Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts
Abstract
:1. Introduction
2. Results
2.1. Nitrogen Physisorption Isotherms
2.2. Powder X-Ray Diffraction
2.3. Thermogravimetric Analysis
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
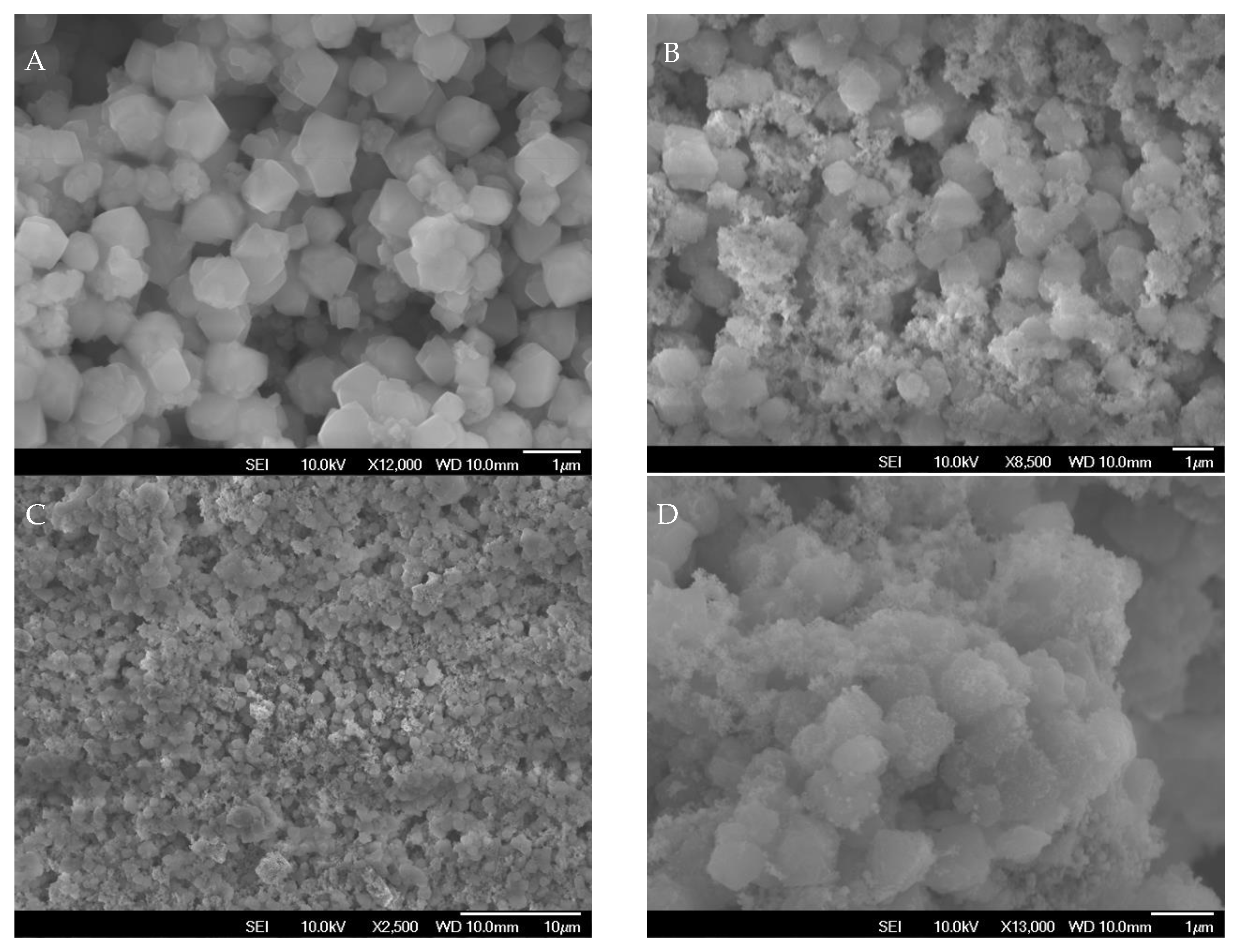
2.5. Field-Emission Scanning Electron Microscopy
2.6. Deoxygenation of Stearic Acid over Co/NaX in N2
3. Discussion
3.1. Effect of Catalyst Loading and Temperature
3.2. Catalyst Recyclability
3.3. Comparison of the State-of-the-Art of Cobalt-Based Catalysts
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Zeolite NaX Synthesis
4.3. Co/NaX Ion-Exchange Synthesis
4.4. Catalyst Characterization
4.5. Catalytic Reaction: Deoxygenation Experiments
4.6. Liquid Product Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, N.; Cao, Y.; Li, J.; Guo, W.; Zhao, Z. Preparation of Ni2P/Zr-MCM-41 catalyst and its performance in the hydrodeoxygenation of Jatropha curcas oil. J. Fuel Chem. Technol. 2016, 44, 76–83. [Google Scholar] [CrossRef]
- Yasir, M.; Azizan, M.T.; Ramli, A.; Ameen, M. Hydroprocessing of Crude Jatropha Oil Using Hierarchical Structured TiO2 Nanocatalysts. Procedia Eng. 2016, 148, 275–281. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Demirbas, A. Diesel-like fuel from tallow by pyrolysis and supercritical water liquefaction. Energy Sources Part A 2009, 31, 824–830. [Google Scholar] [CrossRef]
- Huber, G.W.; O’Connor, P.; Corma, A. Processing biomass in conventional oil refineries: Production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A Gen. 2007, 329, 120–129. [Google Scholar] [CrossRef]
- Miao, C.; Marin-Flores, O.; Dong, T.; Gao, D.; Wang, Y.; Garcia-Pérez, M.; Chen, S. Hydrothermal Catalytic Deoxygenation of Fatty Acid and Bio-oil with in Situ H2. ACS Sustain. Chem. Eng. 2018, 6, 4521–4530. [Google Scholar] [CrossRef]
- Crawford, J.M.; Carreon, M.A. Decarboxylation of Diunsaturated Linoleic Acid to Heptadecane over Zeolite Supported Pt/ZIF-67 Catalysts. Ind. Eng. Chem. Res. 2018, 57, 15991–15997. [Google Scholar] [CrossRef]
- Snåre, M.; Kubičková, I.; Mäki-Arvela, P.; Chichova, D.; Eränen, K.; Murzin, D.Y. Catalytic deoxygenation of unsaturated renewable feedstocks for production of diesel fuel hydrocarbons. Fuel 2008, 87, 933–945. [Google Scholar] [CrossRef]
- Besse, X.; Schuurman, Y.; Guilhaume, N. Hydrothermal conversion of linoleic acid and ethanol for biofuel production. Appl. Catal. A Gen. 2016, 524, 139–148. [Google Scholar] [CrossRef]
