The PT/S-Box of Modular Cellulase AcCel12B Plays a Key Role in the Hydrolysis of Insoluble Cellulose
Abstract
:1. Introduction
2. Results and Discussion
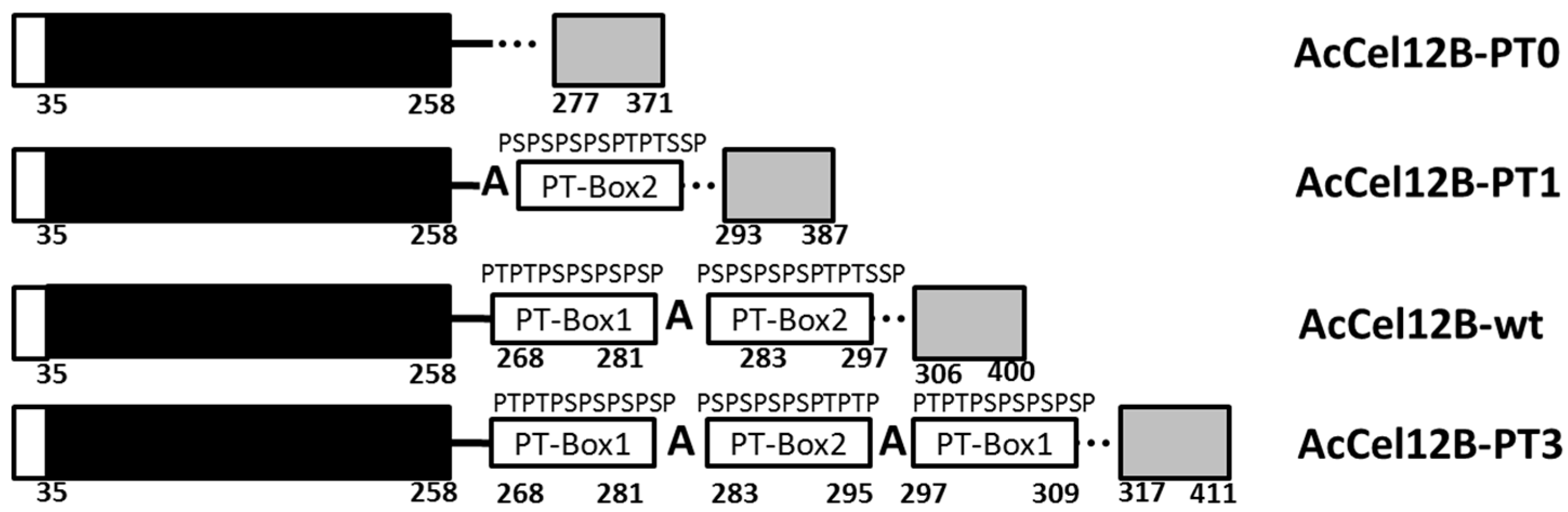
2.1. Mutant Design of AcCel12B Linker
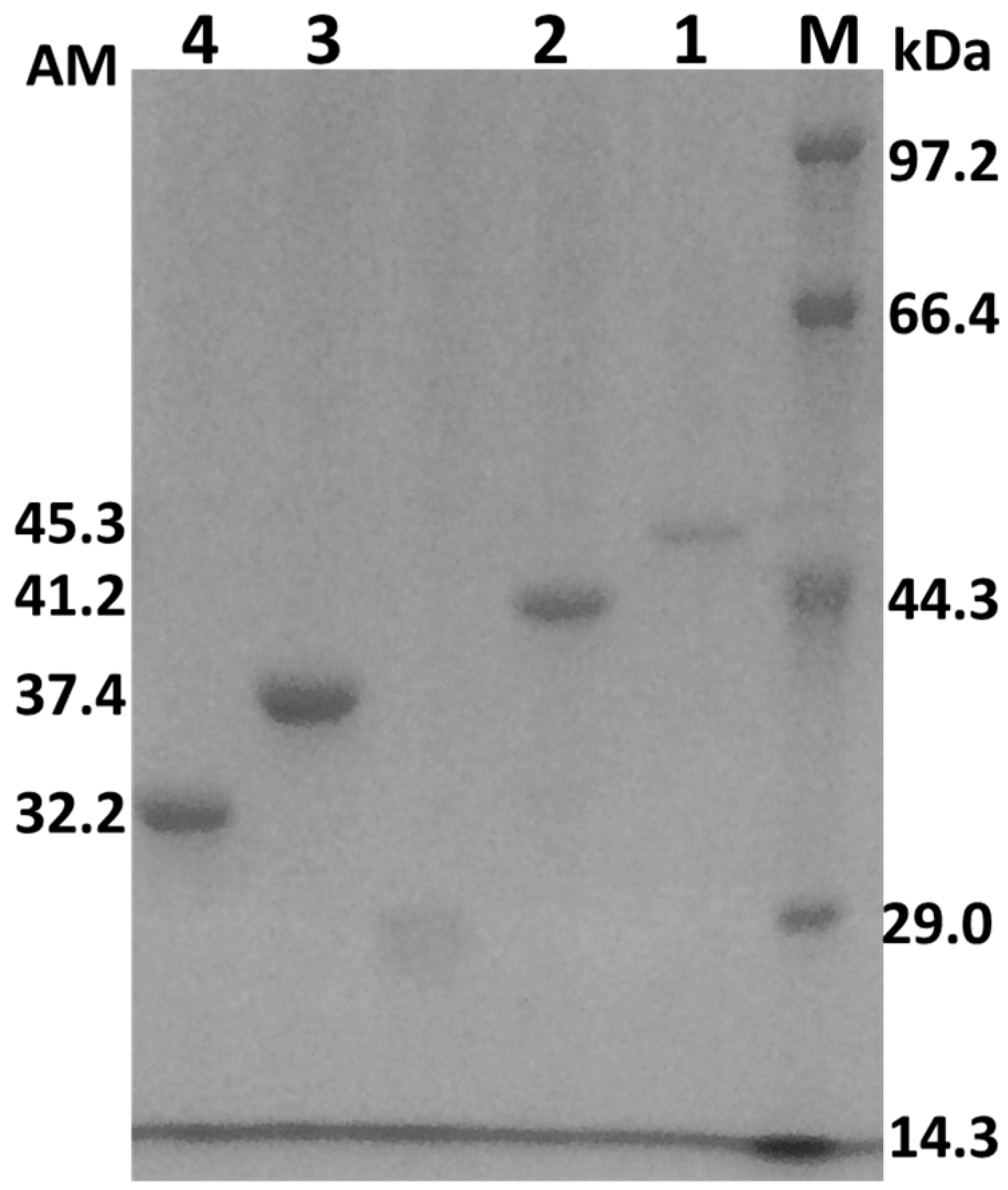
2.2. Cloning, Expression, and Purification of the AcCel12B and Its Mutants
2.3. Enzymatic Activities
2.4. Time Course of Hydrolysis of AcCel12B and Its Mutants
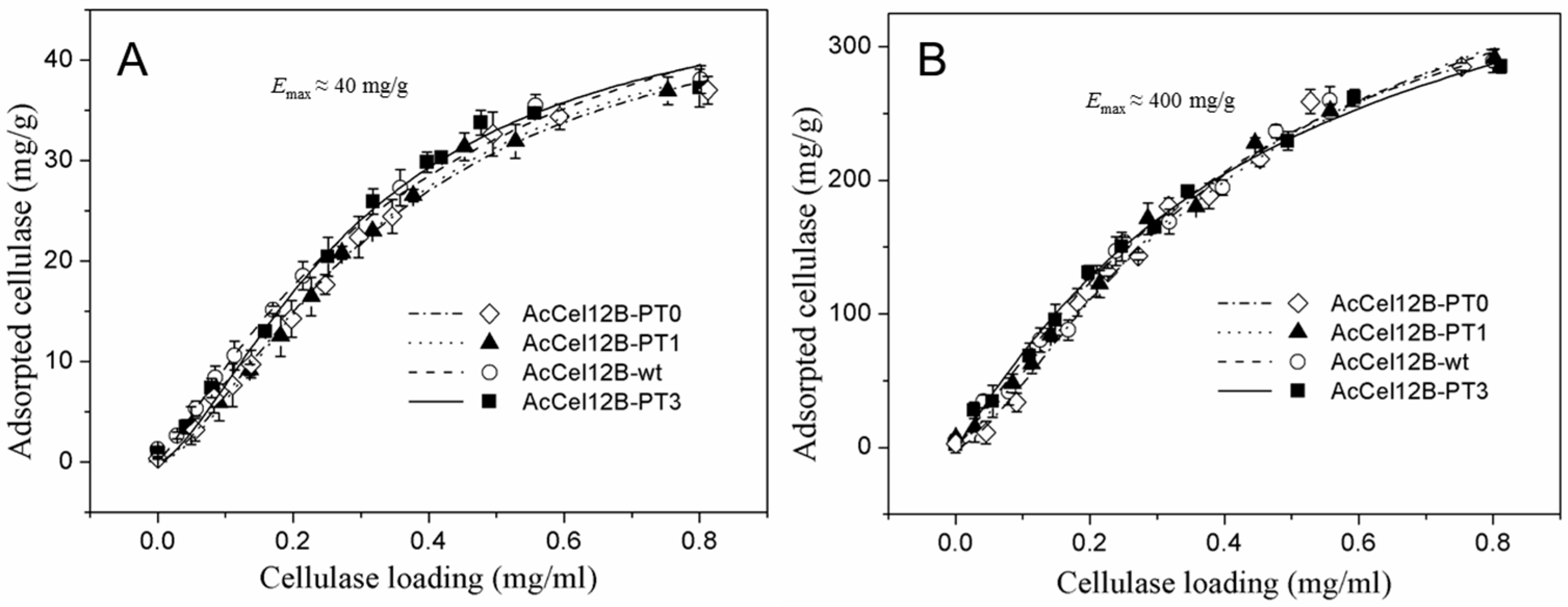
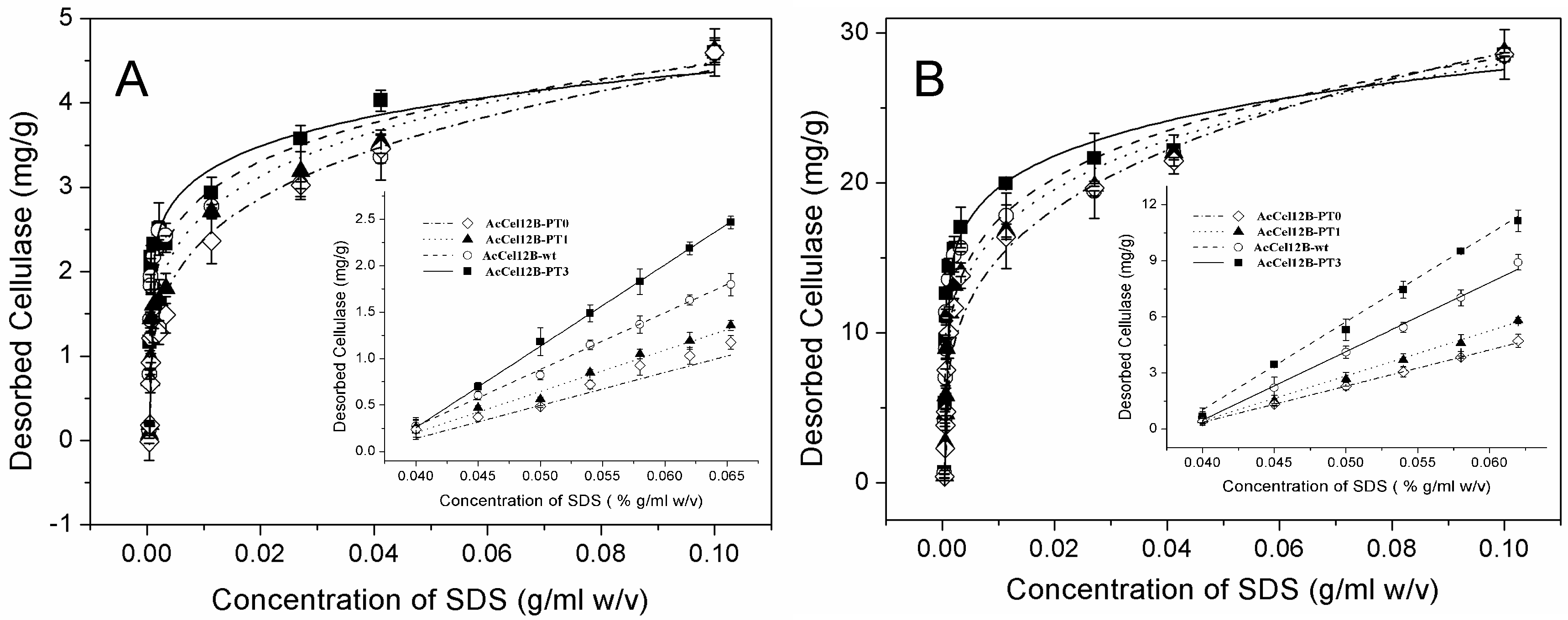
2.5. Adsorption and Desorption of Wild-Type and Mutant AcCel12B
3. Materials and Methods
3.1. Strains, Vectors, and Materials
3.2. Construction of Mutant Plasmids
3.3. Expression and Purification of Wild-Type and Mutant AcCel12B
3.4. Enzyme Activity Assay
3.5. Enzyme Characterization
3.6. Time Course of Hydrolysis
3.7. Enzyme Adsorption and Desorption Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Béguin, P.; Aubert, J.P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.M.; McCrae, S.I.; Bhat, K.M. The mechanism of fungal cellulase action. Synergism between enzyme components of Penicillium pinophilum cellulase in solubilizing hydrogen bond-ordered cellulose. Biochem. J. 1989, 260, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P. Molecular Biology of Cellulose Degradation. Annu. Rev. Microbiol. 1990, 44, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Sonan, G.K.; Receveur-Brechot, V.; Duez, C.; Aghajari, N.; Czjzek, M.; Haser, R.; Gerday, C. The linker region plays a key role in the adaptation to cold of the cellulase from an Antarctic bacterium. Biochem. J. 2007, 407, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.P.; Lynd, L.R. Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol. Bioeng. 2004, 88, 797–824. [Google Scholar]
