Effect of In-Situ Dehydration on Activity and Stability of Cu–Ni–K2O/Diatomite as Catalyst for Direct Synthesis of Dimethyl Carbonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of the Dehydrating Agent
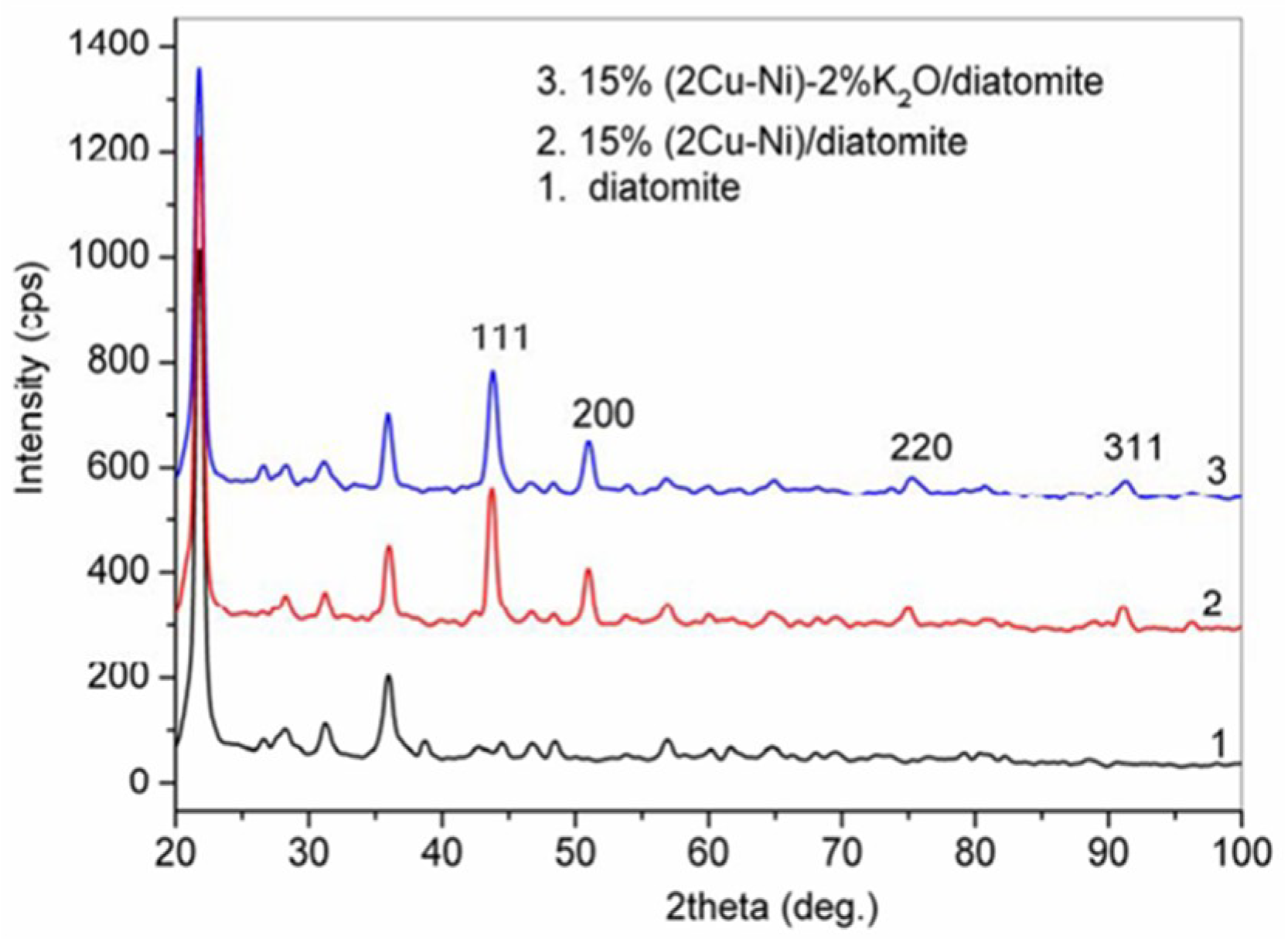
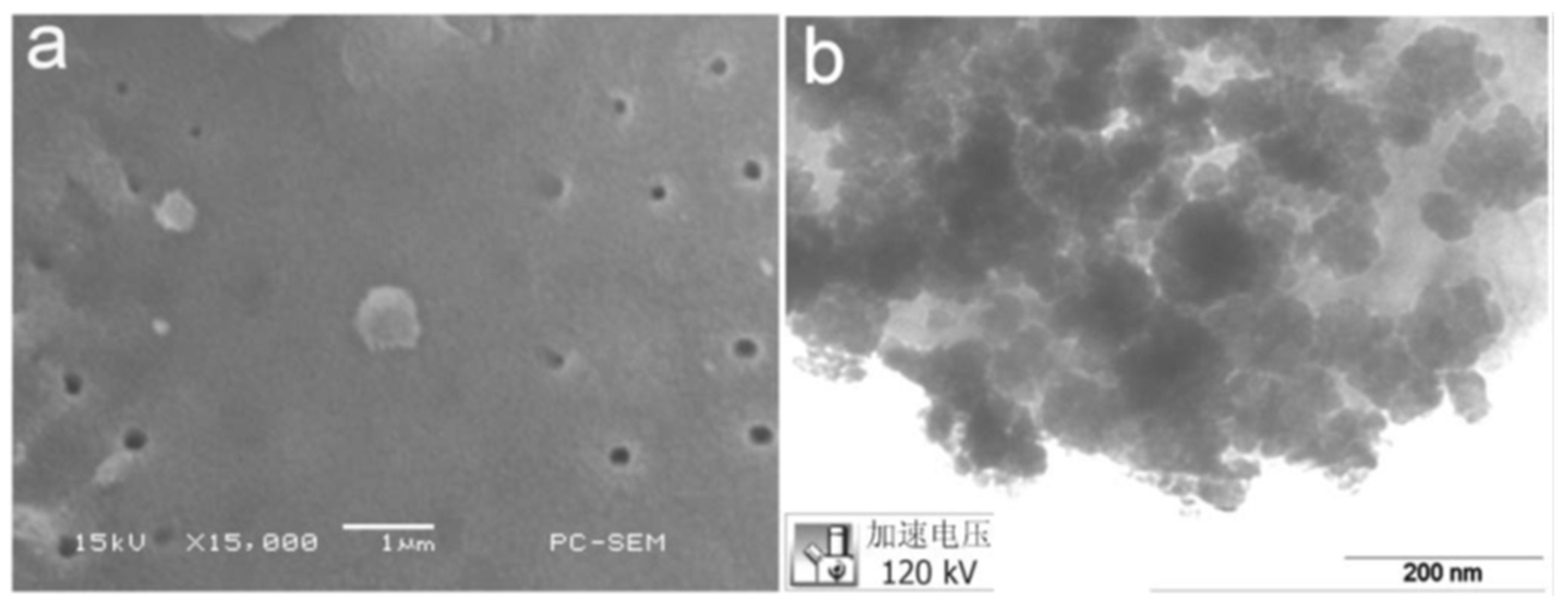
2.2. Characterization of the Catalyst
2.3. Effect of Dehydration on Properties of the Catalyst
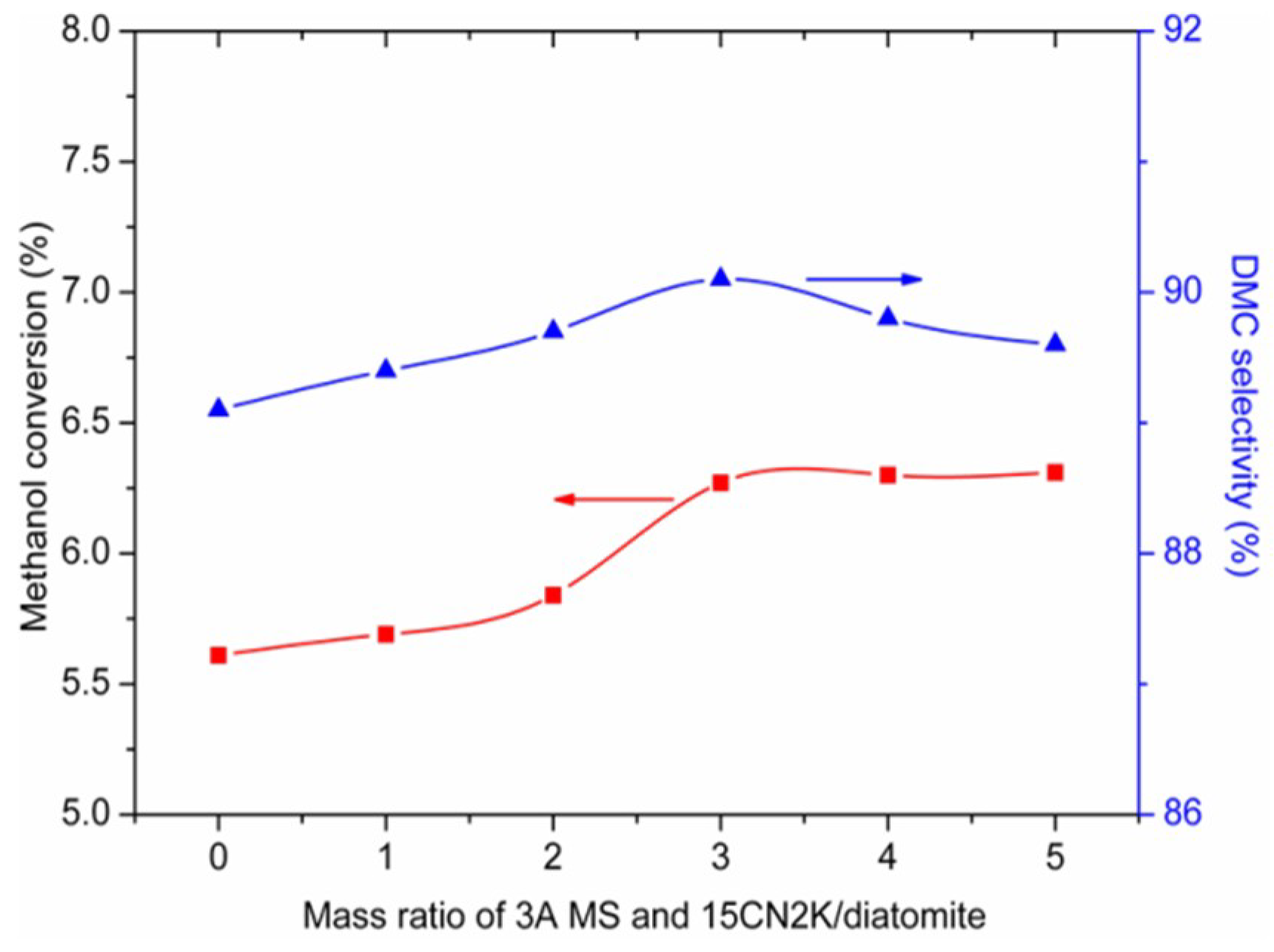
2.3.1. Effect of Mass Ratio of 3A MS and 15CN2K/Diatomite on the Activity of the Catalyst
2.3.2. Effect of Dehydrating Temperature and Pressure on Properties of the Catalyst
2.3.3. Effect of Space Velocity on Dehydrating the Catalyst
2.3.4. Effect of In-Situ Dehydration on Stability of the Catalyst
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalyst Evaluation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fu, Z.W.; Meng, Y.Z. Research Progress in the Phosgene-Free and Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol. Chem. Beyond Chlorine 2016, 363–385. [Google Scholar] [CrossRef]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A 1997, 155, 133–166. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Fu, Z.W.; Wang, S.J.; Xiao, M.; Han, D.M.; Meng, Y.Z. Electrochemical synthesis of dimethyl carbonate from CO2 and methanol over carbonaceous material supported DBU in a capacitor-like cell reactor. RSC Adv. 2016, 6, 40010–40016. [Google Scholar] [CrossRef]
