, M.A.; Bensaid, S.; Fino, D.; Russo, N. Influence of the MgCo2O4 Preparation Method on N2O Catalytic Decomposition. Ind. Eng. Chem. Res. 2011, 50, 2622–2627. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Kapteijn, F.; Schoffel, K.; Moulijn, J.A. Formation and control of N2O in nitric acid production. Where do we stand today. Appl. Catal. B 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Perez-Ramirez, J. Prospects of N2O emission regulations in the European fertilizer industry. Appl. Catal. B 2007, 18, 31–35. [Google Scholar] [CrossRef]
- Kumar, S.; Vinu, A.; Subrt, J.; Bakardjieva, S.; Rayalu, S.; Teraoka, Y.; Labhsetwar, N. Catalytic N2O decomposition on Pr0.8Ba0.2MnO3 type perovskite catalyst for industrial emission control. Catal. Today 2012, 198, 125–132. [Google Scholar] [CrossRef]
- Russo, N.; Fino, D.; Saracco, G.; Specchia, V. N2O catalytic decomposition over various spinel-type oxides. Catal. Today 2007, 119, 228–232. [Google Scholar] [CrossRef]
- Haber, J.; Nattich, M.; Machej, T. Alkali-metal promoted rhodium-on-alumina catalysts for nitrous oxide decomposition. Appl. Catal. B 2008, 77, 278–283. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.; Li, J.; Tian, H.; Hao, Z. A study on N2O catalytic decomposition over Co/MgO catalysts. J. Hazard. Mater. 2009, 163, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zied, B.M.; Schiwieger, W.; Unger, A. Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion—Exchange method. Appl. Catal. B 2008, 84, 277–288. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, P.; Huang, Z.; Qin, F.; Shen, W.; Xu, H. Preparation of NiCe Mixed Oxides for Catalytic Decomposition of N2O. Ind. Eng. Chem. Res. 2013, 52, 4504–4509. [Google Scholar] [CrossRef]
- Konsolakisa, M.; Yentekakisa, I.V.; Pekridisb, G.; Kaklidis, N.; Psarras, A.C.; Marnellosb, G.E. Insights into the role of SO2 and H2O on the surface characteristics and de-N2O efficiency of Pd/Al2O3 catalysts during N2O decomposition in the presence of CH4 and O2 excess. Appl. Catal. B 2013, 138–139, 191–198. [Google Scholar] [CrossRef]
- Kapteijn, F.; Rodriquez-Mirasol, J.; Moulijn, J.A. Heterogeneous catalytic decomposition of nitrous oxide. Appl. Catal. B 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Pasha, N.; Lingaiah, N.; Redd, P.S.S.; Prasad, P.S.S. Direct Decomposition of N2O over Cesium-doped CuO Catalysts. Catal. Lett. 2009, 127, 101–106. [Google Scholar] [CrossRef]
- Gao, L.Z.; Au, C.T. A study on the decomposition of N2O over YBa2Cu3O7. Appl. Catal. B 2001, 30, 35–47. [Google Scholar] [CrossRef]
- Gao, L.Z.; Au, C.T. Studies on the decomposition of N2O over Nd2CuO4, Nd1.6Ba0.4CuO4 and Nd1.8Ce0.2CuO4. J. Mol. Catal. A Chem. 2001, 168, 173–186. [Google Scholar] [CrossRef]
- Smeets, P.J.; Groothaert, M.H.; van Teeffelen, R.M.; Leeman, H.; Hensen, E.J.M.; Schoonheydt, R.A. Direct NO and N2O decomposition and NO-assisted N2O decomposition over Cu-zeolites: Elucidating the influence of the CuCu distance on oxygen migration. J. Catal. 2007, 245, 358–368. [Google Scholar] [CrossRef]
- Gorla, C.R.; Mayo, W.E.; Liang, S.; Lu, Y. Structure and interface-controlled growth kinetics of ZnA2O4 formed at the (1120) ZnO/(0112) Al2O3 interface. J. Appl. Phys. 2000, 87, 3736–3743. [Google Scholar] [CrossRef]
- Nilsson, M.; Jansson, K.; Jozsa, P.; Pettersson, L.J. Catalytic properties of Pd supported on ZnO/ZnAl2O4/Al2O3 mixtures in dimethyl ether autothermal reforming. Appl. Catal. B 2009, 86, 18–26. [Google Scholar] [CrossRef]


| Catalyst | BET Area (m2·g−1) | Pore Diameter (nm) |
|---|---|---|
| fresh | 81.77 | 77.0 |
| used | 94.20 | 75.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhang, R.; Bai, F.; Hua, C. Catalytic Decomposition of N2O over Cu–Zn/ZnAl2O4 Catalysts. Catalysts 2017, 7, 166. https://doi.org/10.3390/catal7050166
Zheng X, Zhang R, Bai F, Hua C. Catalytic Decomposition of N2O over Cu–Zn/ZnAl2O4 Catalysts. Catalysts. 2017; 7(5):166. https://doi.org/10.3390/catal7050166
Chicago/Turabian StyleZheng, Xiaoying, Runhu Zhang, Fang Bai, and Chao Hua. 2017. "Catalytic Decomposition of N2O over Cu–Zn/ZnAl2O4 Catalysts" Catalysts 7, no. 5: 166. https://doi.org/10.3390/catal7050166






