Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion
Abstract
:1. Introduction
2. Results and Discussion
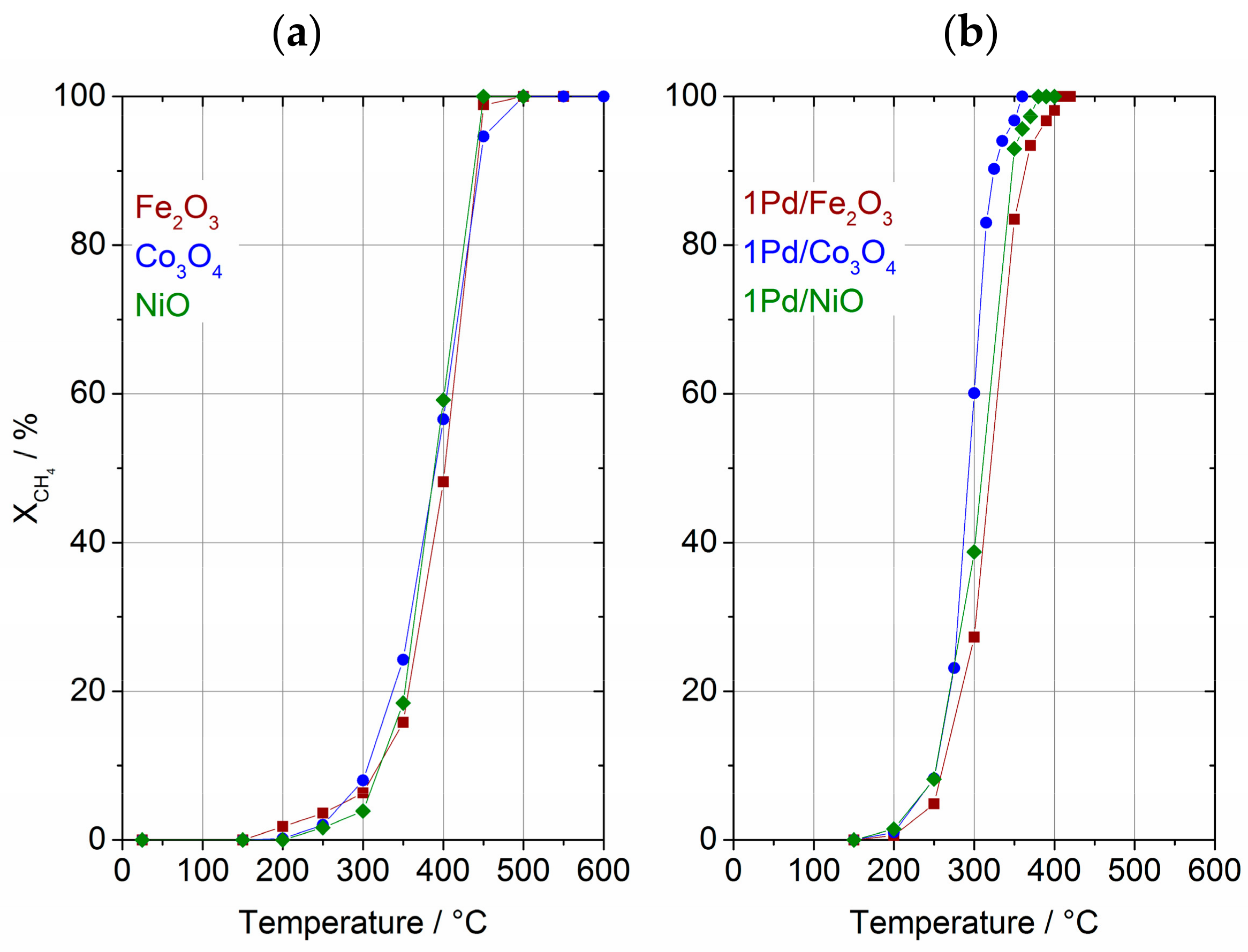
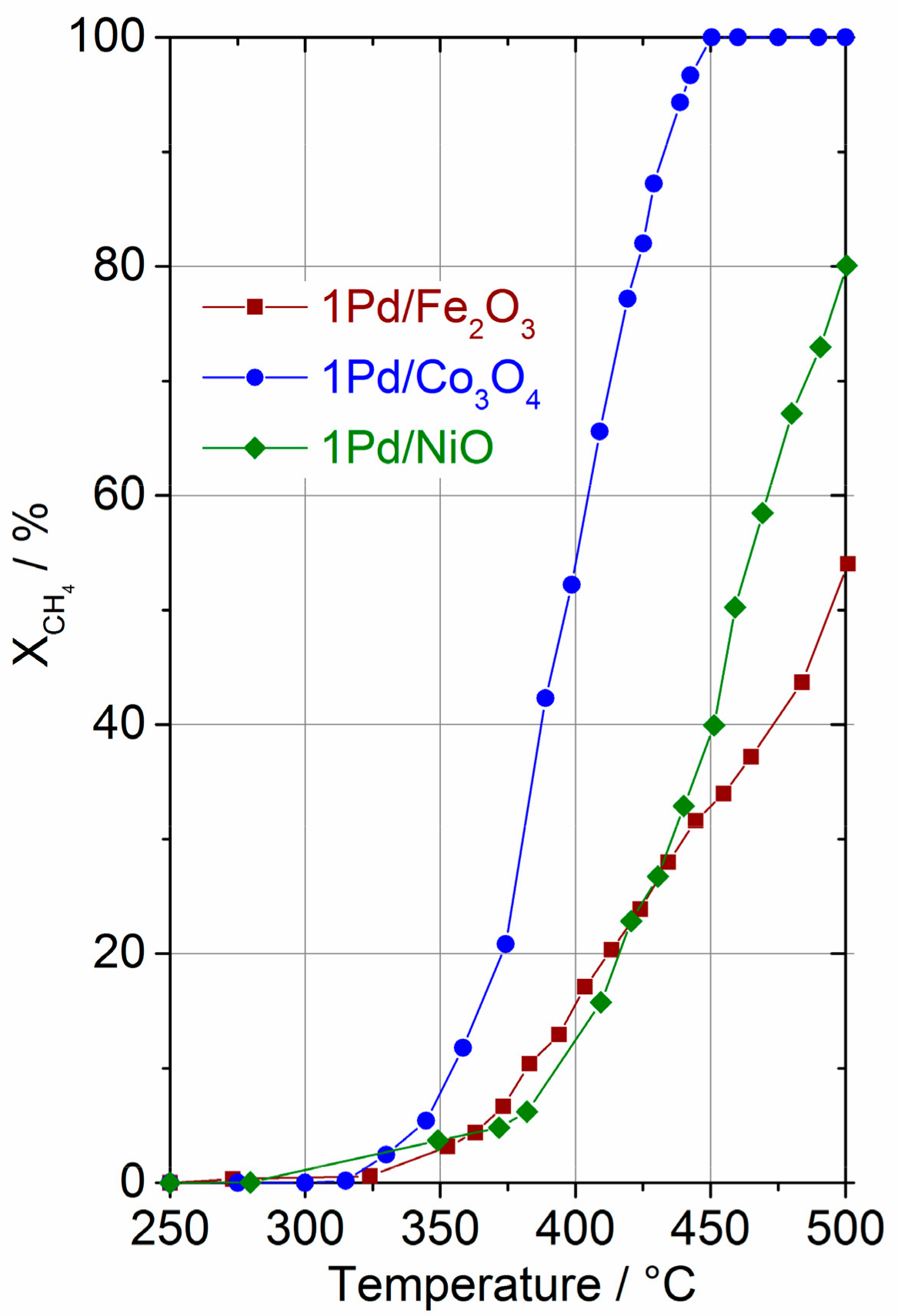
2.1. Catalytic Performance in Methane Combustion
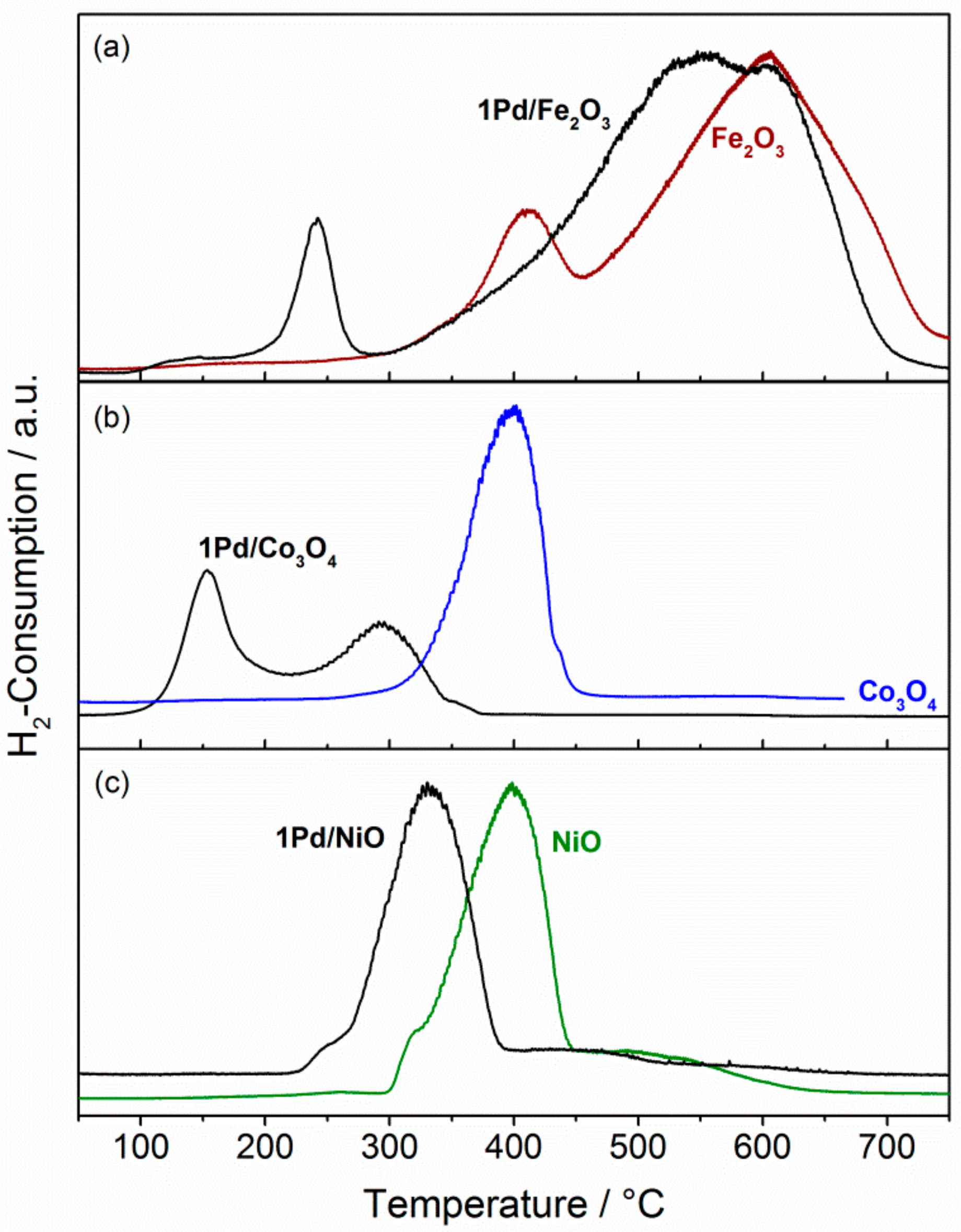
2.2. Structural Properties and Reducibility of the Catalytic Materials
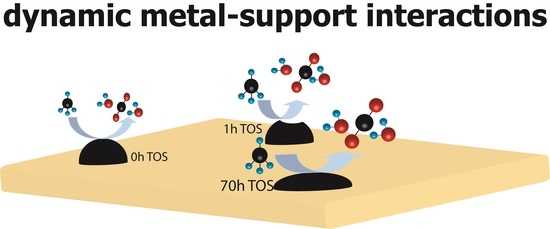
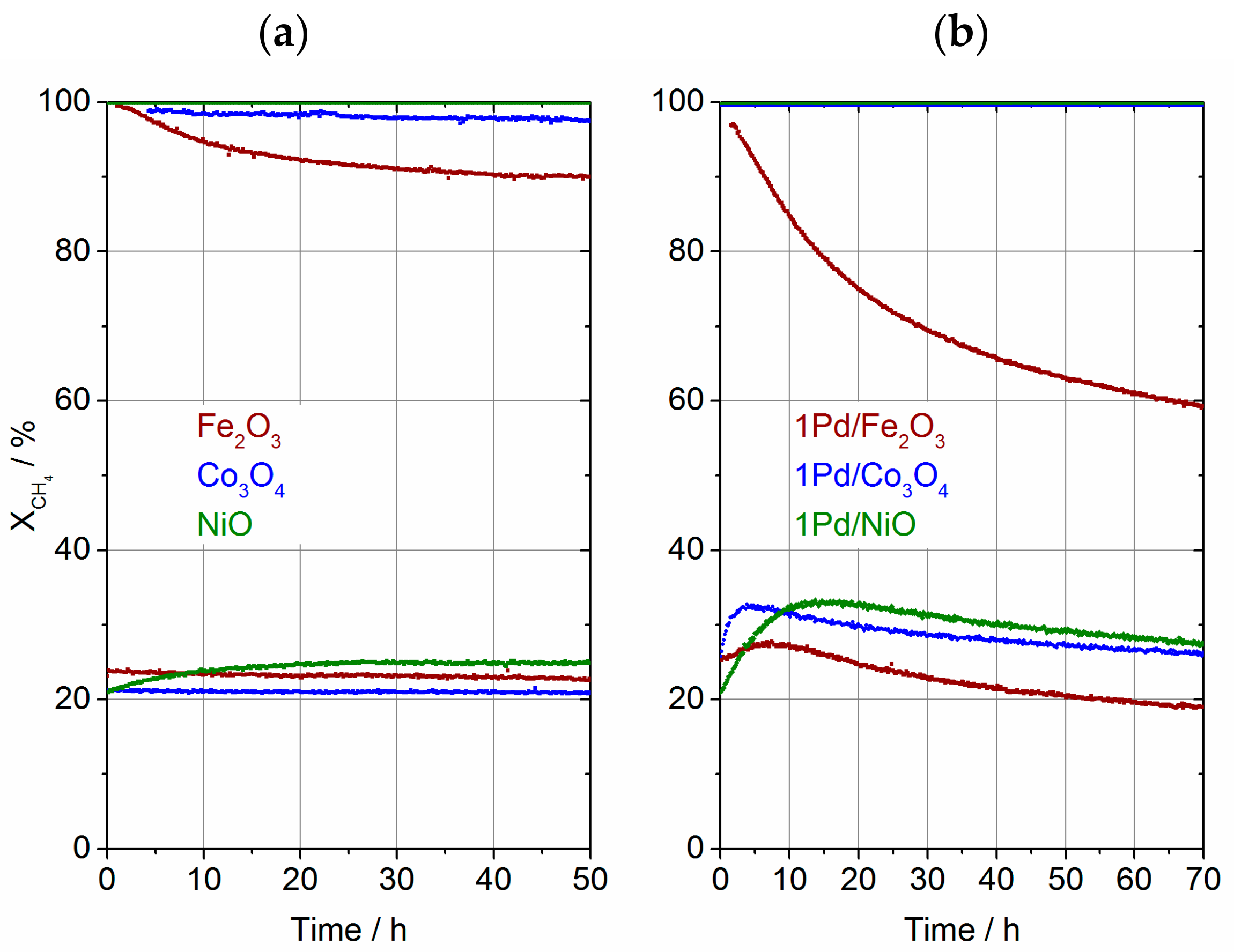
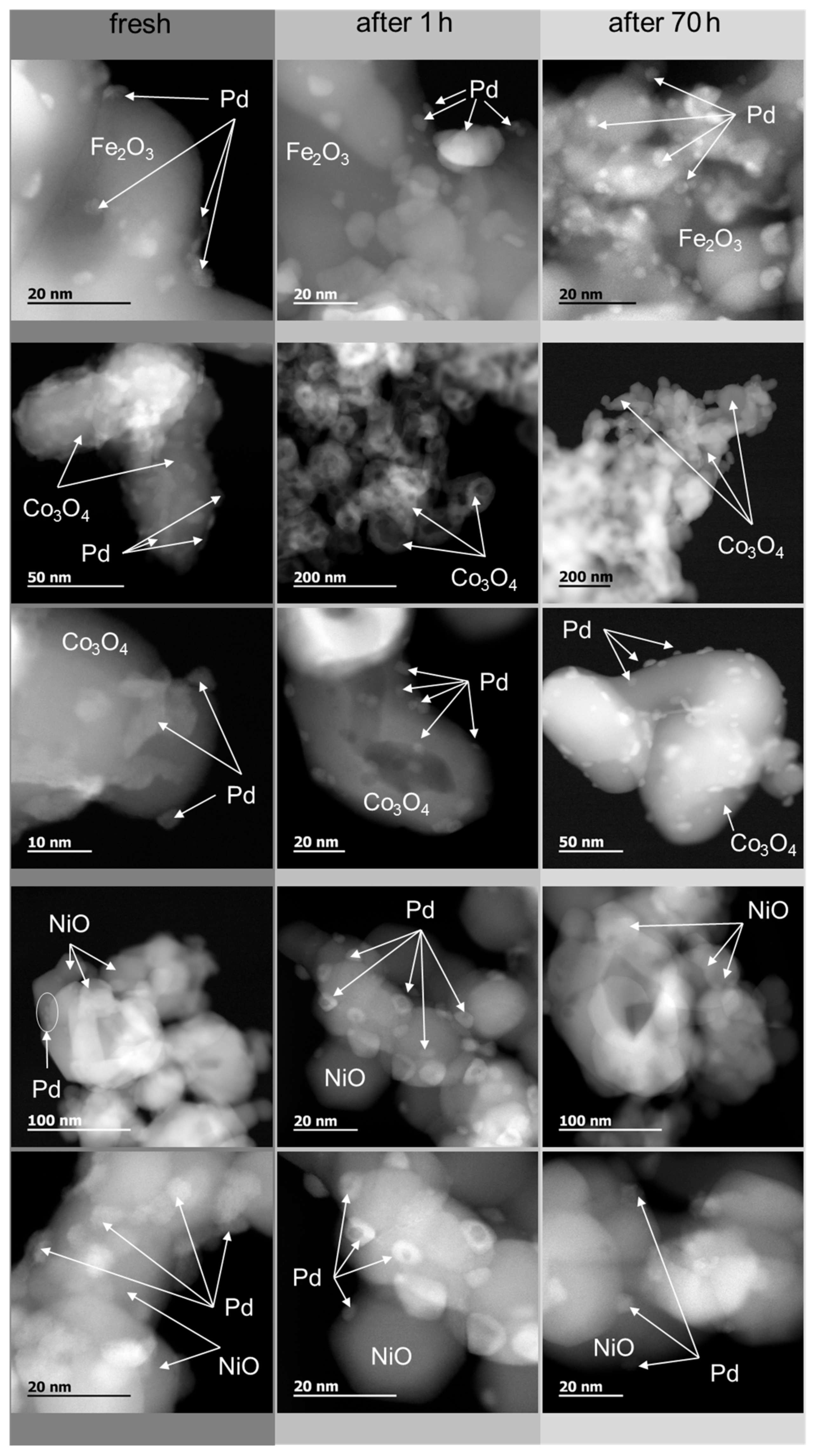
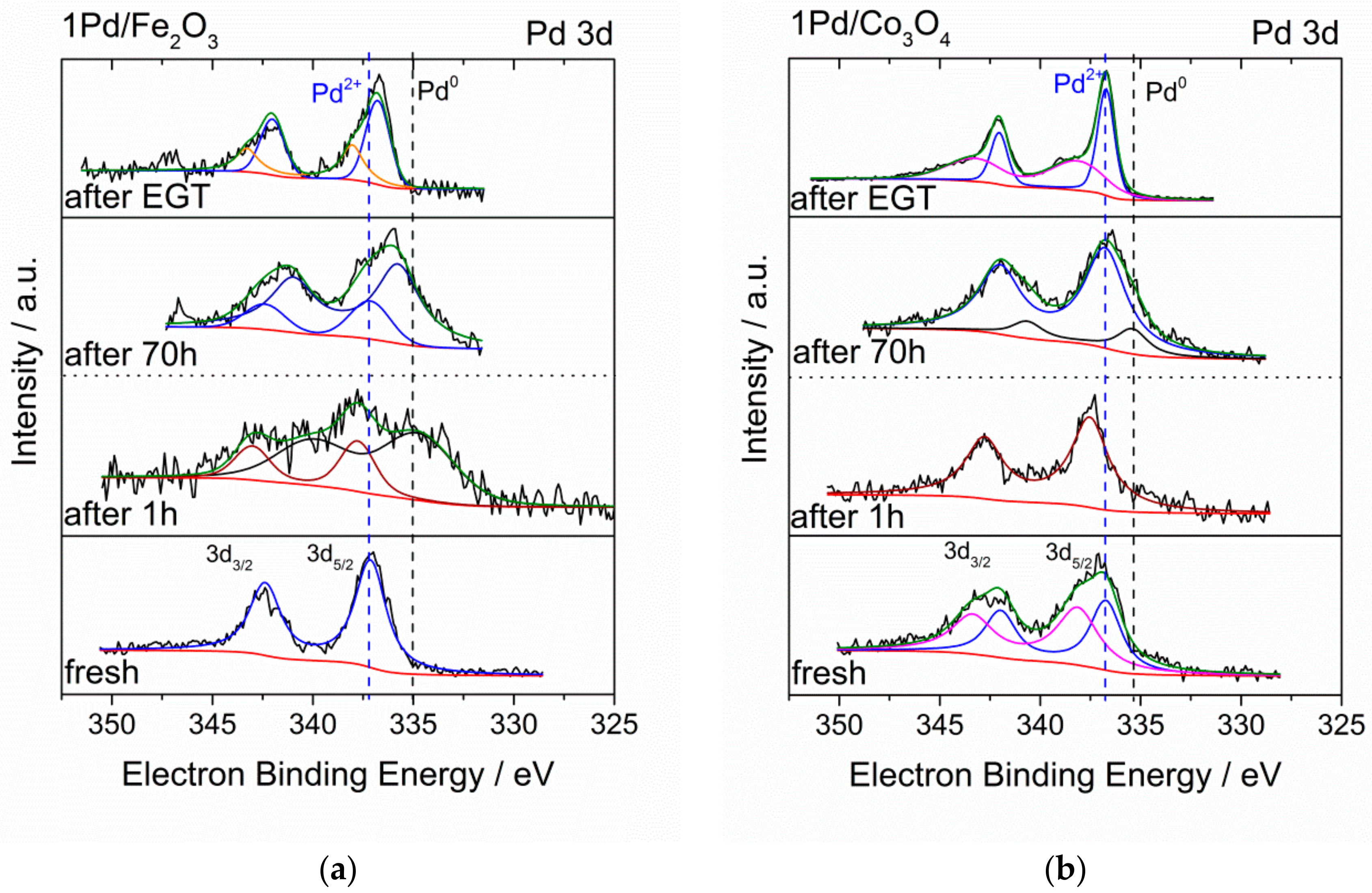
2.3. Catalyst Transformation during Time on Stream
3. Materials and Experimental Details
3.1. Catalyst Preparation
3.2. Catalysts Characterization
3.2.1. Kr Corption
3.2.2. X-ray Powder Diffraction (XRD)
3.2.3. Element Analysis
3.2.4. In Situ X-ray Diffraction (In Situ XRD)
3.2.5. Temperature Programmed Hydrogen Reduction (H2-TPR)
3.2.6. Scanning Electron Microscopy (SEM)
3.2.7. Transmission Electron Microscopy (TEM)
3.2.8. X-ray Photoelectron Spectroscopy (XPS)
3.3. Catalyst Testing for CH4 Combustion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 212. [Google Scholar]
