Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts
Abstract
:1. Introduction
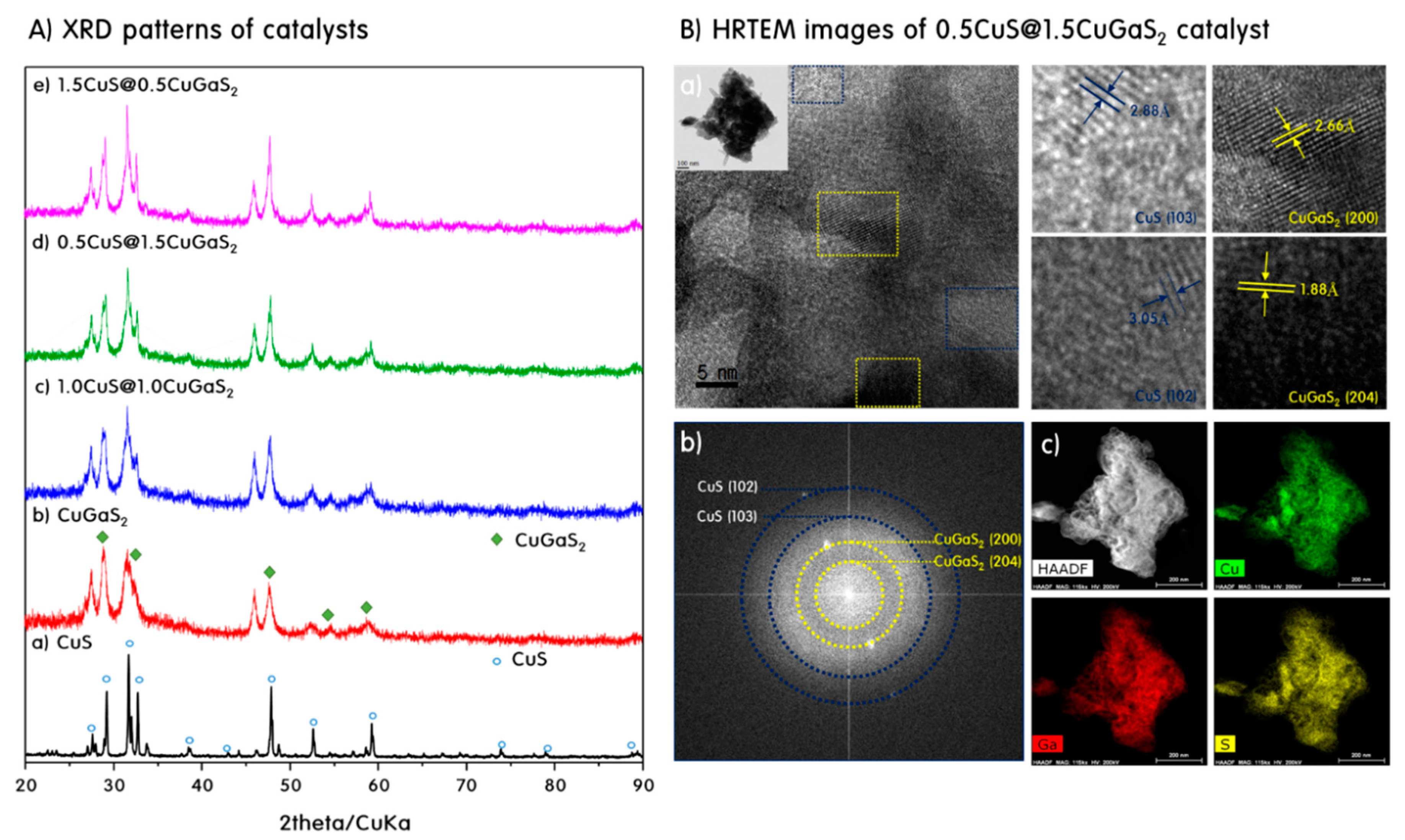
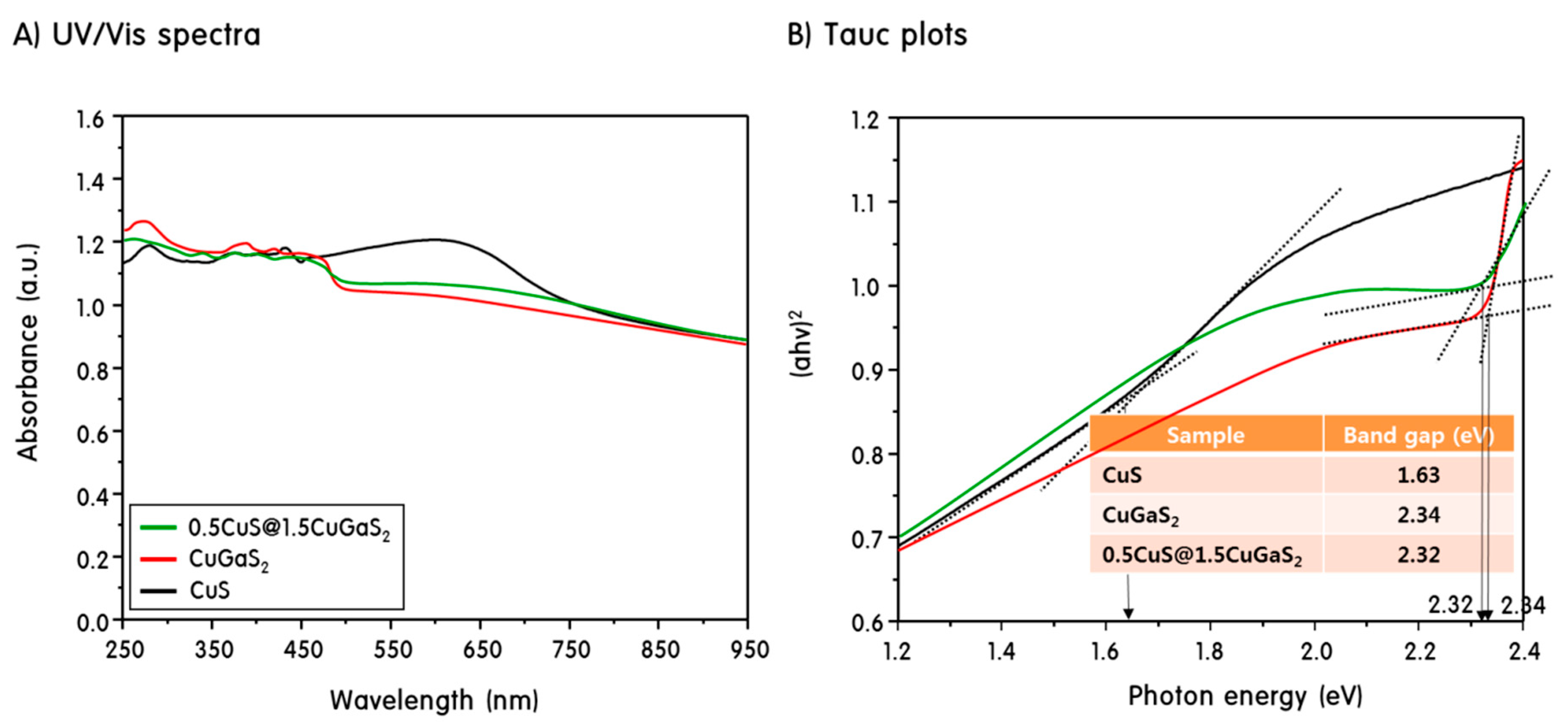
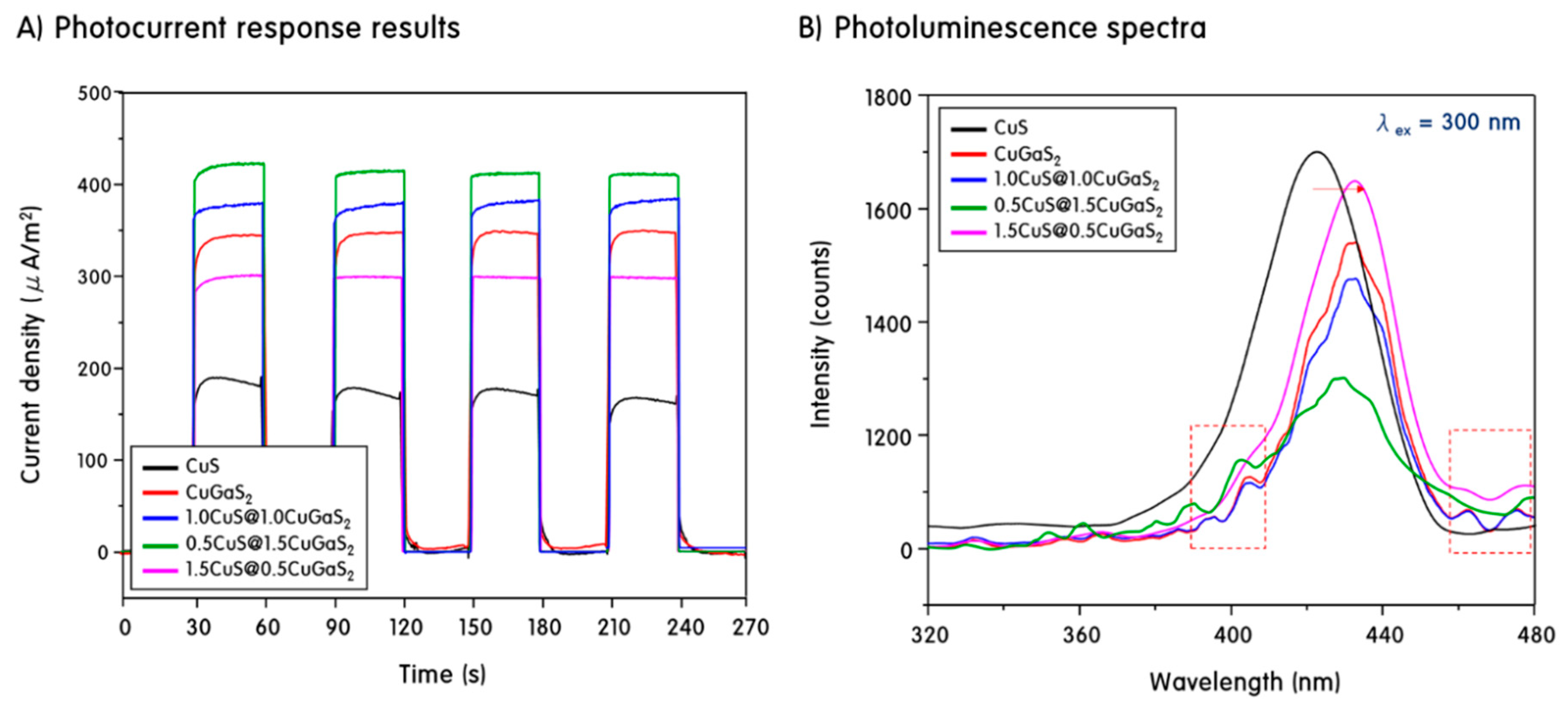
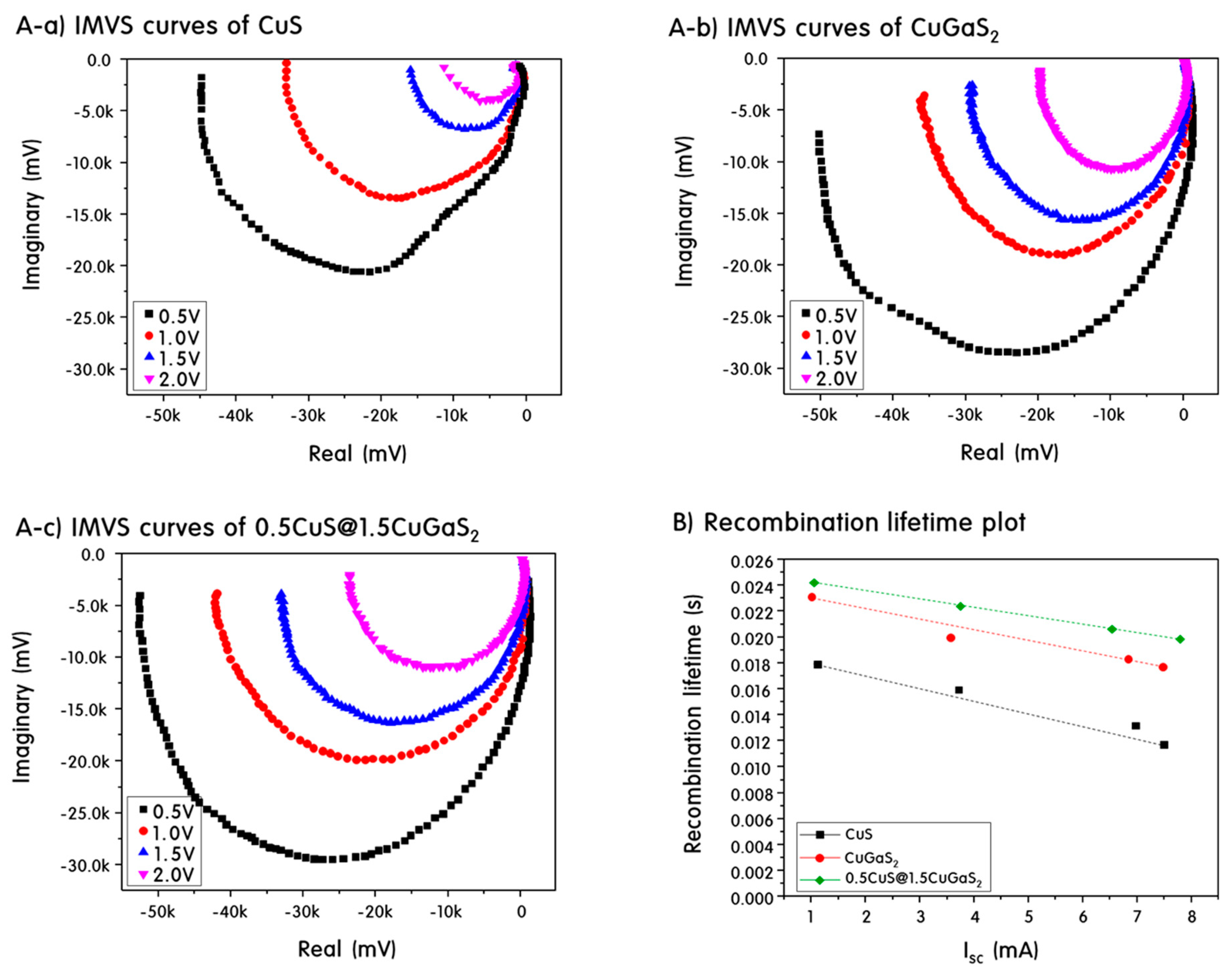
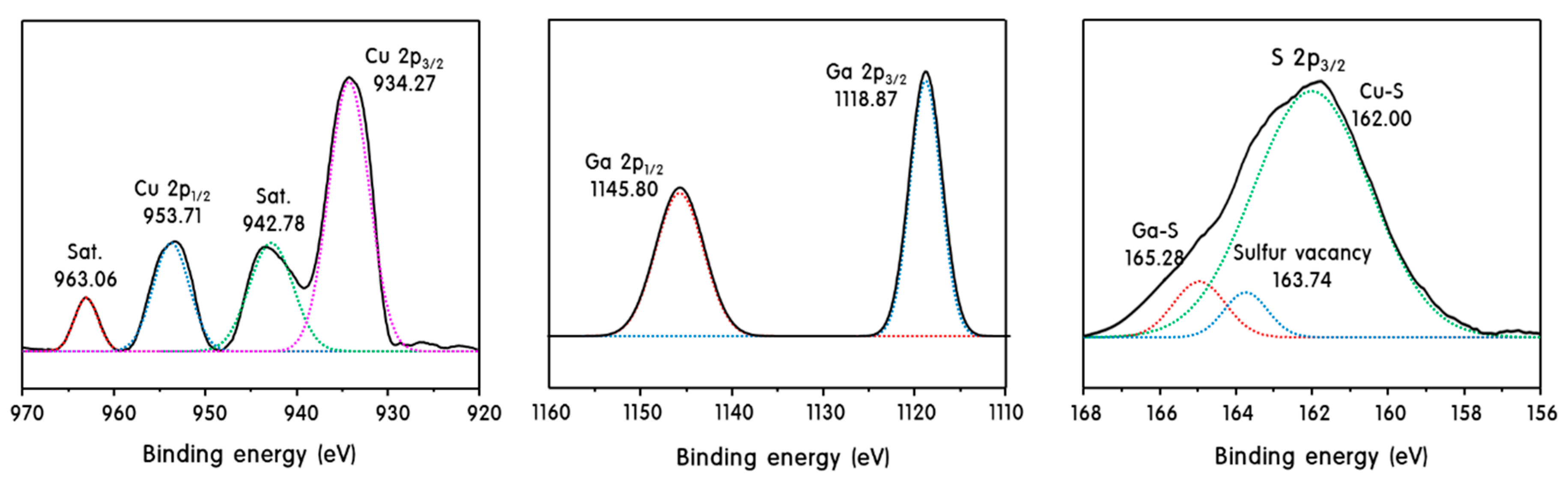
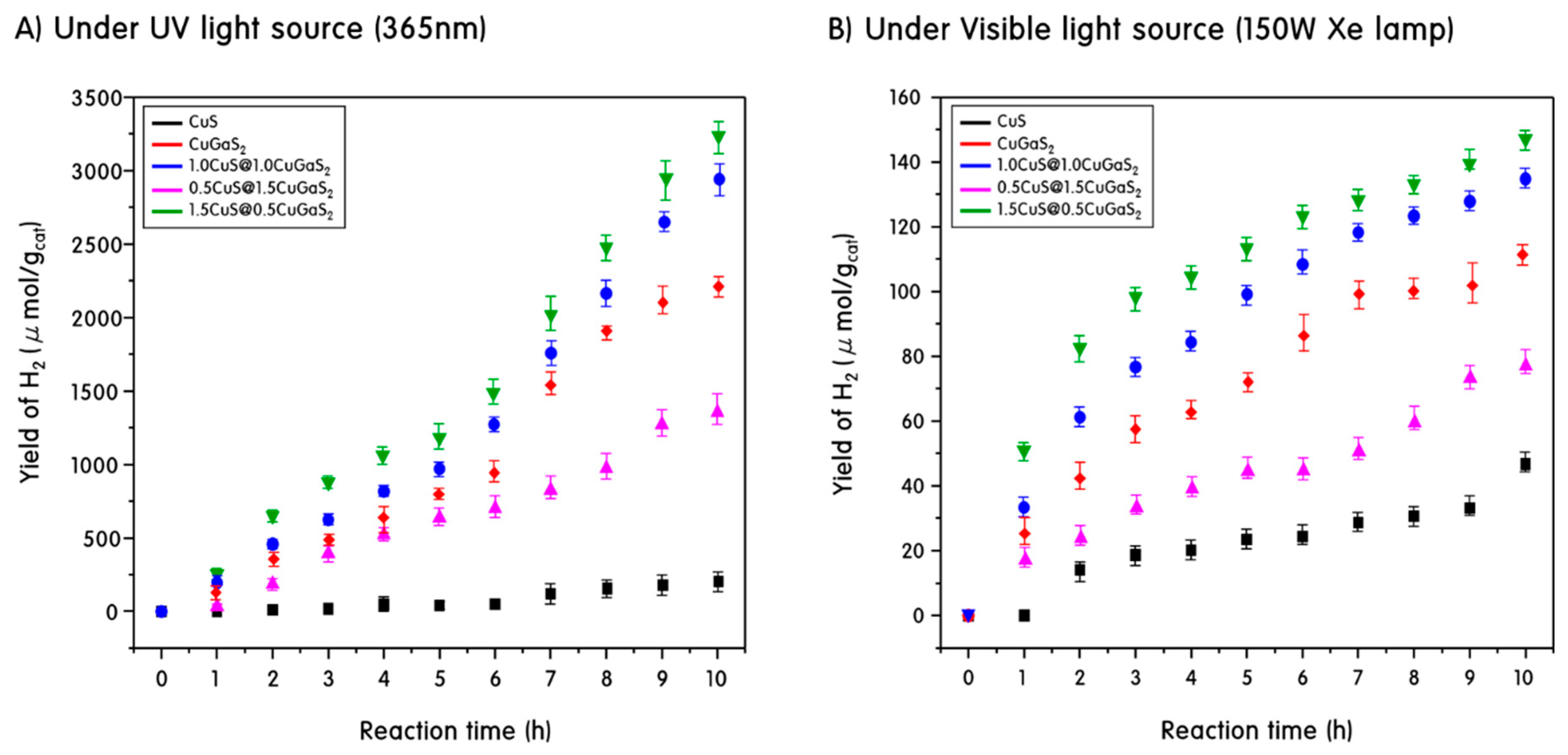
2. Results and Discussion
Characteristics of CuS, CuGaS2 and CuS@CuGaS2 Nanoparticles
- (1)
- 2 S2− + 2 h+ → S22−
- (2)
- S2− + 2h+ → S
3. Experimental
3.1. Preparation of CuS, CuGaS2 and CuS@CuGaS2 Nanoparticles
3.2. Characterization of CuS, CuGaS2 and CuS@CuGaS2 Nanoparticles
3.3. Hydrogen Production by Photo Splitting of Methanol Aqueous Solution Using CuS, CuGaS2 and Heterojunction CuS@CuGaS2 Catalyst
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stam, W.; Gradmann, S.; Altantzis, T.; Ke, X.; Baldus, M.; Bals, S.; Donega, C. Shape Control of Colloidal Cu2-xS Polyhedral Nanocrystals by Tuning the Nucleation Rates. Chem. Mater. 2016, 28, 6705–6715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Burda, C. Development of plasmonic semiconductor nanomaterials with copper chalcogenides for a future with sustainable energy materials. Energy Environ. Sci. 2012, 5, 5564–5577. [Google Scholar] [CrossRef]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified copper sulfide (Cu2-xS) micro /nanostructures: A comprehensive review on synthesis, modifications and applications. Nanoscale 2017, 9, 11357–11404. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 nm Fe3O4@Cu2-xS Core−Shell Nanoparticles for Dual-Modal Imaging and Photothermal Therapy. J. Am. Chem. Soc. 2013, 135, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, A.L.; Ramasamy, K.; Malik, M.A.; O’Brien, P.; Haigh, S.J.; Raftery, J. New routes to copper sulfide nanostructures and thin films. J. Mater. Chem. 2011, 21, 17888–17895. [Google Scholar] [CrossRef]
- Wang, N.; Gao, C.; Han, Y.; Huang, X.; Xu, Y.; Cao, X. Detection of human immunoglobulin G by label-free electrochemical immunoassay modified with ultralong CuS nanowires. J. Mater. Chem. B 2015, 3, 3254–3259. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Ding, Y.; Xiong, Y.; Xie, Y. Solution-phase template approach for the synthesis of Cu2S nanoribbons. Dalton Trans. 2006, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Rosei, F. Hollow micro/nanostructured materials prepared by ion exchange synthesis and their potential applications. New J. Chem. 2014, 38, 1883–1904. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Z.; Lei, Y.; Ge, S.; Zhang, Y.; Zhang, Y.; Wong, K.W.; Yang, R.; Lau, W.M. Design and growth of dendritic Cu2-xSe and bunchy CuSe hierarchical crystalline aggregations. CrystEngComm 2010, 12, 1856–1861. [Google Scholar] [CrossRef]
- Jayasekharan, T.; Gupta, S.L.; Dhiman, V. Binding of Cu+ and Cu2+ with peptides: Peptides = oxytocin, Arg8-vasopressin, bradykinin, angiotensin-I, substance-P, somatostatin, and neurotensin. J. Mass Spectrom. 2018, 53, 296–313. [Google Scholar] [CrossRef]
