Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate
Abstract
:1. Introduction
2. Results
2.1. The Effect of Preparation Conditions on the Physicochemical Properties of NixPy/γ-Al2O3 Catalysts
2.2. Catalytic Properties of NixPy/γ-Al2O3 Catalysts in Methyl Palmitate HDO
2.2.1. The Effect of Preparation Conditions on the Catalytic Properties of NixPy/γ-Al2O3 Catalysts in Methyl Palmitate HDO
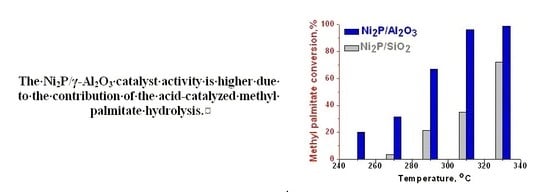
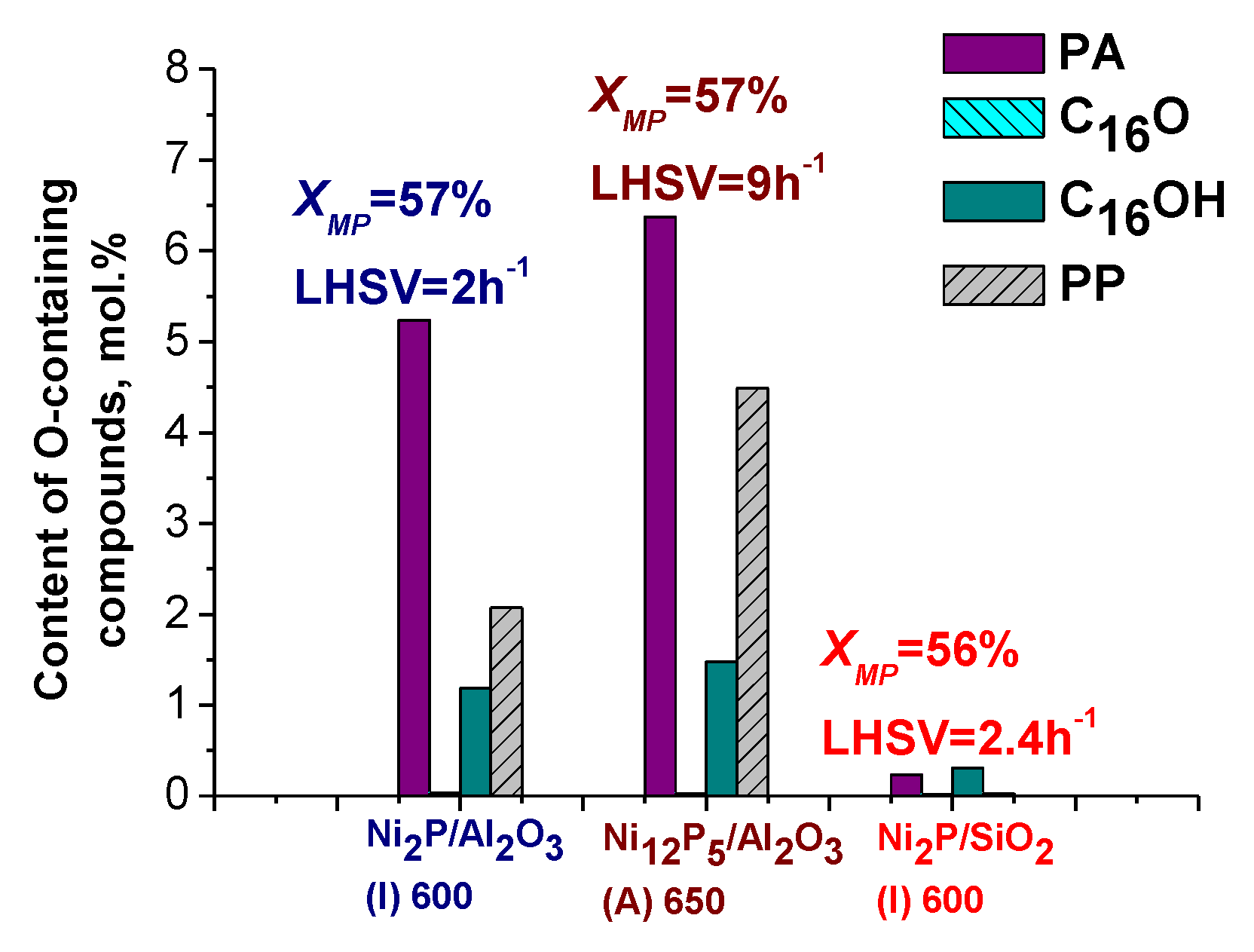
2.2.2. Comparison of Ni2P/Al2O3 and Ni2P/SiO2 Catalysts in the HDO of Methyl Palmitate
2.2.3. Reaction Scheme
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Activity Measurements
3.5. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part II. Renew. Sustain. Energy Rev. 2015, 43, 1446–1466. [Google Scholar] [CrossRef] [Green Version]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.Y.; Jin, Y.B.; Li, J.Y.; Zhang, H.D.; Zhang, A.P.; Xie, J. Heterogeneous sulfur-free hydrodeoxygenation catalysts for selectively upgrading the renewable bio-oils to second generation biofuels. Renew. Sustain. Energy Rev. 2018, 82, 3762–3797. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Mohammad, M.; Hari, T.K.; Yaakob, Z.; Sharma, Y.C.; Sopian, K. Overview on the production of paraffin based-biofuels via catalytic hydrodeoxygenation. Renew. Sustain. Energy Rev. 2013, 22, 121–132. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Lapuerta, M.; Villajos, M.; Agudelo, J.R.; Boehman, A.L. Key properties and blending strategies of hydrotreated vegetable oil as biofuel for diesel engines. Fuel Process. Technol. 2011, 92, 2406–2411. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Garg, M.O. Comprehensive review of combustion, performance and emissions characteristics of a compression ignition engine fueled with hydroprocessed renewable diesel. Renew. Sustain. Energy Rev. 2018, 81, 2947–2954. [Google Scholar] [CrossRef]
- Ryymin, E.M.; Honkela, M.L.; Viljava, T.R.; Krause, A.O.I. Insight to sulfur species in the hydrodeoxygenation of aliphatic esters over sulfided NiMo/γ-Al2O3 catalyst. Appl. Catal. A Gen. 2009, 358, 42–48. [Google Scholar] [CrossRef]
- Kubicka, D.; Horacek, J. Deactivation of HDS catalysts in deoxygenation of vegetable oils. Appl. Catal. A Gen. 2011, 394, 9–17. [Google Scholar] [CrossRef]
- Deliy, I.V.; Vlasova, E.N.; Nuzhdin, A.L.; Gerasimov, E.Y.; Bukhtiyarova, G.A. Hydrodeoxygenation of methyl palmitate over sulfided Mo/Al2O3, CoMo/Al2O3 and NiMo/Al2O3 catalysts. RSC Adv. 2014, 4, 2242–2250. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Influence of Water in the Deactivation of a Sulfided NiMo γ-Al2O3 Catalyst during Hydrodeoxygenation. J. Catal. 1994, 146, 281–291. [Google Scholar] [CrossRef]
- Pelardy, F.; Daudin, A.; Devers, E.; Dupont, C.; Raybaud, P.; Brunet, S. Deep HDS of FCC gasoline over alumina supported CoMoS catalyst: Inhibiting effects of carbon monoxide and water. Appl. Catal. B Environ. 2016, 183, 317–327. [Google Scholar] [CrossRef]