- Srisodsuk, M.; Reinikainen, T.; Penttilä, M.; Teeri, T.T. Role of the interdomain linker peptide of Trichoderma reesei cellobiohydrolase I in its interaction with crystalline cellulose. J. Biol. Chem. 1993, 268, 20756–20761. [Google Scholar] [PubMed]
- Strobel, K.L.; Pfeiffer, K.A.; Blanch, H.W.; Clark, D.S. Engineering Cel7A carbohydrate binding module and linker for reduced lignin inhibition. Biotechnol. Bioeng. 2016, 113, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Kont, R.; Kari, J.; Borch, K.; Westh, P.; Väljamäe, P. Inter-domain Synergism Is Required for Efficient Feeding of Cellulose Chain into Active Site of Cellobiohydrolase Cel7A. J. Biol. Chem. 2016, 291, 26013–26023. [Google Scholar] [CrossRef] [PubMed]
- Sammond, D.W.; Payne, C.M.; Brunecky, R.; Himmel, M.E.; Crowley, M.F.; Beckham, G.T. Cellulase linkers are optimized based on domain type and function: Insights from sequence analysis, biophysical measurements, and molecular simulation. PLoS ONE 2012, 7, e48615. [Google Scholar] [CrossRef] [PubMed]
- Raymond-Wong, W.K.; Gerhard, B.; Guo, Z.M.; Kilburn, D.G.; Anthony, R.; Warren, J.; Miller, R.C. Characterization and structure of an endoglucanase gene cenA of Cellulomonas fimi. Gene 1986, 44, 315–324. [Google Scholar] [CrossRef]
- Shen, H.; Schmuck, M.; Pilz, I.; Gilkes, N.R.; Kilburn, D.G.; Miller, R.C.; Warren, R.A. Deletion of the linker connecting the catalytic and cellulose-binding domains of endoglucanase A (CenA) of Cellulomonas fimi alters its conformation and catalytic activity. J. Biol. Chem. 1991, 266, 11335–11340. [Google Scholar] [PubMed]
- Poon, D.K.; Withers, S.G.; Mcintosh, L.P. Direct demonstration of the flexibility of the glycosylated proline-threonine linker in the Cellulomonas fimi Xylanase Cex through NMR spectroscopic analysis. J. Biol. Chem. 2007, 282, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Sunna, A.; Gibbs, M.D.; Chin, C.W.; Nelson, P.J.; Bergquist, P.L. A gene encoding a novel multidomain beta-1,4-mannanase from Caldibacillus cellulovorans and action of the recombinant enzyme on kraft pulp. Appl. Environ. Microbiol. 