- Sant, S.B. Nanoparticles: From Theory to Applications, Gunter Schmid, 2nd ed.; Wiley-VCH Verlag: Weinheim, Germany, 2010; ISBN 978-3-527-32589-4. [Google Scholar]
- Saranya, M.; Santhosh, C.; Ramachandran, R.; Kollu, P.; Saravanan, P.; Vinoba, M.; Jeong, S.K.; Grace, A.N. Hydrothermal growth of CuS nanostructures and its photocatalytic properties. Powder Technol. 2014, 252, 25–32. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, J.; Zhang, Y.; Li, Q.; Gong, J.R. Visible light photocatalytic H2-production activity of CuS/ZnS porous nanosheets based on photoinduced interfacial charge transfer. Nano Lett. 2011, 11, 4774–4779. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwak, B.S.; Park, N.-K.; Baek, J.-I.; Ryu, H.-J.; Kang, M. Assembly of a check-patterned CuSx-TiO2 film with an electron-rich pool and its application for the photoreduction of carbon dioxide to methane. Appl. Surf. Sci. 2017, 393, 385–396. [Google Scholar] [CrossRef]
- Salak, A.N.; Ignatenko, O.V.; Zhaludkevich, A.L.; Lisenkov, A.D.; Starykeyich, M.; Zheludkevich, M.; Ferreira, M.G.S. High-pressure zinc oxysulphide phases in the ZnO–ZnS system. Phys. Status Solidi 2015, 212, 791–795. [Google Scholar] [CrossRef]