- Han, M.S.; Lee, B.G.; Suh, I.; Kim, H.S.; Ahn, B.S.; Hong, S.I. Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol over Cu-based catalysts. J. Mol. Catal. A Chem. 2001, 170, 225–234. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsumi, T. Hydrotalcite-type materials as catalysts for the synthesis of dimethyl carbonate from ethylene carbonate and methanol. Microporous Mesoporous Mater. 1998, 22, 399–407. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ikariya, T.; Noyori, R. Homogeneous catalysis in supercritical fluids. Chem. Rev. 1999, 99, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Puga, J.; Jones, M.E.; Molzahn, D.C.; Hartwell, G.E. Production of Dialkyl Carbonates from Alkanol, Carbon Monoxide and Oxygen Using a Novel Copper Containing Catalyst, or a Known Catalyst with a Chloro-Carbon Promoter. U.S. Patent 5,387,708, 7 February 1995. [Google Scholar]
- Pimprom, S.; Sriboonkham, K.; Dittanet, P.; Fottinger, K.; Rupprechter, G.; Kongkachuichay, P. Synthesis of copper-nickel/SBA-15 from rice husk ash catalyst for dimethyl carbonate production from methanol and carbon dioxide. J. Ind. Eng. Chem. 2015, 31, 156–166. [Google Scholar] [CrossRef]
- Fu, Z.W.; Zhong, Y.Y.; Yu, Y.H.; Long, L.Z.; Xiao, M.; Han, D.M.; Wang, S.J.; Meng, Y.Z. TiO2-Doped CeO2 Nanorod Catalyst for Direct Conversion of CO2 and CH3OH to Dimethyl Carbonate: Catalytic Performance and Kinetic Study. ACS Omega 2018, 3, 198–207. [Google Scholar] [CrossRef]
- Ionescu, R.O.; Peres-Lucchese, Y.; Camy, S.; Tassaing, T.; Blanco, J.F.; Anne-Archard, G.; Riboul, D.; Condoret, J.S. Activation of CH3OH and CO2 by metallophthalocyanine complexes: Potential route to dimethyl carbonate. Rev. Roum. Chim. 2013, 58, 759–763. [Google Scholar]
