A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification
Abstract
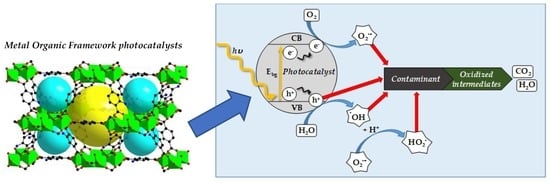
1. Water Purification by Photocatalysis
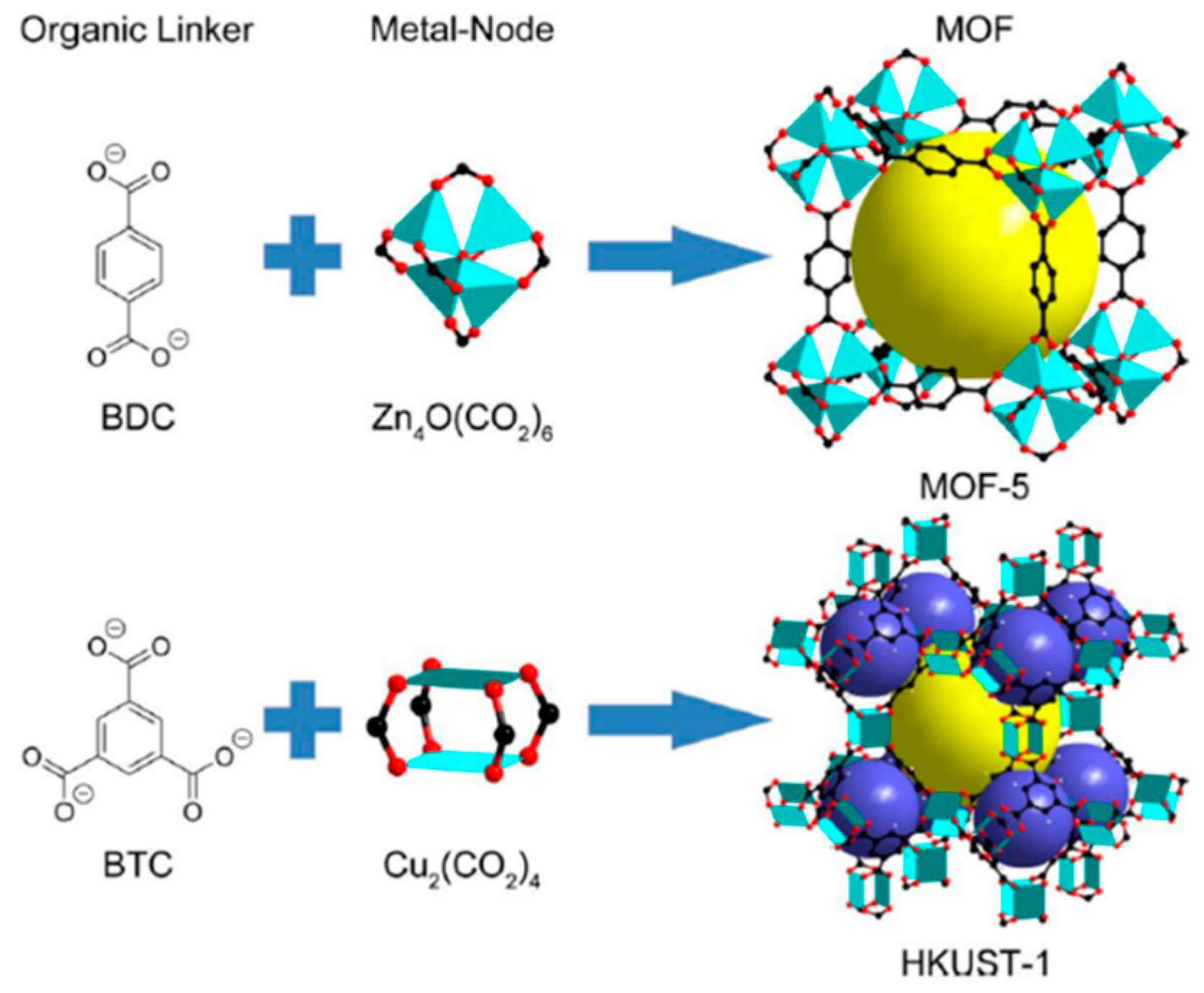
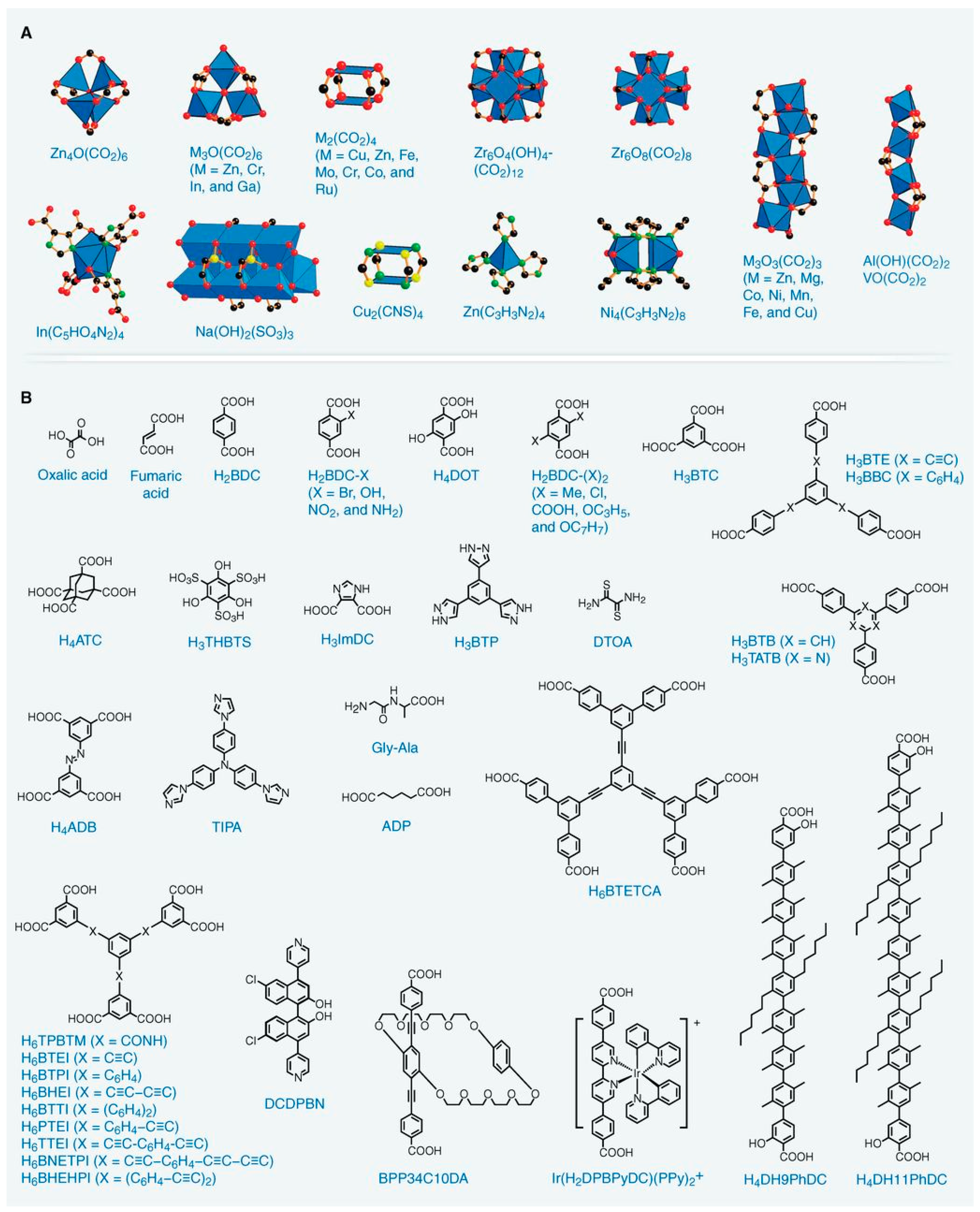
2. Metal Organic Frameworks (MOFs)
3. Synthesis of MOFs
Modification of MOFs
4. Characterization of MOFs
4.1. Powder X-ray Diffraction (PXRD)
4.2. Nitrogen Adsorption-Desorption at −196 °C
4.3. Ultraviolet-Visible Diffuse Reflectance Spectroscopy (UV-vis DRS)
4.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
4.5. Thermogravimetric Analysis (TGA)
4.6. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
4.7. Aqueous Stability Testing
4.8. Photoluminescence (PL) Spectroscopy
5. Experimental Procedures for Photocatalytic Tests
5.1. Photocatalytic Reactors
5.1.1. Slurry Reactors
5.1.2. Photocatalytic Membrane Reactor (PMR)
5.1.3. Annular Photoreactor
5.2. Parameters Affecting the Photocatalytic Behavior
5.2.1. Photocatalyst Loading
5.2.2. pH
5.2.3. Temperature
5.2.4. Dissolved Oxygen
5.2.5. Contaminant Initial Concentration
5.2.6. Light Wavelength
5.2.7. Light Intensity
5.3. Kinetics and Modelling
6. Water Purification by Photocatalysis Using MOFs
6.1. Photodegradation of Dyes
6.1.1. Metal Doping
6.1.2. Metal Nanoparticles Decoration
6.1.3. Heterostructures Combining Different Components
6.2. Photoreduction of Heavy Metals
6.3. Photodegradation of Priority Pollutants
6.4. Photodegradation of Emerging Pollutants
6.5. Stability and Reusability of MOFs during Water Purification by Photocatalysis
7. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wada, Y.; Flörke, M.; Hanasaki, N.; Eisner, S.; Fischer, G.; Tramberend, S.; Satoh, Y.; Van Vliet, M.T.H.; Yillia, P.; Ringler, C.; et al. Modeling global water use for the 21st century: The Water Futures and Solutions (WFaS) initiative and its approaches. Geosci. Model Dev. 2016, 9, 175–222. [Google Scholar] [CrossRef]
- WWAP (United Nations World Water Assessment Programme)/UN-Water. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; WWAP: Paris, France, 2018. [Google Scholar]
- Veldkamp, T.I.E.; Wada, Y.; Aerts, J.C.J.H.; Döll, P.; Gosling, S.N.; Liu, J.; Masaki, Y.; Oki, T.; Ostberg, S.; Pokhrel, Y.; et al. Water scarcity hotspots travel downstream due to human interventions in the 20th and 21st century. Nat. Commun. 2017, 8, 15697. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Environmental Performance Reviews: Chile 2016; OECD Environmental Performance Reviews; OECD Publishing: Paris, France, 2016. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; United Nations: New York, NY, USA, 2017. [Google Scholar]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer Briefs in Molecular Science; Springer: New Delhi, India, 2014. [Google Scholar]