- Yue, M.; Wang, R.; Ma, B.; Cong, R.; Gao, W.; Yang, T. Superior performance of CuInS2 for photocatalytic water treatment: Full conversion of highly stable nitrate ions into harmless N2 under visible light. Catal. Sci. Technol. 2016, 6, 8300–8309. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Jajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Do, J.Y.; Chava, R.K.; Kim, S.K.; Nahm, K.; Park, N.-K.; Hong, J.-P.; Lee, S.J.; Kang, M. Fabrication of core@interface:shell structured CuS@CuInS2:In2S3 particles for highly efficient solar hydrogen production. Appl. Surf. Sci. 2018, 451, 86–98. [Google Scholar] [CrossRef]
- Chae, J.; Lee, J.; Jeong, J.H.; Kang, M. Hydrogen Production from Photo Splitting of Water Using the Ga-incorporated TiO2s Prepared by a Solvothermal Method and Their Characteristics. Bull. Korean Chem. Soc. 2009, 30, 302–309. [Google Scholar]
- Ravi, S.; Gopi, C.V.V.M.; Kim, H. Enhanced electrochemical capacitance of polyimidazole coated covellite CuS dispersed CNT composite materials for application in supercapacitors. Dalton Trans. 2016, 45, 12362–12372. [Google Scholar] [CrossRef]
- Kundu, J.; Pradhan, D. Influence of precursor concentration, surfactant and temperature on the hydrothermal synthesis of CuS: Structural, thermal and optical properties. New J. Chem. 2013, 37, 1470–1479. [Google Scholar] [CrossRef]
- Liu, S.; Nie, L.; Yuan, R. Growth, structure and optical characterization of CuGaS2 thin films obtained by spray pyrolysis. Chalcogenide Lett. 2015, 12, 111–116. [Google Scholar]