2000, 66, 664–670. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.L.; Adney, W.S.; Vinzant, T.B.; Jennings, E.; Eddy, F.P.; Decker, S.R.; Baker, J.O.; Sakon, J.; Himmel, M.E. Exploration of cellulose surface-binding properties of acidothermus cellulolyticus Cel5A by site-specific mutagenesis. Appl. Biochem. Biotechnol. 2002, 98–100, 273–287. [Google Scholar] [CrossRef]
- Yeh, M.; Craig, S.; Lum, M.G.; Foong, F.C. Effects of the PT region of EngD and HLD of CbpA on solubility, catalytic activity and purification characteristics of EngD-CBD(CbpA) fusions from Clostridium cellulovorans. J. Biotechnol. 2005, 116, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Boze, H.; Marlin, T.; Durand, D.; Pérez, J.; Vernhet, A.; Canon, F.; Sarni-Manchado, P.; Cheynier, V.; Cabane, B. Proline-Rich Salivary Proteins Have Extended Conformations. Biophys. J. 2010, 99, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Receveur, V.; Czjzek, M.; Schülein, M.; Panine, P.; Henrissat, B. Dimension, shape, and conformational flexibility of a two domain fungal cellulase in solution probed by small angle X-ray scattering. J. Biol. Chem. 2002, 277, 40887–40892. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Nouwens, A.S.; Jardine, D.R.; Zachara, N.E.; Gooley, A.A.; Nevalainen, H.; Packer, N.H. Modified glycosylation of cellobiohydrolase I from a high cellulase-producing mutant strain of Trichoderma reesei. Eur. J. Biochem. 