1. Introduction
Graphitic carbon nitride (g-C
3N
4), as a fascinating conjugated polymer, has become a hot research topic [
1]. As a metal-free photocatalytic semiconductor, g-C
3N
4 is very promising in energy storage and environmental remediation [
2]. When g-C
3N
4 is implemented in photocatalytic water treatment, one of the hindrances is the hydrophobic properties of the surface. Photocatalysis is a process that occurs at the interface between the liquid and the solid phase, therefore, the adsorption of water as well as of the target pollutants is the first step and very significant. Surface modification may be an effective approach to improve the adsorption of water onto the surface of g-C
3N
4. Hydroxyl functional groups may be potential candidates in improving the hydrophilicity of C
3N
4 [
3]. Our group had reported on the surface modification of C
3N
4 with hydroxyls using hydrogen peroxide (H
2O
2), and found that the photocatalytic activity of C
3N
4 was promoted [
4]. Additionally, as reported by Li et al., that the photocatalytic activity of C
3N
4 treated in an ammonia solution was improved due to the hydroxyl surface modification [
5]. Motivated by this work, we herein developed a hydrothermal method to modify C
3N
4 by the addition of hydroxyls onto the surface. The pH of the hydrothermal treatment solution was explored, and the insight into the photocatalytic activity improvement was performed and discussed in this work.
2. Results and Discussions
The morphology of the as-prepared samples was investigated through TEM. As shown in the TEM image of C
3N
4 (
Figure 1a), a layered structure was observed. Due to the ultrathin structure, the edge of the prepared C
3N
4 is partially curly, which is intended to decrease the surface tension. This is typical of the construction of exfoliated carbon scrolls by peeling graphite [
6]. After hydrothermal treatment, the morphology as shown in
Figure 1b was negligibly influenced. The crystal structure of pure C
3N
4 as shown in
Figure 1c, two obvious peaks can be observed at the 2θ of 13.0° and 27.5°, which agree well with the hexagonal phase of graphitic C
3N
4 (JCPDS 87-1526) [
2]. With hydroxyl group modifications, as shown in
Figure 1c, the crystal structure of C
3N
4 was negligibly influenced, indicating that the hydrothermal treatment may not influence the crystal structure of C
3N
4. The O 1s high-resolution spectra in
Figure 1d can be fitted with a single peak located at 529.44 eV for g-C
3N
4, corresponding to the O-H bond. After hydrothermal treatment, the peak was negatively shifted by 0.66 eV, which may have resulted from the hydroxyl group being bonded with g-C
3N
4. The optical properties of the as-prepared samples were investigated using DRS, as plotted in
Figure 1e, and a rapid increase of the intensity was found with a decrease in the wavelength of the incident to 490 nm. This may be due to the band transition. To evaluate the band gap energy for each sample, the classical Tauc equation was used as plotted in
Figure 1f. It was found that the band gap for C
3N
4, as well as for hydroxyl-modified C
3N
4, was approximately equivalent to 2.54 eV. This confirms that there are no doped impurity energy levels within the band gap after the hydroxyl surface modification.
To further explore the composition of the hydroxyl-modified C
3N
4, FT-IR spectra were used, and plotted in
Figure 2a. The peak located at 810 cm
−1 was assigned to the typical breathing mode of a s-triazine ring, and the peak located at 890 cm
−1 may be due to the out-of-plane bending mode of N-H. A bunch of peaks located at the range of 1200–1700 cm
−1 were ascribed to the stretching vibration modes of C-N and C=N heterocycles. The broad peak at 3000–3250 cm
−1 may have resulted from the N-H stretching vibration of uncondensed amine and hydroxyl groups [
7]. It can be found that the intensity of this peak increased when the pH of the solution increased. This phenomenon may be due to more hydroxyl groups being adsorbed on the surface of C
3N
4 in a solution with a higher pH; and the amine groups being possibly dissolved in a solution with a lower pH during the hydrothermal treatment, resulting in the reduction of the intensity of this peak. This is also confirmed by the fact that hydrothermal treatment in a solution with a high pH favors the addition of more hydroxyls onto C
3N
4. Zeta potentials for the as-prepared samples (
Figure 2b) were negatively shifted with an increase in the pH of the solution in the hydrothermal treatment. This suggests that the zeta potentials became more negative as the amount of hydroxyl groups increased. The more negatively charged surface of the catalyst favors the adsorption of organic pollutants in water, which improves the photocatalytic activity.