- Freyria, F.; Geobaldo, F.; Bonelli, B.; Freyria, F.S.; Geobaldo, F.; Bonelli, B. Nanomaterials for the Abatement of Pharmaceuticals and Personal Care Products from Wastewater. Appl. Sci. 2018, 8, 170. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Good Practices for Regulating Wastewater Treatment: Legislation, Policies and Standards; United Nations Environment Programme: Nairobi, Kenya, 2015. [Google Scholar]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Abu-Ghunmi, D.; Abu-Ghunmi, L.; Kayal, B.; Bino, A. Circular economy and the opportunity cost of not ‘closing the loop’ of water industry: The case of Jordan. J. Clean. Prod. 2016, 131, 228–236. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. DES 2014, 356, 226–254. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P. Linking Water Quality to Human Health and Environment: The Fate of Micropollutants; Institute of Water Policy, National University of Singapore: Singapore, 2011; 62p. [Google Scholar]
- Thomaidi, V.S.; Stasinakis, A.S.; Borova, V.L.; Thomaidis, N.S. Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case-study. J. Hazard. Mater. 2015, 283, 740–747. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Fe3O4 embedded ZnO nanocomposites for the removal of toxic metal ions, organic dyes and bacterial pathogens. J. Mater. Chem. A 2013, 1, 3325. [Google Scholar] [CrossRef]
- Litter, M.I. Mechanisms of removal of heavy metals and arsenic from water by TiO2-heterogeneous photocatalysis. Pure Appl. Chem. 2015, 87, 557–567. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Zhu, J.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Küppers, S. Simultaneous removal of several pharmaceuticals and arsenic on Zn-Fe mixed metal oxides: Combination of photocatalysis and adsorption. Chem. Eng. J. 2017, 328, 141–151. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Shao, Q.; Guo, X.; Hao, L.; Zheng, Y.; et al. Heterostructured TiO2/WO3 Nanocomposites for Photocatalytic Degradation of Toluene under Visible Light. J. Electrochem. Soc. 2017, 164, H1086–H1090. [Google Scholar] [CrossRef]
- Bilgin Simsek, E. Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Ahmed, N. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Hopfield, J.J. On the energy dependence of the absorption constant and photoconductivity near a direct band gap. J. Phys. Chem. Solids 1961, 22, 63–72. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Llabres i Xamena, F.; Gascon, J. (Eds.) Metal Organic Frameworks as Heterogeneous Catalysts; Catalysis Series; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Gimeno, O.; García-Araya, J.F.; Beltrán, F.J.; Rivas, F.J.; Espejo, A. Removal of emerging contaminants from a primary effluent of municipal wastewater by means of sequential biological degradation-solar photocatalytic oxidation processes. Chem. Eng. J. 2016, 290, 12–20. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, L.; Dionysio, D. A parametric study of visible-light sensitive TiO2 photocatalysts synthesis via a facile sol-gel N-doping method. J. Exp. Nanosci. 2015, 10, 1153–1165. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Mondal, C.; Singh, A.; Sahoo, R.; Sasmal, A.K.; Negishi, Y.; Pal, T. Preformed ZnS nanoflower prompted evolution of CuS/ZnS p–n heterojunctions for exceptional visible-light driven photocatalytic activity. New J. Chem. 2015, 39, 5628–5635. [Google Scholar] [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Cheng, H.; Zhang, C. One-step hydrothermal synthesis of flowerlike MoS2/CdS heterostructures for enhanced visible-light photocatalytic activities. RSC Adv. 2015, 5, 15621–15626. [Google Scholar] [CrossRef]
- Mourão, H.A.J.L.; Lopes, O.F.; Ribeiro, C.; Mastelaro, V.R. Rapid hydrothermal synthesis and pH-dependent photocatalysis of strontium titanate microspheres. Mater. Sci. Semicond. Process. 2015, 30, 651–657. [Google Scholar] [CrossRef]
- Rastogi, M.; Kushwaha, H.S.; Vaish, R. Highly efficient visible light mediated azo dye degradation through barium titanate decorated reduced graphene oxide sheets. Electron. Mater. Lett. 2016, 12, 281–289. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Bai, X.; Lv, L.; Ye, Z.; Huang, X. Nitrogen-doped perovskite-type La2Ti2O7 decorated on graphene composites exhibiting efficient photocatalytic activity toward bisphenol A in water. J. Colloid Interface Sci. 2015, 450, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Wang, H.; Zeng, G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 511, 296–306. [Google Scholar] [CrossRef]
- Sethi, Y.A.; Praveen, C.S.; Panmand, R.P.; Ambalkar, A.; Kulkarni, A.K.; Gosavi, S.W.; Kulkarni, M.V.; Kale, B.B. Perforated N-doped monoclinic ZnWO4 nanorods for efficient photocatalytic hydrogen generation and RhB degradation under natural sunlight. Catal. Sci. Technol. 2018, 8, 2909–2919. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W- and Mo-doped BiVO4 oriented along the {0 4 0} facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Meng, X.; Liu, H.; Ye, J. An amine-functionalized iron(III) Metal–Organic Framework as efficient visible-light photocatalyst for Cr(VI) reduction. Adv. Sci. 2015, 2, 1500006. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B Environ. 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Yan, T.; Ji, P.; Zhao, X.; Yuan, K.; Wei, D.; Du, B. Fabrication of heterostructured Bi2O2CO3/Bi2O4 photocatalyst and efficient photodegradation of organic contaminants under visible-light. J. Hazard. Mater. 