- Deliy, I.V.; Vlasova, E.N.; Aleksandrov, P.V.; Aleshina, G.I.; Bukhtiyarov, V.I.; Gerasimov, E.Y.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Inhibition of SRGO Hydrodesulfurization over CoMo/Al2O3 Catalyst: Comparison of Rapeseed Oil and Carbon Monoxide Effects. Int. J. Environ. Sci. 2017, 2, 386–391. [Google Scholar]
- Alvarez-Galvan, M.C.; Blanco-Brieva, G.; Capel-Sanchez, M.; Morales-DelaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Metal phosphide catalysts for the hydrotreatment of non-edible vegetable oils. Catal. Today 2018, 302, 242–249. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Chen, J.X.; Shi, H.; Li, L.; Li, K.L. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ochoa-Hernandez, C.; O′Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Ni2P/SBA-15 As a Hydrodeoxygenation Catalyst with Enhanced Selectivity for the Conversion of Methyl Oleate Into n-Octadecane. ACS Catal. 2012, 2, 592–598. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.X.; Shi, H. Deoxygenation of Methyl Laurate as a Model Compound to Hydrocarbons on Ni2P/SiO2, Ni2P/MCM-41, and Ni2P/SBA-15 Catalysts with Different Dispersions. Energy Fuels 2013, 27, 3400–3409. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ochoa-Hernandez, C.; Pizarro, P.; O′Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Influence of the Ni/P ratio and metal loading on the performance of NixPy/SBA-15 catalysts for the hydrodeoxygenation of methyl oleate. Fuel 2015, 144, 60–70. [Google Scholar] [CrossRef]
- Xue, Y.A.; Guan, Q.X.; Li, W. Synthesis of bulk and supported nickel phosphide using microwave radiation for hydrodeoxygenation of methyl palmitate. RSC Adv. 2015, 5, 53623–53628. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.X.; Yang, Y.; Tian, S.S. Catalytic deoxygenation of methyl laurate as a model compound to hydrocarbons on nickel phosphide catalysts: Remarkable support effect. Fuel Process. Technol. 2014, 118, 161–170. [Google Scholar] [CrossRef]
- Guan, Q.X.; Han, F.; Li, W. Catalytic performance and deoxygenation path of methyl palmitate on Ni2P/SiO2 synthesized using the thermal decomposition of nickel hypophosphite. RSC Adv. 2016, 6, 31308–31315. [Google Scholar] [CrossRef]
- Guan, Q.X.; Wan, F.F.; Han, F.; Liu, Z.H.; Li, W. Hydrodeoxygenation of methyl palmitate over MCM-41 supported nickel phosphide catalysts. Catal. Today 2016, 259, 467–473. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Pakharukova, V.P.; Gerasimov, E.Y.; Rogov, V.A.; Bukhtiyarova, G.A. Effect of the preparation conditions on the physicochemical and catalytic properties of Ni2P/SiO2 catalysts. Russ. Chem. Bull. 2015, 64, 2361–2370. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Ayupov, A.B.; Andreev, A.S.; Lapina, O.B.; Bukhtiyarova, G.A. Effect of precursor on the catalytic properties of Ni2P/SiO2 in methyl palmitate hydrodeoxygenation. RSC Adv. 2016, 6, 30372–30383. [Google Scholar] [CrossRef]
