Effect of Microwave Drying, Calcination and Aging of Pt/Al2O3 on Platinum Dispersion
Abstract
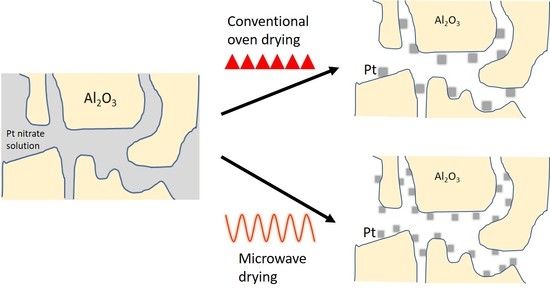
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Microwave Set-Up
2.3. Pt Dispersion Measurement
2.4. Surface Area Measurements
3. Results
3.1. Effect of Microwave Drying
3.2. Effect of Microwave Calcination
3.3. Effect of Microwave Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Den Berg, G.H.; Rijnten, H.T. The Impregnation and Drying Step in Catalyst Manufacturing. Stud. Surf. Sci. Catal. 1979, 3, 265–277. [Google Scholar]
- Lekhal, A.; Glasser, B.J.; Khinast, J.G. Impact of drying on the catalyst profile in supported impregnation catalysts. Chem. Eng. Sci. 2001, 56, 4473–4487. [Google Scholar] [CrossRef]
- Santiago, M.; Restuccia, A.; Gramm, F.; Pérez-Ramírez, J. Spray deposition method for the synthesis of supported catalysts with superior metal dispersion. Microporous Mesoporous Mater. 2011, 146, 76–81. [Google Scholar] [CrossRef]
- Auvray, X.; Pingel, T.; Olsson, E.; Olsson, L. The effect gas composition during thermal aging on the dispersion and NO oxidation activity over Pt/Al2O3 catalysts. Appl. Catal. B 2013, 129, 517–527. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Munnik, P.; Talsma, H.; de Jongh, P.E.; de Jong, K.P. Freeze-drying for controlled nanoparticle distribution in Co/SiO2 Fischer–Tropsch catalysts. J. Catal. 2013, 297, 306–313. [Google Scholar] [CrossRef]
- Liu, X.; Khinast, J.G.; Glasser, B.J. Drying of supported catalysts for low melting point precursors: Impact of metal loading and drying methods on the metal distribution. Chem. Eng. Sci. 2012, 79, 187–199. [Google Scholar] [CrossRef]
- Vergunst, T.; Kapteijn, F.; Moulijn, J.A. Monolithic catalysts—Non-uniform active phase distribution by impregnation. Appl. Catal. A 2001, 213, 179–187. [Google Scholar] [CrossRef]
- Choi, K.Y.; Tompsett, G.; Conner, W.C. Microwave assisted synthesis of silicalite–Power delivery and energy consumption. Green Chem. 2008, 10, 1313–1317. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. Chem. Phys. Chem. 2006, 7, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Brar, T.; France, P.; Smirniotis, P.G. Control of Crystal Size and Distribution of Zeolite A. Ind. Eng. Chem. Res. 2001, 40, 1133–1139. [Google Scholar] [CrossRef]
- Gharibeh, M.; Tompsett, G.A.; Conner, W.C. Microwave reaction enhancement: The rapid synthesis of SAPO-11 molecular sieves. Top. Catal. 2008, 49, 157–166. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, J.; Huang, Y. Densification and grain growth of alumina by microwave processing. Mater. Lett. 1998, 37, 215–220. [Google Scholar] [CrossRef]
- Presenda, Á.; Salvador, M.D.; Penaranda-Foix, F.L.; Catalá-Civera, J.M.; Pallone, E.; Ferreira, J.; Borrell, A. Effects of microwave sintering in aging resistance of zirconia-based ceramics. Chem. Eng. Process. 2017, 122, 404–412. [Google Scholar] [CrossRef]
- Heuguet, R.; Marinel, S.; Thuault, A.; Badev, A. Effects of the Susceptor Dielectric Properties on the Microwave Sintering of Alumina. J. Am. Ceram. Soc. 2013, 96, 3728–3736. [Google Scholar] [CrossRef]
- Vallee, S.J.; Conner, W.C. Microwaves and Sorption on Oxides: A Surface Temperature Investigation. J. Phys. Chem. B 2006, 110, 15459–15470. [Google Scholar] [CrossRef] [PubMed]