1998, 256, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, I.V.; Eaton, J.T.; Czjzek, M.; Perkins, S.J.; Frandsen, T.P.; Schülein, M.; Panine, P.; Henrissat, B.; Receveurbréchot, V. Protein Disorder: Conformational Distribution of the Flexible Linker in a Chimeric Double Cellulase. Biophys. J. 2005, 88, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.M.; Resch, M.G.; Chen, L.; Crowley, M.F.; Himmel, M.E.; Nd, T.L.; Sandgren, M.; Ståhlberg, J.; Stals, I.; Tan, Z. Glycosylated linkers in multimodular lignocellulose-degrading enzymes dynamically bind to cellulose. Proc. Natl. Acad. Sci. USA 2013, 110, 14646–14651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, G.; Li, Y.; Yang, L.; Liang, Y.; Jin, H.; Han, W.; Feng, Y.; Zhang, Z. Cloning, Expression, and Characterization of a Thermophilic Endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B. Int. J. Mol. Sci. 2015, 16, 25080–25095. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Mason, D.A.; Matthews, B.W. Flexible-geometry conformational energy maps for the amino acid residue preceding a proline. Biopolymers 1992, 32, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.A.; Rubenstein, E. Proline: The distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.P. The structure and function of proline-rich regions in proteins. Biochem. J. 1994, 297, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic force microscopy characterization of cellulose nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef] [PubMed]
- Zimm, B.H.; Bragg, J.K. Theory of the Phase Transition between Helix and Random Coil in Polypeptide Chains. J. Chem. Phys. 1959, 31, 526–535. [Google Scholar] [CrossRef]
- Ohkubo, Y.Z.; Brooks, C.L. Exploring Flory’s Isolated-Pair Hypothesis: Statistical Mechanics of Helix-Coil Transitions in Polyalanine and the C-Peptide from RNase A. Proc. Natl. Acad. Sci. USA 2003, 100, 13916–13921. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.R.; Costa, M.G.; Pascutti, P.G.; Bisch, P.M.; de-Souza, W. High temperatures enhance cooperative motions between CBM and catalytic domains of a thermostable cellulase: Mechanism insights from essential dynamics. Phys. Chem. Chem. Phys. 2011, 13, 13709–13720. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.L.; Makarov, D.E.; Wang, Z.G. A kinetic model for the enzymatic action of cellulase. J. Phys. Chem. B 2009, 113, 4970–4977. [Google Scholar] [CrossRef] [PubMed]
- Abuja, P.M.; Schmuck, M.; Pilz, I.; Tomme, P.; Claeyssens, M.; Esterbauer, H. Structural and functional domains of cellobiohydrolase I from Trichoderma reesei. Eur. Biophys. J. 1988, 15, 339–342. [Google Scholar] [CrossRef]
- Kostylev, M.; Wilson, D. Two-parameter kinetic model based on a time-dependent activity coefficient accurately describes enzymatic cellulose digestion. Biochemistry 2013, 52, 5656–5664. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.C.; Zhang, S.; Wilson, D.B. Cloning, expression and characterization of a family 48 exocellulase, Cel48A, from Thermobifida fusca. Eur. J. Biochem. 2000, 267, 4988–4997. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.; Shin, D.H.; Zhang, S.; Barr, B.K.; Sakon, J.; Karplus, P.A.; Wilson, D.B. Roles of the catalytic domain and two cellulose binding domains of Thermomonospora fusca E4 in cellulose hydrolysis. J. Bacteriol. 1998, 180, 1709–1714. [Google Scholar] [PubMed]
- Hong, J.; Ye, X.; Wang, Y.; Zhang, Y.H.P. Bioseparation of recombinant cellulose-binding module-proteins by affinity adsorption on an ultra-high-capacity cellulosic adsorbent. Anal. Chim. Acta 2008, 621, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.L.; Moreira-Neto, J.; da-Cruz, J.G.; Bonomi, A.; Rabelo, S.C.; da-Costa, A.C. Adsorption characteristics of cellulase and β-glucosidase on Avicel, pretreated sugarcane bagasse, and lignin. Biotechnol. Appl. Biochem. 