- Deliy, I.V.; Shamanaev, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Yakovlev, I.V.; Lapina, O.B.; Aleksandrov, P.V.; Bukhtiyarova, G.A. HDO of Methyl Palmitate over Silica-Supported Ni Phosphides: Insight into Ni/P Effect. Catalysts 2017, 7, 298. [Google Scholar] [CrossRef]
- Chen, J.X.; Han, M.M.; Zhao, S.; Pan, Z.Y.; Zhang, Z.N. An in situ approach to preparing Ni2P/SiO2 catalyst under mild conditions and its performance for the deoxygenation of methyl laurate to hydrocarbons. Catal. Sci. Technol. 2016, 6, 3938–3949. [Google Scholar] [CrossRef]
- Chen, J.X.; Yang, Y.; Shi, H.; Li, M.F.; Chu, Y.; Pan, Z.Y.; Yu, X.B. Regulating product distribution in deoxygenation of methyl laurate on silica-supported Ni-Mo phosphides: Effect of Ni/Mo ratio. Fuel 2014, 129, 1–10. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Wang, R.J.; Li, M.F.; Chu, Y.; Chen, J.X. Deoxygenation of methyl laurate to hydrocarbons on silica-supported Ni-Mo phosphides: Effect of calcination temperatures of precursor. J. Energy Chem. 2015, 24, 77–86. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Aleksandrov, P.V.; Bukhtiyarova, G.A. Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts 2017, 7, 329. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Study of the Hydrodeoxygenation of Carbonyl, Carboxylic and Guaiacyl Groups over Sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 Catalyst.2. Influence of Water, Ammonia and Hydrogen-Sulfide. Appl. Catal. A Gen. 1994, 109, 97–115. [Google Scholar] [CrossRef]
- Senol, O.I.; Viljava, T.R.; Krause, A.O.I. Hydrodeoxygenation of methyl esters on sulphided NiMo/γ-Al2O3 and CoMo/γ-Al2O3 catalysts. Catal. Today 2005, 100, 331–335. [Google Scholar] [CrossRef]
- Mehrabadi, B.A.T.; Eskandari, S.; Khan, U.; White, R.D.; Regalbuto, J.R. A Review of Preparation Methods for Supported Metal Catalysts. Adv. In Catal. 2017, 61, 1–35. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A real support effect on the hydrodeoxygenation of methyl oleate by sulfided NiMo catalysts. Catal. Today 2017, 298, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Bukhtiyarova, G.A.; Mart'yanov, O.N.; Yakushkin, S.S.; Shuvaeva, M.A.; Bayukov, O.A. State of iron in nanoparticles prepared by impregnation of silica gel and aluminum oxide with FeSO4 solutions. Phys. Solid State 2010, 52, 826–837. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Tang, M.X.; Chen, J.X. Effects of P/Ni ratio and Ni content on performance of γ-Al2O3-supported nickel phosphides for deoxygenation of methyl laurate to hydrocarbons. Appl. Surf. Sci. 2016, 360, 353–364. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of gamma-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: Optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Motokura, K. Synergistic Catalysis by Multifunctionalized Solid Surfaces for Nucleophilic Addition Reactions. J. Jpn. Petrol. Inst. 2014, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Peroni, M.; Mancino, G.; Barath, E.; Gutierrez, O.Y.; Lercher, J.A. Bulk and γ-Al2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid. Appl. Catal. B Environ. 