- Zanoletti, M.; Klvana, D.; Kirchnerova, J.; Perrier, M.; Guy, C. Auto-cyclic reactor: Design and evaluation for the removal of unburned methane from emissions of natural gas engines. Chem. Eng. Sci. 2009, 64, 945–954. [Google Scholar] [CrossRef]
- Ladavos, A.; Pomonis, P. Methane Combustion on Perovskites in Perovskites and Related Mixed Oxides: Concepts and Applications; Wiley: Weinheim, Germany, 2016; Volume 2, pp. 369–388. [Google Scholar]
- Li, Z.; Hoflund, G.B. A Review on Complete Oxidation of Methane at Low Temperatures. J. Nat. Gas Chem. 2003, 12, 153–160. [Google Scholar]
- Garbowski, E.; Feumi-Jantou, C.; Mouaddib, N.; Primet, M. Catalytic combustion of methane over palladium supported on alumina catalysts: Evidence for reconstruction of particles. Appl. Catal. A 1994, 109, 277–291. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic Combustion of Methane over Palladium-Based Catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Escandón, L.S.; Ordóñez, S.; Vega, A.; Dı́ez, F.V. Oxidation of methane over palladium catalysts: Effect of the support. Chemosphere 2005, 58, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Van Vegten, N.; Maciejewski, M.; Krumeich, F.; Baiker, A. Structural properties, redox behaviour and methane combustion activity of differently supported flame-made Pd catalysts. Appl. Catal. B 2009, 93, 38–49. [Google Scholar] [CrossRef]