- Zheng, H.; Hong, Y.; Xu, J.; Xue, B.; Li, Y.X. Transesterification of ethylene carbonate to dimethyl carbonate catalyzed by CeO2 materials with various morphologies. Catal. Commun. 2018, 106, 6–10. [Google Scholar] [CrossRef]
- Prymak, I.; Prymak, O.; Wang, J.H.; Kalevaru, V.N.; Martin, A.; Bentrup, U.; Wohlrab, S. Phosphate functionalization of CeO2–ZrO2 solid solutions for the catalytic formation of dimethyl carbonate from methanol and carbon dioxide. ChemCatChem 2018, 10, 391–394. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, Z.W.; Lu, J.; Liu, Z.T. Synthesis of dimethyl carbonate from carbon dioxide and methanol over CexZr1−xO2 and [EMIM]Br/Ce0.5Zr0.5O2. Ind. Eng. Chem. Res. 2011, 50, 1981–1988. [Google Scholar] [CrossRef]
- Aouissi, A.; Al-Othman, Z.A.; Al-Amro, A. Gas-phase synthesis of dimethyl carbonate from methanol and carbon dioxide over Co1.5PW12O40 keggin-type heteropolyanion. Int. J. Mol. Sci. 2010, 11, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Xiao, M.; Meng, Y.Z.; Lu, Y.X. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mol. Catal. A Chem. 2005, 238, 158–162. [Google Scholar] [CrossRef]
- Chen, H.L.; Xiao, M.; Wang, S.J.; Han, D.M.; Meng, Y.Z. Direct synthesis of dimethyl carbonate from CO2 and CH3OH using 4A molecular sieve supported Cu-Ni bimetal catalyst. Chin. J. Chem. Eng. 2012, 20, 906–913. [Google Scholar]