2015, 62, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Beckham, G.T.; Bomble, Y.J.; Matthews, J.F.; Taylor, C.B.; Resch, M.G.; Yarbrough, J.M.; Decker, S.R.; Bu, L.; Zhao, X.; McCabe, C. The O-glycosylated linker from the Trichoderma reesei Family 7 cellulase is a flexible, disordered protein. Biophys. J. 2010, 99, 3773–3781. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K.; Bisaria, V.S. Studies on the mechanism of enzymatic hydrolysis of cellulosic substances. Biotechnol. Bioeng. 1979, 21, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Castanon, M.; Wilke, C.R. Adsorption and recovery of cellulases during hydrolysis of newspaper. Biotechnol. Bioeng. 1980, 22, 1037–1053. [Google Scholar] [CrossRef]
- Otter, D.E.; Munro, P.A.; Scott, G.K. Desorption of Trichoderma reesei cellulase from cellulose by a range of desorbents. Biotechnol. Bioeng. 1989, 34, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.T. Elution of Celluiase from Cellulose. Process Biochem. 1982, 17, 2. [Google Scholar]
- Sankararamakrishnan, R.; Vishveshwara, S. Characterization of proline-containing α-helix (helix F model of bacteriorhodopsin) by molecular dynamics studies. Proteins Struct. Funct. Inform. 1993, 15, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Young, T.M. A Distributed Reactivity Model for Sorption by Soils and Sediments. 6. Mechanistic Implications of Desorption under Supercritical Fluid Conditions. Environ. Sci. Technol. 1997, 31, 1686–1691. [Google Scholar] [CrossRef]
- Furuya, E.G.; Chang, H.T.; Miura, Y.; Noll, K.E. A fundamental analysis of the isotherm for the adsorption of phenolic compounds on activated carbon. Sep. Purif. Technol. 1997, 11, 69–78. [Google Scholar] [CrossRef]
- Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.; Arias-Estévez, M. Cr(VI) Sorption/Desorption on Pine Sawdust and Oak Wood Ash. Int. J. Environ. Res. Public Health 2015, 12, 8849–8860. [Google Scholar] [CrossRef] [PubMed]