- Eguchi, K.; Arai, H. Low temperature oxidation of methane over Pd-based catalysts—Effect of support oxide on the combustion activity. Appl. Catal. A 2001, 222, 359–367. [Google Scholar] [CrossRef]
- Xiao, L.-H.; Sun, K.-P.; Xu, X.-L.; Li, X.-N. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition-precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic diesel particulate filters with highly dispersed ceria: Effect of the soot-catalyst contact on the regeneration performance. Appl. Catal. B 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Roth, D.; Gélin, P.; Kaddouri, A.; Garbowski, E.; Primet, M.; Tena, E. Oxidation behaviour and catalytic properties of Pd/Al2O3 catalysts in the total oxidation of methane. Catal. Today 2006, 112, 134–138. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kreft, S.; Georgi, G.; Fulda, G.; Pohl, M.-M.; Seeburg, D.; Berger-Karin, C.; Kondratenko, E.V.; Wohlrab, S. Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl. Catal. B 2015, 179, 313–320. [Google Scholar] [CrossRef]
- Li, Z.; Hoflund, G.B. Catalytic oxidation of methane over Pd/Co3O4. React. Kinet. Catal. Lett. 1999, 66, 367–374. [Google Scholar] [CrossRef]
- Ishihara, T.; Shigematsu, H.; Abe, Y.; Takita, Y. Effects of Additives on the Activity of Palladium Catalysts for Methane Combustion. Chem. Lett. 1993, 22, 407–410. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Arandiyan, H.; Peng, Y.; Chang, H.; Li, J. Low temperature complete combustion of methane over cobalt chromium oxides catalysts. Catal. Today 2013, 201, 12–18. [Google Scholar] [CrossRef]
- Ercolino, G.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A.; Specchia, V. Pd/Co3O4-based catalysts prepared by solution combustion synthesis for residual methane oxidation in lean conditions. Catal. Today 2015, 257, 66–71. [Google Scholar] [CrossRef]
- Lu, N.; Wu, Z.-W.; Lei, L.-J.; Qin, Z.-F.; Zhu, H.-Q.; Luo, L.; Fan, W.-B.; Wang, J.-G. Catalytic combustion of lean methane over a core-shell structured Pd-Co3O4@SiO2 catalyst. Ranliao Huaxue Xuebao 2015, 43, 1120–1127. [Google Scholar]
