Microcystis aeruginosa Synergistically Facilitate the Photocatalytic Degradation of Tetracycline Hydrochloride and Cr(VI) on PAN/TiO2/Ag Nanofiber Mats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of M. aeruginosa-Decorated PAN/TiO2/Ag Nanofiber Mats
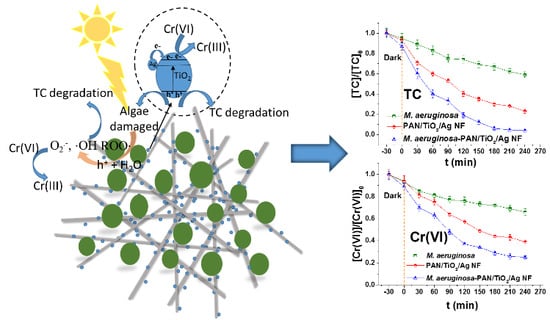
2.2. Photocatalytic Decontamination of TC and Cr(VI)
2.3. Photodegradation of Algae
2.4. Analysis of the Active Species and Discussion of the Mechanism
2.5. Repeated Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of M. aeruginosa-Decorated PAN/TiO2/Ag Nanofiber Mats
3.3. Characterizations of M. aeruginosa-Decorated PAN/TiO2/Ag Nanofiber Mats
3.4. Photocatalytic Activity Measurement
3.5. Photodegradation of Algae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, J.; Sohail, A.; Wang, L.; Haider, M.R.; Mulk, S.; Pan, G. Electro-microbiology as a promising approach towards renewable energy and environmental sustainability. Energies 2018, 11, 1822. [Google Scholar] [CrossRef]
- Deng, L.; Wang, H.; Deng, N. Photoreduction of chromium(VI) in the presence of algae, Chlorella vulgaris. J. Hazard. Mater. 2006, 138, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wu, F.; Deng, N.; Zuo, Y. Photoreduction of mercury(II) in the presence of algae, Anabaena cylindrical. J. Photochem. Photobiol. B 2008, 91, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wu, F.; Deng, N. Photoproduction of hydroxyl radicals in aqueous solution with algae under high-pressure mercury lamp. Environ. Sci. Technol. 2004, 38, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Zepp, R.G.; Schlotzhauer, P.F. Influence of algae on photolysis rates of chemicals in water. Environ. Sci. Technol. 1983, 17, 462–468. [Google Scholar] [CrossRef]
- Peng, Z.e.; Wu, F.; Deng, N. Photodegradation of bisphenol A in simulated lake water containing algae, humic acid and ferric ions. Environ. Pollut. 2006, 144, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Ma, L. Photodegradation mechanism of sulfadiazine catalyzed by Fe(III), oxalate and algae under UV irradiation. Environ. Technol. 2013, 34, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, G. Simultaneous removal of harmful algal blooms and microcystins using microorganism- and chitosan-modified local soil. Environ. Sci. Technol. 2015, 49, 6249–6256. [Google Scholar] [CrossRef]
- Pan, G.; Dai, L.; Li, L.; He, L.; Li, H.; Bi, L.; Gulati, R.D. Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in eutrophic lakes. Environ. Sci. Technol. 2012, 46, 5077–5084. [Google Scholar] [CrossRef]
- Eroglu, E.; Agarwal, V.; Bradshaw, M.; Chen, X.; Smith, S.M.; Raston, C.L.; Iyer, K.S. Nitrate removal from liquid effluents using microalgae immobilized on chitosan nanofiber mats. Green Chem. 2012, 14, 2682–2685. [Google Scholar] [CrossRef]
- Nalbandian, M.J.; Zhang, M.L.; Sanchez, J.; Kim, S.; Choa, Y.H.; Cwiertny, D.M.; Myung, N.V. Synthesis and optimization of Ag-TiO2 composite nanofibers for photocatalytic treatment of impaired water sources. J. Hazard. Mater. 2015, 299, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Amarjargal, A.; Tijing, L.D.; Ruelo, M.T.G.; Lee, D.H.; Kim, C.S. Facile synthesis and immobilization of Ag-TiO2 nanoparticles on electrospun PU nanofibers by polyol technique and simple immersion. Mater. Chem. Phys. 2012, 135, 277–281. [Google Scholar] [CrossRef]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.I.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Veres, A.; Rica, T.; Janovak, L.; Domok, M.; Buzas, N.; Zollmer, V.; Seemann, T.; Richardt, A.; Dekany, I. Silver and gold modified plasmonic TiO2 hybrid films for photocatalytic decomposition of ethanol under visible light. Catal. Today 2012, 181, 156–162. [Google Scholar] [CrossRef]