- Kiméné, A.; Wojcieszak, R.; Paul, S.; Dumeignil, F. Catalytic decarboxylation of fatty acids to hydrocarbons over non-noble metal catalysts: The state of the art. J. Chem. Technol. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.W.; Van Haveren, J.; De Jong, K.P.; Bitter, J.H.; Van Es, D.S. Reaction pathways for the deoxygenation of vegetable oils and related model compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Chowdhury, M.B.; Jhawar, A.K.; Xu, W.Z.; Charpentier, P.A. Continuous low pressure decarboxylation of fatty acids to fuel-range hydrocarbons with in situ hydrogen production. Fuel 2018, 212, 470–478. [Google Scholar] [CrossRef]
- Ma, B.; Hu, J.; Wang, Y.; Zhao, C. Ni nanoparticles encapsulated into mesoporous single-crystalline HBEA: Application for drainage oil hydrodeoxygenation to diesel. Green Chem. 2015, 17, 4610–4617. [Google Scholar] [CrossRef]
- Ma, B.; Yi, X.; Chen, L.; Zheng, A.; Zhao, C. Interconnected hierarchical HUSY zeolite-loaded Ni nano-particles probed for hydrodeoxygenation of fatty acids, fatty esters, and palm oil. J. Mater. Chem. A 2016, 4, 11330–11341. [Google Scholar] [CrossRef]
- Ma, B.; Zhao, C. High-grade diesel production by hydrodeoxygenation of palm oil over a hierarchically structured Ni/HBEA catalyst. Green Chem. 2015, 17, 1692–1701. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.; Yao, S.; Ma, D.; Yan, N. Effective deoxygenation of fatty acids over Ni(OAc)2 in the absence of H2 and solvent. Green Chem. 2015, 17, 4198–4205. [Google Scholar] [CrossRef]
- Chen, N.; Gong, S.; Qian, E.W. Effect of reduction temperature of NiMoO3-x/SAPO-11 on its catalytic activity in hydrodeoxygenation of methyl laurate. Appl. Catal. B Environ. 2015, 174–175, 253–263. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Guan, Y.; Xu, H.; Zhang, L.; Wu, P. Nickel/USY Catalyst Derived from a Layered Double Hydroxide/Zeolite Hybrid Structure with a High Hydrogenation Efficiency. ChemCatChem 2017, 9, 4552–4561. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Townsend, R.P.; Coker, E.N. Ion exchange in zeolites. Stud. Surf. Sci. Catal. 2001, 137, 467–524. [Google Scholar] [CrossRef]
- Garcia, I.; Solache-Rios, M.; Bosch, P.; Bulbulian, S. Cobalt2+ Ion Exchange with NaY. J. Phys. Chem. 1993, 97, 1249–1251. [Google Scholar] [CrossRef]
- Song, W.; Zhao, C.; Lercher, J.A. Importance of size and distribution of Ni nanoparticles for the hydrodeoxygenation of microalgae oil. Chem. Eur. J. 2013, 19, 9833–9842. [Google Scholar] [CrossRef] [PubMed]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use, 1st ed.; Wiley: New York, NY, USA, 1974; ISBN 978-0471099857. [Google Scholar]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A Gen. 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, W.; Chen, C.; Miao, H.; Ma, H.; Xu, J. Ni/NaX: A bifunctional efficient catalyst for selective hydrogenolysis of glycerol. Catal. Lett. 2010, 134, 184–189. [Google Scholar] [CrossRef]