- Bian, J.; Xiao, M.; Wang, S.J.; Lu, Y.X.; Meng, Y.Z. Direct synthesis of DMC from CH3OH and CO2 over V-doped Cu–Ni/AC catalysts. Catal. Commun. 2009, 10, 1142–1145. [Google Scholar] [CrossRef]
- Wang, X.J.; Xiao, M.; Wang, S.J.; Lu, Y.X.; Meng, Y.Z. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance. J. Mol. Catal. A Chem. 2007, 278, 92–96. [Google Scholar] [CrossRef]
- Wu, X.L.; Meng, Y.Z.; Xiao, M.; Lu, Y.X. Direct synthesis of dimethyl carbonate (DMC) using Cu–Ni/VSO as catalyst. J. Mol. Catal. A Chem. 2006, 249, 93–97. [Google Scholar] [CrossRef]
- Zhao, T.X.; Hu, X.B.; Wu, D.S.; Li, R.; Yang, G.Q.; Wu, Y.T. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol at room temperature using imidazolium hydrogen carbonate ionic liquid as a recyclable catalyst and dehydrant. ChemSusChem 2017, 10, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T.; Saito, Y.; Okano, M.; Choi, J.C.; Sako, T. Selective conversion of carbon dioxide to dimethyl carbonate by molecular catalysis. J. Org. Chem. 1998, 63, 7095–7096. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Choi, J.C.; Ohshima, Y.; Yili, A.; Yasuda, H.; Sakakura, T. Reaction of dibutyltin oxide with methanol under CO2 pressure relevant to catalytic dimethyl carbonate synthesis. J. Organomet. Chem. 2008, 693, 1389–1392. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, A.; Noorjahan, B.; Fujimoto, K.; Suzuki, K.; Tomishige, K. Low pressure CO2 to dimethyl carbonate by the reaction with methanol promoted by acetonitrile hydration. Catal. Commun. 2009, 30, 4596–4598. [Google Scholar] [CrossRef] [PubMed]