- Rana, S.; Nazar, U.; Ali, J.; Ali, Q.u.A.; Ahmad, N.M.; Sarwar, F.; Waseem, H.; Jamil, S.U.U. Improved antifouling potential of polyether sulfone polymeric membrane containing silver nanoparticles: Self-cleaning membranes. Environ. Technol. 2018, 39, 1413–1421. [Google Scholar] [CrossRef]
- Chang, J.; Wang, J.; Qu, J.; Li, Y.V.; Ma, L.; Wang, L.; Wang, X.; Pan, K. Preparation of alpha-Fe2O3/polyacrylonitrile nanofiber mat as an effective lead adsorbent. Environ. Sci. Nano 2016, 3, 894–901. [Google Scholar] [CrossRef]
- Morillo, D.; Faccini, M.; Amantia, D.; Perez, G.; Garcia, M.A.; Valiente, M.; Aubouy, L. Superparamagnetic iron oxide nanoparticle-loaded polyacrylonitrile nanofibers with enhanced arsenate removal performance. Environ. Sci. Nano 2016, 3, 1165–1173. [Google Scholar] [CrossRef] [Green Version]
- Abdolmaleki, A.; Mallakpour, S.; Borandeh, S. In situ synthesis of silver nanoparticles in novel L-phenylalanine based poly(amide-benzimidazole-imide) matrix through metal complexation method using N,N′-dimethylformamide as a reaction medium and reducing agent. Polym. Plast. Technol. Eng. 2015, 54, 1002–1008. [Google Scholar] [CrossRef]
- Wang, T.Y.; Quan, W.; Jiang, D.L.; Chen, L.L.; Li, D.; Meng, S.; Chen, M. Synthesis of redox-mediator-free direct Z-scheme AgI/WO3 nanocomposite photocatalysts for the degradation of tetracycline with enhanced photocatalytic activity. Chem. Eng. J. 2016, 300, 280–290. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhang, D.Y.; Mou, S.; Song, W.; Al-Misned, F.A.; Mortuza, M.G.; Pan, X.L. Simultaneous removal of tetracycline hydrochloride and As(III) using poorly-crystalline manganese dioxide. Chemosphere 2015, 136, 102–110. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.R. Preparation and characterization of nitrogen-doped TiO2/diatomite integrated photocatalytic pellet for the adsorption-degradation of tetracycline hydrochloride using visible light. Chem. Eng. J. 2016, 302, 682–696. [Google Scholar] [CrossRef]
- Xu, S.C.; Pan, S.S.; Xu, Y.; Luo, Y.Y.; Zhang, Y.X.; Li, G.H. Efficient removal of Cr(VI) from wastewater under sunlight by Fe(II)-doped TiO2 spherical shell. J. Hazard. Mater. 2015, 283, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhang, D.; Diao, Z.; Huang, X.; He, C.; Morel, J.L.; Xiong, Y. Visible light induced photocatalytic reduction of Cr(VI) over polymer-sensitized TiO2 and its synergism with phenol oxidation. Water Res. 2012, 46, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Ali, N.; Jamil, S.U.U.; Waseem, H.; Khan, K.; Pan, G. Insight into eco-friendly fabrication of silver nanoparticles by Pseudomonas aeruginosa and its potential impacts. J. Environ. Chem. Eng. 2017, 5, 3266–3272. [Google Scholar] [CrossRef]
- Yu, J.G.; Xiong, J.F.; Cheng, B.; Liu, S.W. Fabrication and characterization of Ag-TiO2 multiphase nanocomposite thin films with enhanced photocatalytic activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Ali, J.; Hameed, A.; Ahmed, S.; Ali, M.I.; Zainab, S.; Ali, N. Role of catalytic protein and stabilising agents in the transformation of Ag ions to nanoparticles by Pseudomonas aeruginosa. IET Nanobiotechnol. 2016, 10, 295–300. [Google Scholar] [CrossRef]
- Kim, K.D.; Han, D.N.; Lee, J.B.; Kim, H.T. Formation and characterization of Ag-deposited TiO2 nanoparticles by chemical reduction method. Scr. Mater. 2006, 54, 143–146. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A 2007, 189, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Luo, L.; Lai, X.; Chen, B.; Lin, L.; Fang, L.; Tam, N.F.Y.; Luan, T. Chlorophyll catalyse the photo-transformation of carcinogenic benzo a pyrene in water. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Wang, K.; Garg, S.; Waite, T.D. Light-mediated reactive oxygen species generation and iron redox transformations in the presence of exudate from the cyanobacteriurn microcystis aeruginosa. Environ. Sci. Technol. 2017, 51, 8384–8395. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Wu, F.; Deng, N. Photodegradation of aniline in aqueous suspensions of microalgae. J. Photochem. Photobiol. B 2007, 87, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Varcamonti, M.; Silvestri, B.; Costantini, A.; Branda, F.; Luciani, G. Titania as a driving agent for DHICA polymerization: A novel strategy for the design of bioinspired antimicrobial nanomaterials. J. Mater. Chem. B 2015, 3, 2808–2815. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Costantini, A.; Branda, F.; Luciani, G. 5,6-dihydroxyindole-2-carboxylic-acid-TiO2 charge transfer complexes in the radical polymerization of melanogenic precursor(s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]
- Saeki, A.; Yamamoto, N.; Yoshida, Y.; Kozawa, T. Geminate charge recombination in liquid alkane with concentrated CCl4: Effects of CCl4 radical anion and narrowing of initial distribution of Cl. J. Phys. Chem. A 2011, 115, 10166–10173. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.N.; Lang, J.Y.; Wang, S.W.; Chai, Z.L.; Su, Y.G.; Wang, X.J. Nanospherical composite of WO3 wrapped NaTaO3: Improved photodegradation of tetracycline under visible light irradiation. Appl. Surf. Sci. 2016, 388, 412–419. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Hu, X.X.; Qu, J.H. Indirect photodegradation of amine drugs in aqueous solution under simulated sunlight. Environ. Sci. Technol. 2009, 43, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xiong, X.L.; Liang, N.G.; Long, Q.Y. Highly effective and stable Ag3PO4-WO3/MWCNTs photocatalysts for simultaneous Cr(VI) reduction and orange II degradation under visible light irradiation. Appl. Surf. Sci. 2015, 353, 939–948. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl. Catal. B Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, S.; Yang, S.; Wang, H.; Yu, H.; Zhang, S.; Peng, F. Synthesis and characterization of g-C3N4/Cu2O composite catalyst with enhanced photocatalytic activity under visible light irradiation. Mater. Res. Bull. 2014, 56, 19–24. [Google Scholar] [CrossRef]
- Deng, X.; Wu, F.; Liu, Z.; Luo, M.; Li, L.; Ni, Q.; Jiao, Z.; Wu, M.; Liu, Y. The splenic toxicity of water soluble multi-walled carbon nanotubes in mice. Carbon 2009, 47, 1421–1428. [Google Scholar] [CrossRef]
- Rocchetta, I.; Mazzuca, M.; Conforti, V.; Ruiz, L.; Balzaretti, V.; de Molina, M.d.C.R. Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ. Pollut. 2006, 141, 353–358. [Google Scholar] [CrossRef] [PubMed]










| Reaction System | K (× 10−3, min−1) | Correlation Coefficient R2 | |
|---|---|---|---|
| TC | M. aeruginosa | 1.96 ± 0.15 | 0.99 |
| PAN/TiO2/Ag NF | 5.62 ± 0.36 | 0.99 | |
| M. aeruginosa-PAN/TiO2/Ag NF | 13.21 ± 0.51 | 0.98 | |
| Cr(VI) | M. aeruginosa | 1.41 ± 0.12 | 0.94 |
| PAN/TiO2/Ag NF | 3.72 ± 0.24 | 0.99 | |
| M. aeruginosa-PAN/TiO2/Ag NF | 5.58 ± 0.38 | 0.97 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, C.; Cheng, R.; Ali, J.; Wang, Z.; Mailhot, G.; Pan, G. Microcystis aeruginosa Synergistically Facilitate the Photocatalytic Degradation of Tetracycline Hydrochloride and Cr(VI) on PAN/TiO2/Ag Nanofiber Mats. Catalysts 2018, 8, 628. https://doi.org/10.3390/catal8120628
Wang L, Zhang C, Cheng R, Ali J, Wang Z, Mailhot G, Pan G. Microcystis aeruginosa Synergistically Facilitate the Photocatalytic Degradation of Tetracycline Hydrochloride and Cr(VI) on PAN/TiO2/Ag Nanofiber Mats. Catalysts. 2018; 8(12):628. https://doi.org/10.3390/catal8120628
Chicago/Turabian StyleWang, Lei, Changbo Zhang, Rong Cheng, Jafar Ali, Zhenbo Wang, Gilles Mailhot, and Gang Pan. 2018. "Microcystis aeruginosa Synergistically Facilitate the Photocatalytic Degradation of Tetracycline Hydrochloride and Cr(VI) on PAN/TiO2/Ag Nanofiber Mats" Catalysts 8, no. 12: 628. https://doi.org/10.3390/catal8120628