- Tavagnacco, L.; Mason, P.E.; Schnupf, U.; Pitici, F.; Zhong, L.; Himmel, M.E.; Crowley, M.; Cesàro, A.; Brady, J.W. Sugar-binding sites on the surface of the carbohydrate-binding module of CBH I from Trichoderma reesei. Carbohydr. Res. 2011, 346, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.B.; Talib, M.F.; McCabe, C.; Bu, L.; Adney, W.S.; Himmel, M.E.; Crowley, M.F.; Beckham, G.T. Computational Investigation of Glycosylation Effects on a Family 1 Carbohydrate-binding Module. J. Biol. Chem. 2012, 287, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Baker, J.O.; Zeng, Y.; Himmel, M.E.; Haas, T.; Ding, S.Y. Cellobiohydrolase Hydrolyzes Crystalline Cellulose on Hydrophobic Faces. J. Biol. Chem. 2011, 286, 11195–11201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Reinikainen, T.; Teleman, O.; Teeri, T.T. Effects of pH and high ionic strength on the adsorption and activity of native and mutated cellobiohydrolase I from Trichoderma reesei. Proteins 1995, 22, 392–403. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | Specific Activity | ||
|---|---|---|---|
| CMC * (U/µM) | RAC * (U/µM) | Avicel (mU/µM) | |
| AcCel12B-PT0 | 2.8 ± 0.4 | 2.0 ± 0.1 | 8.3 ± 2.7 |
| AcCel12B-PT1 | 2.2 ± 0.2 | 2.1 ± 0.2 | 12.0 ± 2.9 |
| AcCel12B-wt | 2.6 ± 0.3 | 2.4 ± 0.1 | 16.5 ± 1.1 |
| AcCel12B-PT3 | 2.6 ± 0.3 | 2.7 ± 0.2 | 22.7 ± 3.4 |
| Enzyme | A (μM/min) | b (μM/min) | R2 |
|---|---|---|---|
| AcCel12B-PT0 | 33.7 ± 1.7 | 0.54 ± 0.01 | 0.995 |
| AcCel12B-PT1 | 36.1 ± 1.8 | 0.54 ± 0.01 | 0.995 |
| AcCel12B-wt | 40.3 ± 2.6 | 0.54 ± 0.02 | 0.990 |
| AcCel12B-PT3 | 44.1 ± 3.6 | 0.54 ± 0.02 | 0.990 |
| Enzyme | Generation Rate of IRE * | Generation Rate of SRE * | ||||||
|---|---|---|---|---|---|---|---|---|
| RPI * (µM/min) | R2 | SPI * (µM/min) | R2 | RPS * (µM/min) | R2 | SPS * (µM/min) | R2 | |
| AcCel12B-PT0 | 7.8 ± 1.3 | 0.94 | 1.6 ± 0.03 | 0.97 | 4.5 ± 0.3 | 0.96 | 1.4 ± 0.3 | 0.95 |