2016, 180, 301–311. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Jimenez-Morales, I.; Infantes-Molina, A.; Rodriguez-Castellon, E.; Jimenez-Lopez, A. Influence of the silica support on the activity of Ni and Ni2P based catalysts in the hydrodechlorination of chlorobenzene. Study of factors governing catalyst deactivation. J. Mol. Catal. A Chem. 2013, 368, 78–87. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhou, S.J.; Ci, D.H.; Zhang, J.X.; Wang, R.J.; Zhang, J.Y. Influence of Supports on Structure and Performance of Nickel Phosphide Catalysts for Hydrodechlorination of Chlorobenzene. Ind. Eng. Chem. Res. 2009, 48, 3812–3819. [Google Scholar] [CrossRef]
- Sawhill, S.J.; Layman, K.A.; Van Wyk, D.R.; Engelhard, M.H.; Wang, C.; Bussell, M.E. Thiophene hydrodesulfurization over nickel phosphide catalysts: Effect of the precursor composition and support. J. Catal. 2005, 231, 300–313. [Google Scholar] [CrossRef]
- Wu, S.K.; Lai, P.C.; Lin, Y.C.; Wan, H.P.; Lee, H.T.; Chang, Y.H. Atmospheric Hydrodeoxygenation of Guaiacol over Alumina-, Zirconia-, and Silica-Supported Nickel Phosphide Catalysts. ACS Sustain. Chem. Eng. 2013, 1, 349–358. [Google Scholar] [CrossRef]
- Wu, S.K.; Lai, P.C.; Lin, Y.C. Atmospheric Hydrodeoxygenation of Guaiacol over Nickel Phosphide Catalysts: Effect of Phosphorus Composition. Catal. Lett. 2014, 144, 878–889. [Google Scholar] [CrossRef]
- Stinner, C.; Tang, Z.; Haouas, M.; Weber, T.; Prins, R. Preparation and 31P NMR characterization of nickel phosphides on silica. J. Catal. 2002, 208, 456–466. [Google Scholar] [CrossRef]
- Oyama, S.; Wang, X.; Lee, Y.; Bando, K.; Requejo, F. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Li, K.L.; Wang, R.J.; Chen, J.X. Hydrodeoxygenation of Anisole over Silica-Supported Ni2P, MoP, and NiMoP Catalysts. Energy Fuels 2011, 25, 854–863. [Google Scholar] [CrossRef]
- Oyama, S.; Lee, Y. The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-DMDBT. J. Catal. 2008, 258, 393–400. [Google Scholar] [CrossRef]
- Clark, P.A.; Oyama, S.T. Alumina-supported molybdenum phosphide hydroprocessing catalysts. J. Catal. 2003, 218, 78–87. [Google Scholar] [CrossRef]
- Li, L.L.; Han, C.X.; Han, X.Y.; Zhou, Y.X.; Yang, L.; Zhang, B.G.; Hu, J.L. Catalytic Decomposition of Toxic Chemicals over Metal-Promoted Carbon Nanotubes. Environ. Sci. Technol. 2011, 45, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Song, L.M.; Zhang, S.J.; Wei, Q.W. A new route for synthesizing nickel phosphide catalysts with high hydrodesulfurization activity based on sodium dihydrogenphosphite. Catal. Commun. 2011, 12, 1157–1160. [Google Scholar] [CrossRef]
- Yang, S.F.; Prins, R. New synthesis method for nickel phosphide hydrotreating catalysts. Chem. Commun. 2005, 4178–4180. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Castellanos, A.; Zepeda, T.A.; Pawelec, B.; Fierro, J.L.G.; de los Reyes, J.A. Preparation, characterization, and performance of alumina-supported nanostructured Mo-phosphide systems. Chem. Mater. 2007, 19, 5627–5636. [Google Scholar] [CrossRef]