- Eta, V.; Maki-Arvela, P.; Leino, A.R.; Kordas, K.; Salmi, T.; Murzin, D.Y.; Mikkola, J.P. Synthesis of dimethyl carbonate from methanol and carbon dioxide: circumventing thermodynamic limitations. Ind. Eng. Chem. Res. 2010, 49, 9609–9617. [Google Scholar] [CrossRef]
- Choi, J.C.; He, L.N.; Yasuda, H.; Sakakura, T. Selective and high yield synthesis of dimethyl carbonate directly from carbon dioxide and methanol. Green Chem. 2002, 4, 230–234. [Google Scholar] [CrossRef]
- Han, D.M.; Chen, Y.; Wang, S.J.; Xiao, M.; Lu, Y.X.; Meng, Y.Z. Effect of alkali-doping on the performance of diatomite supported Cu-Ni bimetal catalysts for direct synthesis of dimethyl carbonate. Catalysts 2018, 8, 302. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Chen, Y.; Wang, S.; Xiao, M.; Lu, Y.; Meng, Y. Effect of In-Situ Dehydration on Activity and Stability of Cu–Ni–K2O/Diatomite as Catalyst for Direct Synthesis of Dimethyl Carbonate. Catalysts 2018, 8, 343. https://doi.org/10.3390/catal8090343
Han D, Chen Y, Wang S, Xiao M, Lu Y, Meng Y. Effect of In-Situ Dehydration on Activity and Stability of Cu–Ni–K2O/Diatomite as Catalyst for Direct Synthesis of Dimethyl Carbonate. Catalysts. 2018; 8(9):343. https://doi.org/10.3390/catal8090343
Chicago/Turabian StyleHan, Dongmei, Yong Chen, Shuanjin Wang, Min Xiao, Yixin Lu, and Yuezhong Meng. 2018. "Effect of In-Situ Dehydration on Activity and Stability of Cu–Ni–K2O/Diatomite as Catalyst for Direct Synthesis of Dimethyl Carbonate" Catalysts 8, no. 9: 343. https://doi.org/10.3390/catal8090343