- Benaliouche, F.; Boucheffa, Y.; Ayrault, P.; Mignard, S.; Magnoux, P. NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites. Microporous Mesoporous Mater. 2008, 111, 80–88. [Google Scholar] [CrossRef]
- Ahmadi, M.; Macias, E.E.; Jasinski, J.B.; Ratnasamy, P.; Carreon, M.A. Decarboxylation and further transformation of oleic acid over bifunctional, Pt/SAPO-11 catalyst and Pt/chloride Al2O3 catalysts. J. Mol. Catal. A Chem. 2014, 386, 14–19. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nambo, A.; Jasinski, J.B.; Ratnasamy, P.; Carreon, M.A. Decarboxylation of oleic acid over Pt catalysts supported on small-pore zeolites and hydrotalcite. Catal. Sci. Technol. 2015, 5, 380–388. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; He, T.; Hu, H.; Wu, J. Hydrodeoxygenation of dibenzofuran over noble metal supported on mesoporous zeolite. Catal. Commun. 2011, 12, 1201–1205. [Google Scholar] [CrossRef]
- Hong, D.Y.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Hydrodeoxygenation and coupling of aqueous phenolics over bifunctional zeolite-supported metal catalysts. Chem. Commun. 2010, 46, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, Y.; Luo, H.; Hu, T.; Liu, W. Amorphous Co-Mo-B catalyst with high activity for the hydrodeoxygenation of bio-oil. Catal. Commun. 2011, 12, 436–440. [Google Scholar] [CrossRef]
- Centeno, A.; Maggi, R.; Delmon, B. Use of noble metals in hydrodeoxygenation reactions. Stud. Surf. Sci. Catal. 1999, 127, 77–84. [Google Scholar] [CrossRef]
- Bui, V.N.; Laurenti, D.; Delichère, P.; Geantet, C. Hydrodeoxygenation of guaiacol. Part II: Support effect for CoMoS catalysts on HDO activity and selectivity. Appl. Catal. B 2011, 101, 246–255. [Google Scholar] [CrossRef]
- Valle, B.; Gayubo, A.G.; Alonso, A.; Aguayo, A.T.; Bilbao, J. Hydrothermally stable HZSM-5 zeolite catalysts for the transformation of crude bio-oil into hydrocarbons. Appl. Catal. B Environ. 2010, 100, 318–327. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zheng, Y. The role of cobalt and nickel in deoxygenation of vegetable oils. Appl. Catal. B Environ. 2014, 160–161, 415–422. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Choi, M. Controlled decationization of X zeolite: Mesopore generation within zeolite crystallites for bulky molecular adsorption and transformation. J. Mater. Chem. A 2013, 1, 12096–12102. [Google Scholar] [CrossRef]