- Quartararo, J.; Amoureux, J.P.; Grimblot, J. Sol-gel synthesis, characterization by solid state NMR and HDS activity of Mo-alumina and Mo-P-alumina based catalysts. J. Mol. Catal. A Chem. 2000, 162, 353–365. [Google Scholar] [CrossRef]
- Fitz, C.W.; Rase, H.F. Effects of Phosphorus on Nickel Molybdenum Hydrodesulfurization Hydrodenitrogenation Catalysts of Varying Metals Content. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 40–44. [Google Scholar] [CrossRef]
- Berteau, P.; Delmon, B. Modified Aluminas - Relationship between Activity in 1-Butanol Dehydration and Acidity Measured by NH3 TPD. Catal. Today 1989, 5, 121–137. [Google Scholar] [CrossRef]
- Lee, Y.K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Chen, J.X.; Guo, T.; Li, K.L.; Sun, L.M. A facile approach to enhancing activity of Ni2P/SiO2 catalyst for hydrodechlorination of chlorobenzene: Promoting effect of water and oxygen. Catal. Sci. Technol. 2015, 5, 2670–2680. [Google Scholar] [CrossRef]
- Krawietz, T.R.; Lin, P.; Lotterhos, K.E.; Torres, P.D.; Barich, D.H.; Clearfield, A.; Haw, J.F. Solid phosphoric acid catalyst: A multinuclear NMR and theoretical study. J. Am. Chem. Soc. 1998, 120, 8502–8511. [Google Scholar] [CrossRef]
- Eichele, K.; Wasylishen, R.E. P-31-NMR Study of Powder and Single-Crystal Samples of Ammonium Dihydrogen Phosphate - Effect of Homonuclear Dipolar Coupling. J. Phys. Chem.-US 1994, 98, 3108–3113. [Google Scholar] [CrossRef]
- Koranyi, T.I.; Vit, Z.; Nagy, J.B. Support and pretreatment effects on the hydrotreating activity of SBA-15 and CMK-5 supported nickel phosphide catalysts. Catal. Today 2008, 130, 80–85. [Google Scholar] [CrossRef]
- Bekaert, E.; Bernardi, J.; Boyanov, S.; Monconduit, L.; Doublet, M.L.; Menetrier, M. Direct Correlation between the 31P MAS NMR Response and the Electronic Structure of Some Transition Metal Phosphides. J. Phys. Chem. C 2008, 112, 20481–20490. [Google Scholar] [CrossRef]
- Quartararo, J.; Guelton, M.; Rigole, M.; Amoureux, J.P.; Fernandez, C.; Grimblot, J. Sol-gel synthesis of alumina modified by phosphorus: A solid state NMR characterization study. J. Mater. Chem. 1999, 9, 2637–2646. [Google Scholar] [CrossRef]
- d’Espinose de la Caillerie, J.B.; Fretigny, C.; Massiot, D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model. J. Magn. Reson. 2008, 192, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T.; Gott, T.; Zhao, H.Y.; Lee, Y.K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Vines, F.; Liu, P.; Illas, F. Role of C and P Sites on the Chemical Activity of Metal Carbides and Phosphides: From Clusters to Single-Crystal Surfaces. Model Syst. Catal. 2010, 117–132. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wei, L.; Cheng, S.Y.; Julson, J. Review of Heterogeneous Catalysts for Catalytically Upgrading Vegetable Oils into Hydrocarbon Biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data; JCPDS: Swarthmore, PA, USA, 1997.