- Kimi, M.; Yuliati, L.; Shamsuddin, M. Preparation of High Activity Ga and Cu Doped ZnS by Hydrothermal Method for Hydrogen Production under Visible Light Irradiation. J. Nanomater. 2015, 2015, 200. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Fullmann, T.; Galucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Do, J.Y.; Lee, J.H.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Synthesis and characterization of Ni2-xPdxMnO4/γ-Al2O3 catalysts for hydrogen production via propane steam reforming. Chem. Eng. J. 2018, 334, 1668–1678. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, I.; Tseng, Y.-H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Huxter, V.M.; Mirkovic, T.; Nair, S.; Scholes, G.D. Demonstration of bulk semiconductor optical properties in processable Ag2S and EuS nanocrystalline systems. Adv. Mater. 2008, 20, 2439–2443. [Google Scholar] [CrossRef]
- Maeda, T.; Yu, Y.; Chen, Q.; Ueda, K.; Wada, T. Crystallographic and optical properties and band diagrams of CuGaS2 and CuGa5S8 phases in Cu-poor Cu2S-Ga2S3 pseudo-binary system. Jpn. J. Appl. Phys. 2017, 56, 04CS12. [Google Scholar] [CrossRef]
- Aliaga, J.; Cifuentes, N.; González, G.; Sotomayor-Torres, C.; Benavente, E. Enhancement Photocatalytic Activity of the Heterojunction of Two-Dimensional Hybrid Semiconductors ZnO/V2O5. Catalysts 2018, 8, 374. [Google Scholar] [CrossRef]
- Tessler, N.; Rappaport, N. Excitation density dependence of photocurrent efficiency in low mobility semiconductors. J. Appl. Phys. 2004, 96, 1083–1087. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, W.K.; Chen, H.Y.; Gwo, S. Green, yellow, and orange defect emission from ZnO nanostructures: Influence of excitation wavelength. Appl. Phys. Lett. 2006, 88, 103107–103110. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F- Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Mehmood, F.; Iqbal, J.; Ismail, M.; Mehmood, A. Ni doped WO3 nanoplates: An excellent photocatalyst and novel nanomaterial for enhanced anticancer activities. J. Alloy. Compd. 2018, 746, 729–738. [Google Scholar] [CrossRef]
- Xie, Z.; Sui, Y.; Buckeridge, J.; Catlow, C.R.A.; Keal, T.W.; Sherwood, P.; Walsh, A.; Farrow, M.R.; Scanlon, D.O.; Woodley, S.M.; et al. Donor and Acceptor characteristics of Native Point Defects in GaN. arXiv, 2018; arXiv:1803.06273. [Google Scholar]
- Cen, J.; Wu, Q.; Liu, M.; Orlov, A. Devloping new understanding of photo-electrochemical water splitting via in-situ techniques: A review on recent progress. Green Energy Environ. 2017, 2, 100–111. [Google Scholar] [CrossRef]
- Yang, Y.; Ri, K.; Mei, A.; Liu, L.; Hu, M.; Liu, T.; Li, X.; Han, H. The size effect of TiO2 Nanoparticles on a printable mesoscopic perovskite solar cell. J. Mater. Chem. A 2015, 3, 9103–9107. [Google Scholar] [CrossRef]
- Bavarian, M.; Nejati, S.; Lau, K.K.S.; Lee, D.; Soroush, M. Theoretical and Experimental study of a Dye-Sensitized Solar Cell. Ind. Eng. Chem. Res. 2014, 53, 5234–5247. [Google Scholar] [CrossRef]