2017, 333, 169–178. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.; Zhang, J.; Yu, J.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liang, J.; Wang, X.-S.; Cao, R. Multifunctional metal–organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-T.; Liao, Z.-L.; Jiang, Y.-S.; Li, G.-H.; Li, G.-D.; Chen, J.-S. Construction of a microporous inorganic-organic hybrid compound with uranyl units. Chem. Commun. 2004, 1814–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Wei, Z. New Design and Synthetic Strategies of Metal-Organic Frameworks. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2014. [Google Scholar]
- Sharmin, E.; Zafar, F. Introductory Chapter: Metal Organic Frameworks (MOFs). In Metal-Organic Frameworks; InTech: Munich/Garching, Germany, 2016. [Google Scholar]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Perez, E.; Karunaweera, C.; Musselman, I.; Balkus, K.; Ferraris, J.; Perez, E.V.; Karunaweera, C.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Origins and Evolution of Inorganic-Based and MOF-Based Mixed-Matrix Membranes for Gas Separations. Processes 2016, 4, 32. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Hu, Y.H.; Zhang, L. Hydrogen Storage in Metal-Organic Frameworks. Adv. Mater. 2010, 22, E117–E130. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal–organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Su, Z.-M. Metal-organic frameworks as potential drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Silva, C.G.; Corma, A.; García, H. Metal–organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xiao, X.; Duan, S.; Liu, J.; He, J.; Lei, J.; Wang, L. Synthesis of novel microporous nanocomposites of ZIF-8 on multiwalled carbon nanotubes for adsorptive removing benzoic acid from water. Chem. Eng. J. 2018, 331, 64–74. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, T.; Wang, C.; Yin, X.; Xiong, Z. Introduction of amidoxime groups into metal-organic frameworks to synthesize MIL-53(Al)-AO for enhanced U(VI) sorption. J. Mol. Liq. 2017, 242, 531–536. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Getachew, N.; Díaz, K.; Díaz-García, M.; Chebude, Y.; Díaz, I. Synthesis of metal–organic frameworks in water at room temperature: Salts as linker sources. Green Chem. 2015, 17, 1500–1509. [Google Scholar] [CrossRef]
- Yoo, Y.; Varela-Guerrero, V.; Jeong, H.-K. Isoreticular Metal−Organic Frameworks and Their Membranes with Enhanced Crack Resistance and Moisture Stability by Surfactant-Assisted Drying. Langmuir 2011, 27, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Masel, R.I. Rapid Production of Metal−Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef] [PubMed]