- Wang, Q.; Peng, Y.; Fu, J.; Kyzas, G.Z.; Billah, S.M.R.; An, S. Synthesis, characterization, and catalytic evaluation of Co3O4/γ-Al2O3 as methane combustion catalysts: Significance of Co. species and the redox cycle. Appl. Catal. B 2015, 168–169, 42–50. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, J.; Liu, Y.; Xie, S.; Jiang, Y.; Zhao, X.; Yang, J.; Arandiyan, H.; Guo, G.; Dai, H. Three-dimensionally ordered mesoporous Co3O4-supported Au-Pd alloy nanoparticles: High-performance catalysts for methane combustion. J. Catal. 2015, 332, 13–24. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Hu, W.; Lan, J.; Guo, Y.; Cao, X.-M.; Hu, P. Origin of Efficient Catalytic Combustion of Methane over Co3O4: Active Low-Coordination Lattice Oxygen and Cooperation of Multiple Active Sites. ACS Catal. 2016, 6, 5508–5519. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Song, J.; Huang, S.; Yang, N.; Zhang, J.; Sun, Y.; Zhu, Y. Exploring the Effect of Co3O4 Nanocatalysts with Different Dimensional Architectures on Methane Combustion. ChemCatChem 2016, 8, 540–545. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Xu, L.; Deng, Z.; Shi, W. Low temperature CO oxidation and CH4 combustion over Co3O4 nanosheets. Fuel 2017, 203, 419–429. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Kikuchi, R.; Maeda, S.; Sasaki, K.; Wennerström, S.; Eguchi, K. Low-temperature methane oxidation over oxide-supported Pd catalysts: Inhibitory effect of water vapor. Appl. Catal. A 2002, 232, 23–28. [Google Scholar] [CrossRef]
- Demoulin, O.; Rupprechter, G.; Seunier, I.; Le Clef, B.; Navez, M.; Ruiz, P. Investigation of Parameters Influencing the Activation of a Pd/γ-Alumina Catalyst during Methane Combustion. J. Phys. Chem. B 2005, 109, 20454–20462. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, B.; Tylus, W. Effect of washcoat modification with metal oxides on the activity of a monolithic Pd-based catalyst for methane combustion. Catal. Today 2008, 137, 324–328. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Yan, Y. Investigation of low concentration methane combustion in a fluidized bed with Pd/Al2O3 as catalytic particles. RSC Adv. 2014, 4, 59418–59426. [Google Scholar] [CrossRef]
- Gholami, R.; Alyani, M.; Smith, K. Deactivation of Pd Catalysts by Water during Low Temperature Methane Oxidation Relevant to Natural Gas Vehicle Converters. Catalysts 2015, 5, 561–594. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yu, S.C. Synthesis and structural features of a flux–grown hematite. J. Geol. Soc. China 1999, 42, 349–358. [Google Scholar]