- Li, N.; Bi, Y.; Xia, X.; Chen, H.; Hu, J. Hydrodeoxygenation of Methyl Laurate over Ni Catalysts Supported on Hierarchical HZSM-5 Zeolite. Catalysts 2017, 7, 383. [Google Scholar] [CrossRef]
- Tran, H.-L.; Kuo, M.-S.; Yang, W.-D.; Huang, Y.-C. Study on Modification of NaX Zeolites: The Cobalt (II)-Exchange Kinetics and Surface Property Changes under Thermal Treatment. J. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Yang, L.; Tate, K.L.; Jasinski, J.B.; Carreon, M.A. Decarboxylation of Oleic Acid to Heptadecane over Pt Supported on Zeolite 5A Beads. ACS Catal. 2015, 5, 6497–6502. [Google Scholar] [CrossRef]
- Wei, Y.; Parmentier, T.E.; de Jong, K.P.; Zečević, J. Tailoring and visualizing the pore architecture of hierarchical zeolites. Chem. Soc. Rev. 2015, 44, 7234–7261. [Google Scholar] [CrossRef] [Green Version]
- Díaz, E.; Ordóñez, S.; Vega, A.; Coca, J. Catalytic combustion of hexane over transition metal modified zeolites NaX and CaA. Appl. Catal. B Environ. 2005, 56, 313–322. [Google Scholar] [CrossRef]
- Ilić, B.; Wettstein, S.G. A review of adsorbate and temperature-induced zeolite framework flexibility. Microporous Mesoporous Mater. 2017, 239, 221–234. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Starace, A.K.; Black, B.A.; Lee, D.D.; Palmiotti, E.C.; Orton, K.A.; Michener, W.E.; ten Dam, J.; Watson, M.J.; Beckham, G.T.; Magrini, K.A.; et al. Characterization and Catalytic Upgrading of Aqueous Stream Carbon from Catalytic Fast Pyrolysis of Biomass. ACS Sustain. Chem. Eng. 2017, 5, 11761–11769. [Google Scholar] [CrossRef]
- Xing, S.; Lv, P.; Zhao, C.; Li, M.; Yang, L.; Wang, Z.; Chen, Y.; Liu, S. Solvent-free catalytic deoxygenation of oleic acid via nano-Ni/HZSM-5: Effect of reaction medium and coke characterization. Fuel Process. Technol. 2018, 179, 324–333. [Google Scholar] [CrossRef]
- Król, M.; Mozgawa, W.; Jastrzębski, W. Theoretical and experimental study of ion-exchange process on zeolites from 5-1 structural group. J. Porous Mater. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Regli, L.; Zecchina, A.; Vitillo, J.G.; Cocina, D.; Spoto, G.; Lamberti, C.; Lillerud, K.P.; Olsbye, U.; Bordiga, S. Hydrogen storage in Chabazite zeolite frameworks. Phys. Chem. Chem. Phys. 2005, 7, 3197–3203. [Google Scholar] [CrossRef]
- Bauer, F.; Karge, H.G. Characterization of Coke on Zeolites; Karge, H.G., Weitkamp, J., Eds.; Springer: Berlin, Germany, 2007; ISBN 978-3-540-44825-9. [Google Scholar]