- Klinowski, J.; Almeida Paz, F.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal–Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 47, 6336–6338. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal–organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Pichon, A.; Lazuen-Garay, A.; James, S.L. Solvent-free synthesis of a microporous metal–organic framework. CrystEngComm 2006, 8, 211–214. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical Synthesis of Metal−Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Friščić, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal-Organic Frameworks Reveals Salt Inclusion and Anion Templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Martinez Joaristi, A.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Imaz, I.; Cano-Sarabia, M.; Maspoch, D. A spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures. Nat. Chem. 2013, 5, 203–211. [Google Scholar] [CrossRef]
- Bian, Y.; Xiong, N.; Zhu, G.; Bian, Y.; Xiong, N.; Zhu, G. Technology for the Remediation of Water Pollution: A Review on the Fabrication of Metal Organic Frameworks. Processes 2018, 6, 122. [Google Scholar] [CrossRef]
- Dhanya, V.S.; Sudarsanakumar, M.R.; Suma, S.; Ng, S.W.; Augustine, M.S.; Roy, S.M. Crystal structure, thermal decomposition, photoluminescence and magnetic studies of a new two dimensional metal–organic framework constructed from infinite chains of edge-sharing CeO6(H2O)2(NO3) polyhedron with bullet shaped channels. Inorg. Chem. Commun. 2013, 35, 140–143. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal Synthesis of Zeolites, Metal–Organic Frameworks, and Inorganic–Organic Hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef]
- Nelson, A.P.; Farha, O.K.; Mulfort, K.L.; Hupp, J.T. Supercritical Processing as a Route to High Internal Surface Areas and Permanent Microporosity in Metal−Organic Framework Materials. J. Am. Chem. Soc. 2009, 131, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.; Garzón-Tovar, L.; Carné-Sánchez, A.; Imaz, I.; Maspoch, D. Photothermal Activation of Metal−Organic Frameworks Using a UV-Vis Light Source. ACS Appl. Mater. Interfaces 2018, 10, 9555–9562. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; Wiley-VCH: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Frost, C.G.; Mahon, M.F.; Richardson, C. Post-Synthetic Modification of Tagged Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2008, 47, 8482–8486. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal–Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- Aijaz, A.; Karkamkar, A.; Choi, Y.J.; Tsumori, N.; Rönnebro, E.; Autrey, T.; Shioyama, H.; Xu, Q. Immobilizing Highly Catalytically Active Pt Nanoparticles inside the Pores of Metal–Organic Framework: A Double Solvents Approach. J. Am. Chem. Soc. 2012, 134, 13926–13929. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z. Double-Solvent Method to Pd Nanoclusters Encapsulated inside the Cavity of NH2–UiO-66(Zr) for Efficient Visible-Light-Promoted Suzuki Coupling Reaction. J. Phys. Chem. C 2016, 120, 19744–19750. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized Pt@UiO-66(Zr) metal–organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. 2014, 50, 7063–7066. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Yin, L.-S.; Cao, S.-W.; Xu, G.-S.; Li, C.-H.; Xue, C. Improving photocatalytic hydrogen production of metal–organic framework UiO-66 octahedrons by dye-sensitization. Appl. Catal. B Environ. 2015, 168–169, 572–576. [Google Scholar] [CrossRef]
- Qin, J.; Wang, S.; Wang, X. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst. Appl. Catal. B Environ. 2017, 209, 476–482. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale screening of hypothetical metal–organic frameworks. Nat. Chem. 2012, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and Its Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1987. [Google Scholar]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Citrin, P.H.; Eisenberger, P.; Kincaid, B.M. Extended X-ray absorption fine structure—Its strengths and limitations as a structural tool. Rev. Mod. Phys. 1981, 53, 769–806. [Google Scholar] [CrossRef]
- Alp, E.E.; Mini, S.M.; Ramanathan, M. X-Ray Absorption Spectroscopy: EXAFS and XANES-A Versatile Tool to Study the Atomic and Electronic Structure of Materials. In Synchrotron X-Ray Sources and New Opportunities in the Soil and Environmental Sciences; Schulze, D., Anderson, S., Mattigod, S., Eds.; Argonne National Lab.: Lemont, IL, USA, 1990; pp. 25–36. [Google Scholar]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chemie-Ingenieur-Technik 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.S.; Rouquerol, J.; Siemieniewska, T. International Union of Pure Commission on Colloid and Surface Chemistry Including Catalysis-Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chung, Y.G.; Camp, J.; Haranczyk, M.; Sikora, B.J.; Bury, W.; Krungleviciute, V.; Farha, O.K.; Sholl, D.S.; Snurr, R.Q. Computation-Ready, Experimental Metal−Organic Frameworks: A Tool To Enable High-Throughput Screening of Nanoporous Crystals. Chem. Mater. 2014, 26, 6185–6192. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr6-based MOFs water stable? Linker hydrolysis vs. capillary-force-driven channel collapse. Chem. Commun. 2014, 50, 8944–8946. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro and mesoporous solids by physisorption methods and poro size analysis. Appl. Catal. A Gen. 