- Ludwing, J.; An, L.; Pattengale, B.; Kong, Q.; Zhang, X.; Xi, P.; Huang, J. Ultrafast hole trapping and relaxation dynamics in p-type CuS nanodisks. J. Phys. Chem. Lett. 2015, 6, 2671–2675. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Zhu, L.; Pan, Z.; Jiang, Y.; Wang, P.; Zhou, Y.-N.; Fang, F.; Hu, L.; Sun, D. CuGaS2 nanoplates: A robust and self-healing anode for Li/Na ion batteries in a wide temperature range of 268–318 K. J. Mater. Chem. A 2018, 6, 10886–11093. [Google Scholar] [CrossRef]
- Dong, J.; Zeng, X.; Xia, W.; Zhang, X.; Zhou, M.; Wang, C. Ferromagnetic behavior of non-stoichiometric ZnS microspheres with a nanoplate-netted surface. RSC Adv. 2017, 7, 20874–20881. [Google Scholar] [CrossRef] [Green Version]
- Do, J.Y.; Choi, S.; Nahm, K.; Kim, S.K.; Kang, M. Reliable hydrogen production from methanol photolysis in aqueous solution by a harmony between In and Zn in bimetallic zinc indium sulfide. Mater. Res. Bull. 2018, 100, 234–242. [Google Scholar] [CrossRef]
- Cui, H.; Li, B.; Li, Z.; Li, X.; Xu, S. Z-scheme based CdS/CdWO4 heterojunction visible light photocatalyst for dye degradation and hydrogen evolution. Appl. Surf. Sci. 2018, 455, 831–840. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous Photocatalytic Decomposition of Saturated Carboxylic Acids on TiO2 Powder. Decarboxylative Route to Alkanes. J. Am. Chem. Soc. 1978, 100, 5985–5992. [Google Scholar] [CrossRef]
- Peña-Garcia, R.; Guerra, Y.; Farias, B.V.M.; Martínez Buitrago, D.; Franco, A., Jr.; Padrón-Hernández, E. Effects of temperature and atomic disorder on the magnetic phase transitions in ZnO nanoparticles obtained by sol-gel method. Mater. Lett. 2018, 233, 146–148. [Google Scholar]