- Triantafyllidis, K.; Lappas, A.; Stöcker, M. The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444563309. [Google Scholar]
- Schreiber, M.W.; Rodriguez-Nino, D.; Gutierrez, O.Y.; Lercher, J.A. Hydrodeoxygenation of fatty acid esters catalyzed by Ni on nano-sized MFI type zeolites. Catal. Sci. Technol. 2016, 6, 7976–7984. [Google Scholar] [CrossRef] [Green Version]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of triglycerides to hydrocarbons over supported metal catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Hermida, L.; Amani, H.; Abdullah, Z.A.; Mohamed, R.A. Deoxygenation of Palmitic Acid to Produce Diesel-like Hydrocarbons over Nickel Incorporated Cellular Foam Catalyst: A Kinetic Study. J. Adv. Chem. Eng. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Fu, J.; Shi, F.; Thompson, L.T.; Lu, X.; Savage, P.E. Activated carbons for hydrothermal decarboxylation of fatty acids. ACS Catal. 2011, 1, 227–231. [Google Scholar] [CrossRef]
- Wu, J.; Shi, J.; Fu, J.; Leidl, J.A.; Hou, Z.; Lu, X. Catalytic decarboxylation of fatty acids to aviation fuels over nickel supported on activated carbon. Sci. Rep. 2016, 6, 27820. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Aloise, A.; Bonura, G.; Gómez-Hortigüela, L.; Frusteri, L.; Cannilla, C.; Frusteri, F.; Giordano, G. New insights about coke deposition in methanol-to-DME reaction over MOR-, MFI- and FER-type zeolites. J. Ind. Eng. Chem. 2018, 68, 196–208. [Google Scholar] [CrossRef]
- Guisnet, M.; Magnoux, P. Organic chemistry of coke formation. Appl. Catal. A Gen. 2001, 212, 83–96. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Liu, Y.; Hou, X.; Zhang, R.; Tang, X. Coke deposition on Ni/HZSM-5 in bio-oil hydrodeoxygenation processing. Energy Fuels 2015, 29, 1722–1728. [Google Scholar] [CrossRef]
- Wilcox, J. Carbon Capture; Springer: New York, NY, USA, 2012; ISBN 9781461422150. [Google Scholar]
- Zhang, J.; Zhao, C. Development of a Bimetallic Pd-Ni/HZSM-5 Catalyst for the Tandem Limonene Dehydrogenation and Fatty Acid Deoxygenation to Alkanes and Arenes for Use as Biojet Fuel. ACS Catal. 2016, 6, 4512–4525. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, N.; Dai, W.; Guan, N.; Li, L. Construction of Bifunctional Co/H-ZSM-5 Catalysts for the Hydrodeoxygenation of Stearic Acid to Diesel-Range Alkanes. ChemSusChem 2018, 11, 2179–2188. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, E.; Cao, Y.; Liu, M.; Wu, K.; Yang, M.; Wu, Y. Tailoring product distribution during upgrading of palmitic acid over bi-functional metal/zeolite catalysts. Chem. Eng. Sci. 2017, 166, 262–273. [Google Scholar] [CrossRef]