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Sing, K.S.W. Characterization of porous materials: Past, present and future. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 3–7. [Google Scholar] [CrossRef]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 3–9. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Ye, J. Metal-organic frameworks for photocatalysis. Phys. Chem. Chem. Phys. 2016, 18, 7563–7572. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal–Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Zheng, H.; Wang, P.; Hai, G.; Dong, W.; Wang, G. Aromatic heterocycle-grafted NH2-MIL-125(Ti) via conjugated linker with enhanced photocatalytic activity for selective oxidation of alcohols under visible light. Appl. Catal. B Environ. 2018, 224, 479–487. [Google Scholar] [CrossRef]
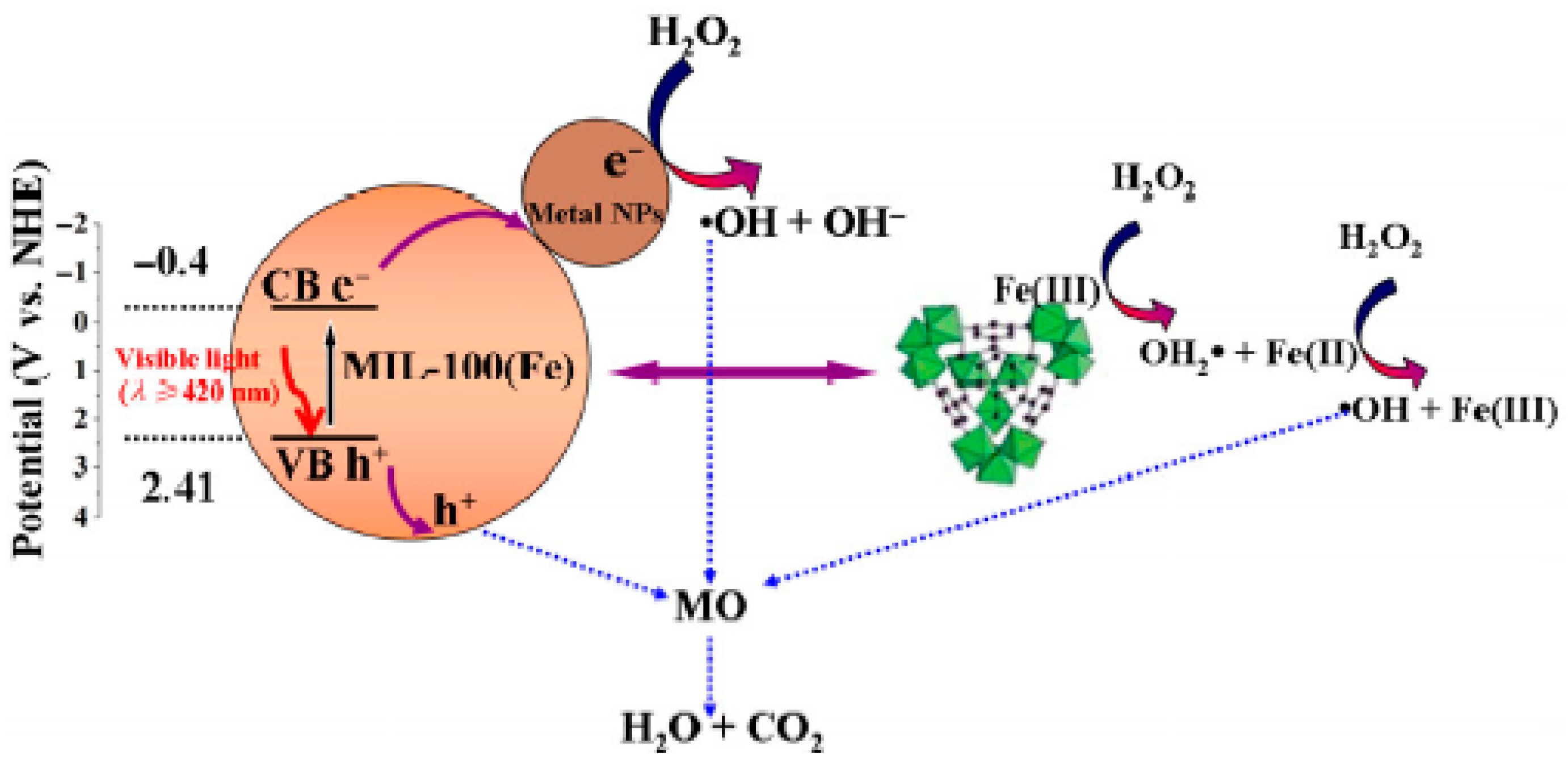
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. M@MIL-100(Fe) (M = Au, Pd, Pt) nanocomposites fabricated by a facile photodeposition process: Efficient visible-light photocatalysts for redox reactions in water. Nano Res. 2015, 8, 3237–3249. [Google Scholar] [CrossRef]
- Gao, G.; Nie, L.; Yang, S.; Jin, P.; Chen, R.; Ding, D.; Wang, X.C.; Wang, W.; Wu, K.; Zhang, Q. Well-defined strategy for development of adsorbent using metal organic frameworks (MOF) template for high performance removal of hexavalent chromium. Appl. Surf. Sci. 2018, 457, 1208–1217. [Google Scholar] [CrossRef]
- Fassel, V.A.; Kniseley, R.N. Inductively coupled plasma. Optical emission spectroscopy. Anal. Chem. 1974, 46, 1110A–1120A. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Singhal, N.K.; Hassan, A.A.; Kim, K.H.; Kumar, S. Metal organic frameworks MIL-100(Fe) as an efficient adsorptive material for phosphate management. Environ. Res. 2019, 169, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Luz, I.; Soukri, M.; Lail, M. Transformation of single MOF nanocrystals into single nanostructured catalysts within mesoporous supports: A platform for pioneer fluidized-nanoreactor hydrogen carriers. Chem. Commun. 2018, 54, 8462–8465. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Avilés, A.; Peñas-Garzón, M.; Bedia, J.; Dionysiou, D.D.; Rodríguez, J.J.; Belver, C. Mixed Ti-Zr metal-organic-frameworks with solar photocatalytic applications. Appl. Catal. B Environ. Environ. 2018. submitted. [Google Scholar]
- Shen, L.; Huang, L.; Liang, S.; Liang, R.; Qin, N.; Wu, L. Electrostatically derived self-assembly of NH2-mediated zirconium MOFs with graphene for photocatalytic reduction of Cr(vi). RSC Adv. 2014, 4, 2546–2549. [Google Scholar] [CrossRef]