- Roth, W.L. The magnetic structure of Co3O4. J. Phys. Chem. Solids 1964, 25, 1–10. [Google Scholar] [CrossRef]
- Slack, G.A. Crystallography and Domain Walls in Antiferromagnetic NiO Crystals. J. Appl. Phys. 1960, 31, 1571–1582. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. In Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse; DigiZeitschriften: Göttingen, Germany, 1918; pp. 98–100. [Google Scholar]
- Conner, W.C.; Falconer, J.L. Spillover in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 759–788. [Google Scholar] [CrossRef]
- Ciuparu, D.; Perkins, E.; Pfefferle, L. In situ DR-FTIR investigation of surface hydroxyls on γ-Al2O3 supported PdO catalysts during methane combustion. Appl. Catal. A 2004, 263, 145–153. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstrom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, NIST SRD 20, Version 4.1 (Web Version). Available online: https://srdata.nist.gov/xps/Default.aspx (accessed on 13 December 2017).
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dahl, M.; Yin, Y. Hollow Nanocrystals through the Nanoscale Kirkendall Effect. Chem. Mater. 2013, 25, 1179–1189. [Google Scholar] [CrossRef]

| Material | SBET 1/m² g−1 | Pd 2/wt % | Crystallite Size 3/nm |
|---|---|---|---|
| Fe2O3 | 18.7 | 49 (60) | |
| 1Pd/Fe2O3 | 1.07 | 52 (61) | |
| Co3O4 | 2.22 | 63 (63) | |
| 1Pd/Co3O4 | 0.85 | 55 (63) | |
| NiO | 5.25 | 40 (52) | |
| 1Pd/NiO | 0.98 | 38 (48) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeburg, D.; Liu, D.; Radnik, J.; Atia, H.; Pohl, M.-M.; Schneider, M.; Martin, A.; Wohlrab, S. Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion. Catalysts 2018, 8, 42. https://doi.org/10.3390/catal8020042
Seeburg D, Liu D, Radnik J, Atia H, Pohl M-M, Schneider M, Martin A, Wohlrab S. Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion. Catalysts. 2018; 8(2):42. https://doi.org/10.3390/catal8020042
Chicago/Turabian StyleSeeburg, Dominik, Dongjing Liu, Joerg Radnik, Hanan Atia, Marga-Martina Pohl, Matthias Schneider, Andreas Martin, and Sebastian Wohlrab. 2018. "Structural Changes of Highly Active Pd/MeOx (Me = Fe, Co, Ni) during Catalytic Methane Combustion" Catalysts 8, no. 2: 42. https://doi.org/10.3390/catal8020042