- Soni, V.K.; Sharma, P.R.; Choudhary, G.; Pandey, S.; Sharma, R.K. Ni/Co-Natural Clay as Green Catalysts for Microalgae Oil to Diesel-Grade Hydrocarbons Conversion. ACS Sustain. Chem. Eng. 2017, 5, 5351–5359. [Google Scholar] [CrossRef]
- Srifa, A.; Viriya-Empikul, N.; Assabumrungrat, S.; Faungnawakij, K. Catalytic behaviors of Ni/γ-Al2O3 and Co/γ-Al2O3 during the hydrodeoxygenation of palm oil. Catal. Sci. Technol. 2015, 5, 3693–3705. [Google Scholar] [CrossRef]
- Liu, Q.; Bie, Y.; Qiu, S.; Zhang, Q.; Sainio, J.; Wang, T.; Ma, L.; Lehtonen, J. Hydrogenolysis of methyl heptanoate over Co based catalysts: Mediation of support property on activity and product distribution. Appl. Catal. B Environ. 2014, 147, 236–245. [Google Scholar] [CrossRef]
- Ochoa-Hernández, C.; Yang, Y.; Pizarro, P.; de la Pena, V.A.; Coronado, J.M.; Serrano, D.P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15. Catal. Today 2013, 210, 81–88. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, D.; Zhang, M.; Yang, R. Synthesis of NaX zeolite: Influence of crystallization time, temperature and batch molar ratio SiO2/Al2O3 on the particulate properties of zeolite crystals. Powder Technol. 2013, 235, 322–328. [Google Scholar] [CrossRef]
- Snåre, M.; Kubičková, I.; Mäki-Arvela, P.; Eränen, K.; Murzin, D.Y. Heterogeneous Catalytic Deoxygenation of Stearic Acid for Production of Biodiesel. Ind. Eng. Chem. Res. 2006, 45, 5708–5715. [Google Scholar] [CrossRef]
- Froment, G.F.; Bischoff, K.B.; De Wilde, J. Chemical Reactor Analysis and Design, 3rd ed.; Wiley: New York, NY, USA, 2011; ISBN 978-0470565414. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connel, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001; ISBN 978-0070116825. [Google Scholar]

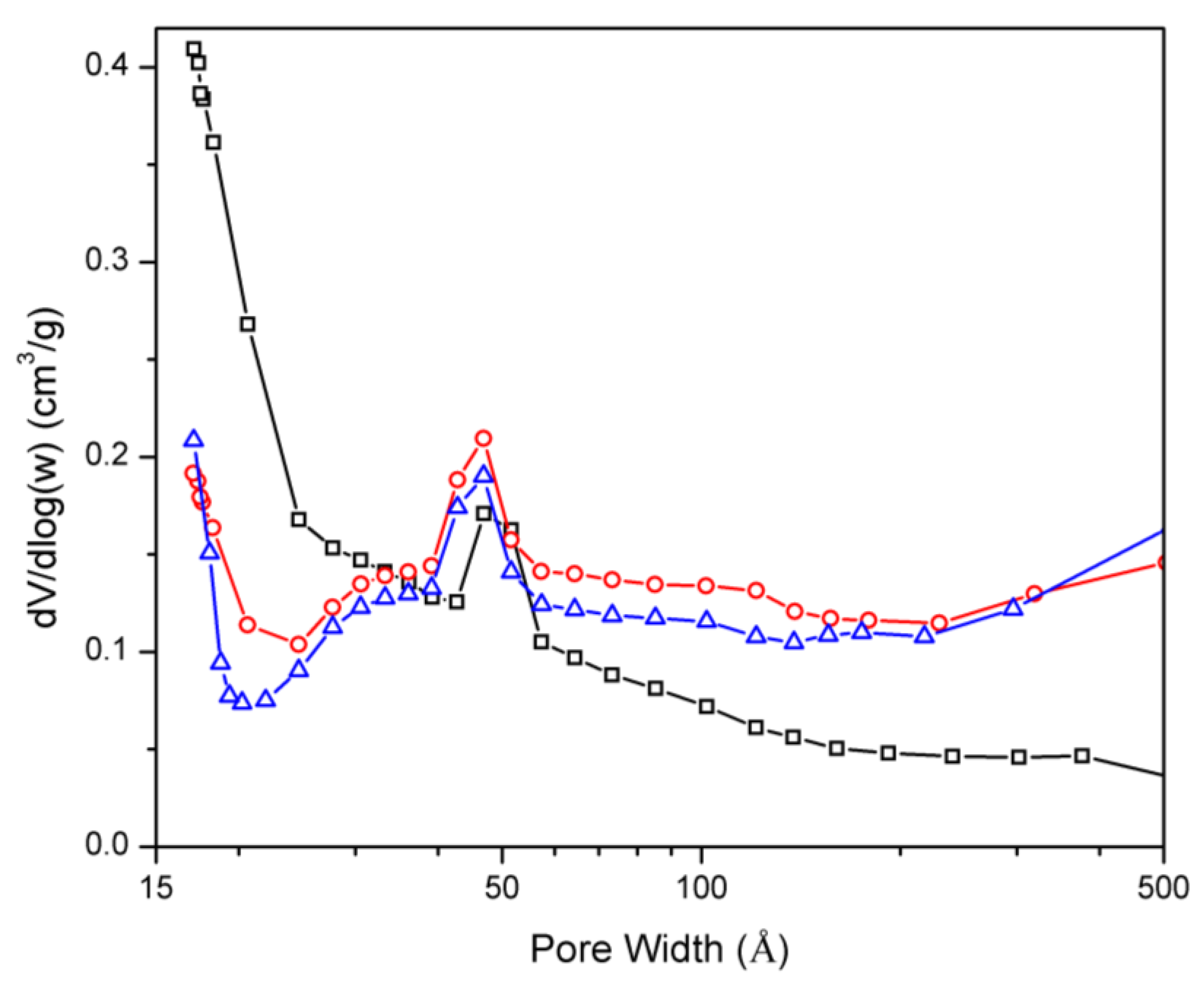





| Catalyst | Surface Area [m2·g−1] | Pore volume [cm3·g−1] | ||||
|---|---|---|---|---|---|---|
| SBET | Sext1 | Vtot2 | Vmicro1 | Vmeso3 | Vmeso4 | |
| NaX | 788 | 106 | 0.40 | 0.28 | 0.12 | 0.15 |
| Co/NaX | 447 | 117 | 0.31 | 0.12 | 0.19 | 0.25 |
| Recycled Co/NaX | 391 | 108 | 0.27 | 0.10 | 0.18 | 0.23 |
| Entry | Catalyst | Loading (mg) | Temp. (°C) | XTOT (%) | Product Distribution (%) | YC17 (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| C17 | C17-ene | C18-ene | Other | ||||||
| 1 | NaX (blank) | 250 | 280 | 23.7 | 21.2 | 21.7 | 6.5 | 50.6 | 5.0 |
| 2 | Co/NaX | 15 | 280 | 30.9 | 71.0 | 16.6 | 0.0 | 12.3 | 22.0 |