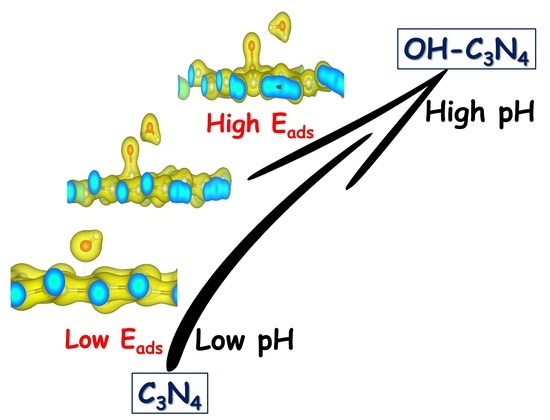
To further determine the mechanism of the hydroxyl modifications, DFT simulation was conducted, and the results were shown in
Table 1. To determine the site on hydroxyl-bonded C
3N
4, the free energies of the crystals were calculated. It was found that the free energy of the crystal with OH that was bonded to the carbon of C
3N
4 (OH-C) was lower than that of the crystal with OH that was bonded to the nitrogen of C
3N
4 (OH-N). This suggests that the crystal may be more stable when the OH was bonded with the carbon of C
3N
4. The distance between H
2O and the site on C
3N
4 was also measured, and it was found that the distance was shortened when the C
3N
4 was modified with –OH. The adsorption energy of H
2O also increased with the modification of –OH onto C
3N
4. This suggests that the hydroxyl modification may be very promising in the improvement of the photocatalytic activity of C
3N
4 by enhancing the adsorption between the H
2O/organic and the photocatalyst surface. To further confirm this verdict, the photocatalytic activity of the as-prepared samples was evaluated in the decomposition of phenol under visible light, the results of which were shown in
Figure 3. It was found that with hydroxyl modification, both the conversion of phenol and the TOC (total organic carbon) in the solution was enhanced. In addition, the improvement also increased with an increase in the number of hydroxyls on the surface. This provides solid evidence that hydroxyl modification was very effective in enhancing the photocatalytic activity of C
3N
4 through the improvement of the adsorption capacity.
3. Experimental
All chemicals were purchased from Fisher Scientific (Canada) unless otherwise mentioned, and used as received. C
3N
4 was prepared by directly heating melamine in a semi-closed system to prevent the sublimation of melamine [
8]. Typically, 10 g of melamine powder, in a crucible with a cover, was heated up to 550 °C in a muffle furnace with a heating rate of 2.5 °C/min, and the further deamination treatment was performed for 4 h. After the crucible was naturally cooled down to room temperature, the yellowish powder was mounted and collected for further use. The surface modification of C
3N
4 with hydroxyls was conducted by hydrothermal treatment. Typically, 0.3 g of C
3N
4 was first dispersed into a 35 mL solution under ultrasonication for 30 min. Then, the suspension was transferred to a 50 mL Teflon-lined autoclave, which was heated up to 160 °C for 4 h. The autoclave was then allowed to naturally cool down to room temperature, and the precipitates in the suspension were separated out through centrifugation, and washed with deionized distilled water, several times. To explore the effect of the pH of the solution in the hydrothermal treatment, it was adjusted using NaOH (1 mol/L) and HNO
3 (1 mol/L).
Morphology of the as-prepared C
3N
4 was investigated by a transmission electron microscope (TEM, JEM-2100F). Crystal structures of the as-prepared samples were explored using an X-ray powder diffraction instrument (Bruker-AXS, Karlsruhe, Germany). An ultraviolet-visible light diffuse reflectance spectrophotometer (DRS) was employed to determine the optical properties of the as-prepared samples. Fourier transform infrared spectroscopy (FTIR) was also done at room temperature with a Cary 630 (Agilent Technologies). Zeta potentials of hydroxyl-modified C
3N
4 were tested using a Zetasizer (Malvern Panalytical). DFT simulations were performed using the Vienna ab initio simulation package (VASP) [
9,
10]. The ion-electron interaction is described with the projector augmented wave (PAW) method [
11]. The electron exchange-correlation is represented by the Perdew, Burke, and Ernzerhof (PBE) function of the generalized gradient approximation (GGA) [
12]. A cut-off energy of 450 eV was used for the plane-wave basis set. The calculations were done on periodical supercells of the C
3N
4 monolayer with a 3 × 3 × 1 Monkhorst Pack k-point sampling. The charge density distribution and the distance between bonds were analyzed using VESTA [
13].
The photocatalytic activity of as-prepared samples was evaluated with regard to the degradation of phenol under visible light irradiation. The light source was provided by a 300-W halogen tungsten projector lamp (Ushio) equipped with a UV cut-off (Kenko Zeta, transmittance > 90%) to filter out wavelength below 400 nm. Typically, in each test, 0.10 g of photocatalyst was added into a 100 mL of phenol solution with an initial concentration of 10 mg (phenol)/L (pH 4.5). The mixture was magnetically stirred in the dark for 1 h to reach an adsorption/desorption equilibrium prior to the photocatalysis experiment. After 3 h, 1 mL aliquots were drawn and centrifuged. The supernatant was analyzed using a high-performance liquid chromatograph (HPLC, Agilent 1100 series) equipped with a UV-Vis detector. The total organic carbon (TOC) was evaluated on an Appollo 9000 TOC analyzer equipped with a non-dispersive Infra-Red (NDIR) detector. The combustion temperature was fixed at 750 °C. To explore the repeatability of the activity test, the photocatalytic activity for each sample was repeated for three times.