- Zhang, R.; Xu, J.; Lu, C.; Xu, Z. Photothermal application of SmCoO3/SBA-15 catalysts synthesized by impregnation method. Mater. Lett. 2018, 228, 199–202. [Google Scholar] [CrossRef]
- Son, N.; Do, J.Y.; Kang, M. Characterization of core@shell-structured ZnO@Sb2S3 particles for effective hydrogen production from water photo spitting. Ceram. Int. 2017, 43, 11250–11259. [Google Scholar] [CrossRef]


| Catalysts/Elements | Cu | Ga | S |
|---|---|---|---|
| CuS | 46.74 | - | 53.26 |
| CuGaS2 | 31.46 | 27.26 | 41.27 |
| [email protected]2 | 32.99 | 23.06 | 43.95 |
| [email protected]2 | 15.00 | 36.58 | 48.43 |
| [email protected]2 | 41.05 | 18.31 | 40.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, N.; Heo, J.N.; Youn, Y.-S.; Kim, Y.; Do, J.Y.; Kang, M. Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts. Catalysts 2019, 9, 41. https://doi.org/10.3390/catal9010041
Son N, Heo JN, Youn Y-S, Kim Y, Do JY, Kang M. Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts. Catalysts. 2019; 9(1):41. https://doi.org/10.3390/catal9010041
Chicago/Turabian StyleSon, Namgyu, Jun Neoung Heo, Young-Sang Youn, Youngsoo Kim, Jeong Yeon Do, and Misook Kang. 2019. "Enhancement of Hydrogen Productions by Accelerating Electron-Transfers of Sulfur Defects in the CuS@CuGaS2 Heterojunction Photocatalysts" Catalysts 9, no. 1: 41. https://doi.org/10.3390/catal9010041