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calve, S.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]






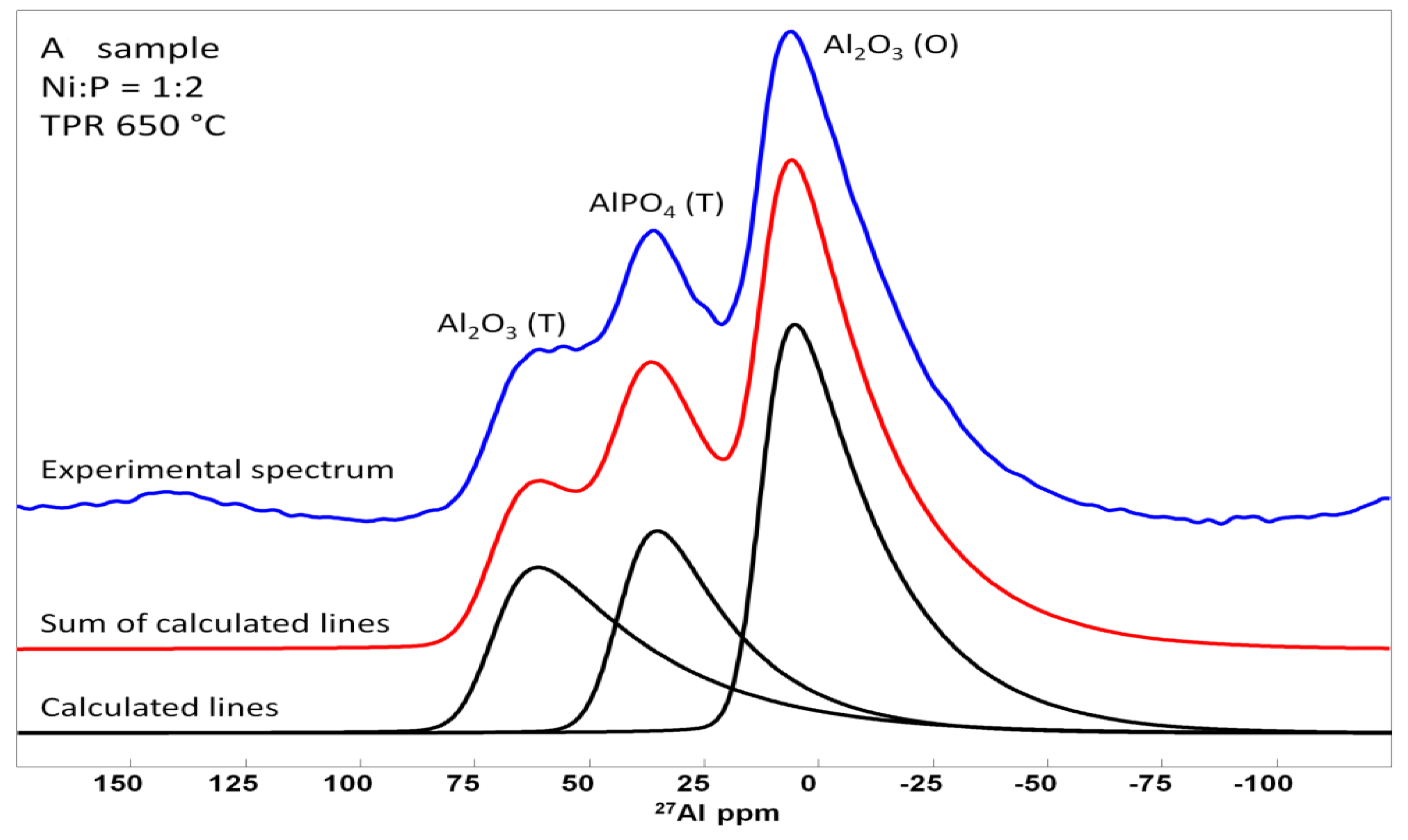







| Sample | Treduction, °C | Elemental Content after TPR, wt% | Molar Ni/P | ABET, m2/g | Dpore, nm | XRD Phase | DXRD, nm | |
|---|---|---|---|---|---|---|---|---|
| Ni | P | |||||||
| NiP_A/Al2O3(550) | 550 | 7.3 | 7.6 | 0.51 | 127 | 12.7 | Ni Ni3P | 5.5 - |
| NiP_A/Al2O3(600) | 600 | 7.3 | 7.6 | 0.51 | 130 | 11.9 | Ni Ni3P Ni12P5 | 5.0 4.5 12.0 |
| NiP_A/Al2O3(650) | 650 | 7.5 | 7.2 | 0.55 | 158 | 10.6 | Ni3P Ni12P5 | 7.6 11.5 |
| NiP_I/Al2O3(550) | 550 | 7.4 | 11.6 | 0.34 | 115 | 8.6 | Ni2P | 3.8 |
| NiP_I/Al2O3(600) | 600 | 7.4 | 11.3 | 0.35 | 120 | 8.3 | Ni2P | 3.8 |
| NiP_I/Al2O3(650) | 650 | 7.6 | 11.5 | 0.35 | 101 | 9.2 | Ni2P | 4.4 |
| NiP_I/SiO2(600) | 600 | 7.5 | 7.4 | 0.53 | 180 | 12.7 | Ni2P | 5.5 |
| Sample | Treduction, oC | NH3-TPD | MAS NMR | |||
|---|---|---|---|---|---|---|
| Tmax, oC | Quantity, μmol/g | Overall Quantity, μmol/g | NixPy, at.% 31P NMR | AlPO4, at.% 27Al NMR | ||
| γ-Al2O3 | - | 237 | 106 | 421 | - | - |
| 335 | 315 | |||||
| NiP_A/Al2O3(550) | 550 | 249 | 298 | 624 | ||
| 311 | 326 | |||||
| NiP_A/Al2O3(600) | 600 | 245 | 287 | 510 | ||
| 334 | 222 | |||||