| AcCel12B-PT1 | 10.8 ± 1.4 | 0.94 | 2.1 ± 0.06 | 0.99 | 4.8 ± 0.1 | 0.99 | 1.3 ± 0.2 | 0.85 |
| AcCel12B-wt | 13.3 ± 1.2 | 0.91 | 2.4 ± 0.1 | 0.98 | 4.8 ± 0.2 | 0.99 | 1.5 ± 0.2 | 0.96 |
| AcCel12B-PT3 | 14.1 ± 1.7 | 0.91 | 2.3 ± 0.05 | 0.99 | 4.5 ± 0.4 | 0.97 | 1.4 ± 0.3 | 0.90 |
| Enzyme | Avicel | RAC | ||
|---|---|---|---|---|
| Slope | R2 | Slope | R2 | |
| AcCel12B-PT0 | 35.5 ± 2.5 | 0.88 | 193.6 ± 4.8 | 0.99 |
| AcCel12B-PT1 | 44.4 ± 2.2 | 0.94 | 240.7 ± 9 | 0.99 |
| AcCel12B-wt | 61.1 ± 3.1 | 0.99 | 367.4 ± 20 | 0.99 |
| AcCel12B-PT3 | 87.6 ± 3.1 | 0.99 | 470.2 ± 12 | 0.99 |
| Enzyme | Avicel | RAC | ||||
|---|---|---|---|---|---|---|
| Kf (mg/g)/(g/mL)n | n | R2 | Kf (mg/g)/(g/mL)n | n | R2 | |
| AcCel12B-PT0 | 8.0 ± 0.5 | 0.26 ± 0.02 | 0.99 | 54.4 ± 3.2 | 0.28 ± 0.02 | 0.98 |
| AcCel12B-PT1 | 7.4 ± 0.9 | 0.22 ± 0.02 | 0.95 | 46.5 ± 2.4 | 0.22 ± 0.02 | 0.99 |
| AcCel12B-wt | 6.8 ± 1.0 | 0.18 ± 0.02 | 0.87 | 45.4 ± 3.3 | 0.20 ± 0.02 | 0.97 |
| AcCel12B-PT3 | 6.0 ± 0.6 | 0.14 ± 0.02 | 0.92 | 38.5 ± 3.9 | 0.14 ± 0.02 | 0.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, J.; Wang, L.; Tong, H.; Bu, M.; Gao, G.; Han, W.; Zhang, Z. The PT/S-Box of Modular Cellulase AcCel12B Plays a Key Role in the Hydrolysis of Insoluble Cellulose. Catalysts 2018, 8, 123. https://doi.org/10.3390/catal8030123
Li Y, Wang J, Wang L, Tong H, Bu M, Gao G, Han W, Zhang Z. The PT/S-Box of Modular Cellulase AcCel12B Plays a Key Role in the Hydrolysis of Insoluble Cellulose. Catalysts. 2018; 8(3):123. https://doi.org/10.3390/catal8030123
Chicago/Turabian StyleLi, Yuwei, Junling Wang, Limei Wang, Hao Tong, Mingwei Bu, Gui Gao, Weiwei Han, and Zuoming Zhang. 2018. "The PT/S-Box of Modular Cellulase AcCel12B Plays a Key Role in the Hydrolysis of Insoluble Cellulose" Catalysts 8, no. 3: 123. https://doi.org/10.3390/catal8030123