| 3 | Co/NaX | 150 | 280 | 67.1 | 17.1 | 30.1 | 36.4 | 16.4 | 11.5 |
| 4 | Co/NaX | 250 | 280 | 78.8 | 32.1 | 30.7 | 9.9 | 27.3 | 25.3 |
| 5 | Co/NaX | 250 | 260 | 54.2 | 27.5 | 21.1 | 7.3 | 44.1 | 14.9 |
| 6 | Co/NaX | 250 | 280 | 83.7 | 33.3 | 31.9 | 19.7 | 15.1 | 27.9 |
| 7 | Co/NaX | 250 | 300 | 95.1 | 27.0 | 32.5 | 28.4 | 12.0 | 25.7 |
| 8 | Co/NaX Recycled 1 | 230 | 280 | 70.5 | 21.9 | 31.7 | 31.8 | 14.6 | 15.4 |
| Compound | Area (%) |
|---|---|
| Aromatic | |
| 1H-trindene, 2,3,4,5,6,7,8,9-octahydro-1,1,4,4,9,9-hexamethyl- | 87 |
| Aldehyde | |
| benzaldehyde, 3-methyl- | 10 |
| Phenol | |
| oxirane, [[4-(1,1-dimethylethyl)phenoxy]methyl]- | 2.5 |
| Ketone | |
| 2,4,6-cycloheptatrien-1-one | 1.5 |
| Ref. | Catalyst | Reactant | Pathway | Synthesis | Rxn Conditions | Major Product | XTOT (%) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| [36] | Co/MoS2 | Canola Oil | HDO | Hydro | 375 °C, 90 bar H2, 8 h | C17-ene | 80 | - |
| [62] | Co/HZSM-5 | Stearic Acid | HDO | SOMC | 260 °C, 30 bar H2, 4 h | C18 | 100 | 80 |
| [63] | Co/HZ-5 | Palmitic Acid | HDO | SS | 260 °C, 40 bar H2, 4 h | C18 | 100 | 49.5 |
| [63] | Co/HZP-5 | Palmitic Acid | HDO | SS | 260 °C, 40 bar H2, 4 h | C16 | 100 | 47.6 |
| [64] | Co2.5/Clay | Methyl Oleate | HDO | WI | 300 °C, 40 bar H2, 6 h | C18 | 100 | 89 |
| [65] | Co/γ-Al2O3 | Palm Oil | HDO | IW | 300 °C, 50 bar H2, 100 h | C16 | 100 | 35 |
| [66] | Co/MgO | Methyl Heptanoate | HDO | IW | 220 °C, 30 bar H2, 6 h | C7 | 73.3 | 1.7 |
| [66] | Co/SiO2 | Methyl Heptanoate | HDO | IW | 220 °C, 30 bar H2, 6 h | C7 | 98.3 | 44.8 |
| [66] | Co/Hβ | Methyl Heptanoate | HDO | IW | 220 °C, 30 bar H2, 6 h | C7 | 99.7 | 38.4 |
| [67] | Co/SBA-15 | Methyl Oleate | HDO | IW | 340 °C, H2, 6 h | C17 | 90 | 49.5 |
| [16] | Co(Oac)2 | Stearic Acid | DCO | No synth | 350 °C, N2, 2 h | C17 | 31 | 1.5 |
| This work | Co/NaX | Stearic Acid | DCO | IE | 280 °C, 10 bar N2, 2 h | C17 | 83.7 | 27.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crawford, J.M.; Smoljan, C.S.; Lucero, J.; Carreon, M.A. Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts. Catalysts 2019, 9, 42. https://doi.org/10.3390/catal9010042
Crawford JM, Smoljan CS, Lucero J, Carreon MA. Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts. Catalysts. 2019; 9(1):42. https://doi.org/10.3390/catal9010042
Chicago/Turabian StyleCrawford, James M., Courtney S. Smoljan, Jolie Lucero, and Moises A. Carreon. 2019. "Deoxygenation of Stearic Acid over Cobalt-Based NaX Zeolite Catalysts" Catalysts 9, no. 1: 42. https://doi.org/10.3390/catal9010042