| NiP_A/Al2O3(650) | 650 | 245 | 246 | 440 | 35 ± 10 | 25 ± 2 |
| 306 | 194 | |||||
| NiP_I/Al2O3(550) | 550 | 244 | 322 | 477 | 39 ± 10 | 22 ± 2 |
| 308 | 155 | |||||
| NiP_I/Al2O3(600) | 600 | 244 | 223 | 354 | 39 ± 10 | 25 ± 2 |
| 285 | 130 | |||||
| NiP_I/Al2O3(650) | 650 | 238 | 193 | 326 | 60 ± 10 | 30 ± 2 |
| 278 | 133 | |||||
| SiO2 | - | 231 | 84 | 84 | - | - |
| NiP_I/SiO2(600) | 600 | 241 | 116 | 163 | 92 | - |
| 299 | 47 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deliy, I.V.; Shamanaev, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Yakovlev, I.V.; Lapina, O.B.; Bukhtiyarova, G.A. Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate. Catalysts 2018, 8, 515. https://doi.org/10.3390/catal8110515
Deliy IV, Shamanaev IV, Aleksandrov PV, Gerasimov EY, Pakharukova VP, Kodenev EG, Yakovlev IV, Lapina OB, Bukhtiyarova GA. Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate. Catalysts. 2018; 8(11):515. https://doi.org/10.3390/catal8110515
Chicago/Turabian StyleDeliy, Irina V., Ivan V. Shamanaev, Pavel V. Aleksandrov, Evgeny Yu. Gerasimov, Vera P. Pakharukova, Evgeny G. Kodenev, Ilya V. Yakovlev, Olga B. Lapina, and Galina A. Bukhtiyarova. 2018. "Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate" Catalysts 8, no. 11: 515. https://doi.org/10.3390/catal8110515