- Vallee, S.J.; Conner, W.C. Effects of microwaves and microwave frequency on the selectivity of sorption for binary mixtures on oxides. J. Phys. Chem. C 2008, 112, 15483–15489. [Google Scholar] [CrossRef]
- Jobic, H.; Santander, J.E.; Conner, W.C.; Wittaker, G.; Giriat, G.; Harrison, A.; Ollivier, J.; Auerbach, S.M. Experimental evidence of selective heating of molecules adsorbed in nanopores under microwave radiation. Phys. Rev. Lett. 2011, 106, 157401. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Aris, R. The Distribution of Active ingredients in Supported Catalysts Prepared by Impregnation. Catal. Rev. Sci. Eng. 1985, 27, 207–340. [Google Scholar] [CrossRef]
- Nelmark, A.V.; Kheifez, L.I.; Fenelonov, V.B. Theory of Preparation of Supported Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 439–450. [Google Scholar] [CrossRef]
- Bond, G.; Moyes, R.B.; Pollington, S.D.; Whan, D.A. The Advantageous use of Microwave Radiation in the Preparation of Supported Nickel Catalysts. Stud. Surf. Sci. Catal. 1993, 75, 1805–1808. [Google Scholar]
- Olevsky, E.A.; Maximenko, A.L.; Grigoryev, E.G. Ponderomotive effects during contact formation in microwave sintering. Modell. Simul. Mater. Sci. Eng. 2013, 21, 055022. [Google Scholar] [CrossRef]
- Stuerga, D.; Gaillard, P. Microwave heating as a new way to induce localized enhancements of reaction rate. Non-isothermal and heterogeneous kinetics. Tetrahedron 1996, 52, 5505–5510. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Microwave dielectric heating behavior of supported MoS2 and Pt catalysts. Ind. Eng. Chem. Res. 2001, 40, 2810–2817. [Google Scholar] [CrossRef]
- Auvray, X.P.; Olsson, L. Sulfur dioxide exposure: A way to improve the oxidation catalyst performance. Ind. Eng. Chem. Res. 2013, 52, 14556–14566. [Google Scholar] [CrossRef]
- Mihai, O.; Fathali, A.; Auvray, X.; Olsson, L. DME, propane and CO: The oxidation, steam reforming and WGS over Pt/Al2O3. The effect of aging and presence of water. Appl. Catal. B 2014, 160–161, 480–491. [Google Scholar] [CrossRef]
- Durka, T.; van Gerven, T.; Stankiewicz, A. Microwaves in heterogeneous gas-phase catalysis: Experimental and numerical approaches. Chem. Eng. Technol. 2009, 32, 1301–1312. [Google Scholar] [CrossRef]
- Atlas, L.M.; Nagao, H.; Nakamura, H.H. Control of Dielectric Constant and Loss in Alumina Ceramics. J. Am. Ceram. Soc. 1962, 45, 464–471. [Google Scholar] [CrossRef]



| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Drying | MW | MW | MW | MW | CH | CH | CH |
| Calcination | CH | MW | MW | MW | CH | CH | CH |
| Aging (MW) | – | – | 700 °C | 800 °C | * 600 °C | 700 °C | 800 °C |
| Catalyst | Drying | CH | MW | MW | CH | CH | CH | MW |
| Calcination | CH | CH | MW | CH | CH | CH | MW | |
| Aging (°C) | – | – | – | MW600 | MW700 | MW800 | MW800 | |
| BET Surface Area (m2/g) | 147 | 147 | 151 | 144 | 144 | 138 | 137 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auvray, X.; Thuault, A. Effect of Microwave Drying, Calcination and Aging of Pt/Al2O3 on Platinum Dispersion. Catalysts 2018, 8, 348. https://doi.org/10.3390/catal8090348
Auvray X, Thuault A. Effect of Microwave Drying, Calcination and Aging of Pt/Al2O3 on Platinum Dispersion. Catalysts. 2018; 8(9):348. https://doi.org/10.3390/catal8090348
Chicago/Turabian StyleAuvray, Xavier, and Anthony Thuault. 2018. "Effect of Microwave Drying, Calcination and Aging of Pt/Al2O3 on Platinum Dispersion" Catalysts 8, no. 9: 348. https://doi.org/10.3390/catal8090348