- Sofi, F.A.; Majid, K.; Mehraj, O. The visible light driven copper based metal-organic-framework heterojunction:HKUST-1@Ag-Ag3PO4 for plasmon enhanced visible light photocatalysis. J. Alloys Compd. 2018, 737, 798–808. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H.; Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Palmisano, L. Photocatalytic membrane reactors for water treatment. In Advances in Membrane Technologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2015; pp. 205–238. [Google Scholar]
- Palmisano, G.; Loddo, V.; Augugliaro, V.; Bellardita, M.; Camera Roda, G.; Parrino, F. Validation of a two-dimensional modeling of an externally irradiated slurry photoreactor. Chem. Eng. J. 2015, 262, 490–498. [Google Scholar] [CrossRef]
- Leblebici, M.E.; Stefanidis, G.D.; Van Gerven, T. Comparison of photocatalytic space-time yields of 12 reactor designs for wastewater treatment. Chem. Eng. Process. Process Intensif. 2015, 97, 106–111. [Google Scholar] [CrossRef]
- Zeghioud, H.; Khellaf, N.; Djelal, H.; Amrane, A.; Bouhelassa, M. Photocatalytic Reactors Dedicated to the Degradation of Hazardous Organic Pollutants: Kinetics, Mechanistic Aspects, and Design—A Review. Chem. Eng. Commun. 2016, 203, 1415–1431. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S. Photoreactors for wastewater treatment: A review. Recent Pat. Eng. 2010, 4, 242–266. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Photoreactors for heterogeneous photocatalysis for wastewater treatment. Int. Res. J. Eng. Technol. 2016, 3, 1011–1014. [Google Scholar]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Chong, M.N.; Lei, S.; Jin, B.; Saint, C.; Chow, C.W.K. Optimisation of an annular photoreactor process for degradation of Congo Red using a newly synthesized titania impregnated kaolinite nano-photocatalyst. Sep. Purif. Technol. 2009, 67, 355–363. [Google Scholar] [CrossRef]
- Saquib, M.; Muneer, M. TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dyes Pigment. 2003, 56, 37–49. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z. Iron-based metal–organic frameworks (MOFs) for visible-light-induced photocatalysis. Res. Chem. Intermed. 2017, 43, 5169–5186. [Google Scholar] [CrossRef]
- Chi, L.; Xu, Q.; Liang, X.; Wang, J.; Su, X. Iron-Based Metal–Organic Frameworks as Catalysts for Visible Light-Driven Water Oxidation. Small 2016, 12, 1351–1358. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, J.; Lu, S.; Cen, C.; Chen, Y.; Ren, L.; Feng, W.; Fang, Z. Zirconium metal-organic framework supported highly-dispersed nanosized BiVO4 for enhanced visible-light photocatalytic applications. J. Chem. Technol. Biotechnol. 2016, 91, 2785–2792. [Google Scholar] [CrossRef]
- Glatzmaier, G.C.; Nix, R.G.; Mehos, M.S. Solar destruction of hazardous chemicals. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1990, 25, 571–581. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.; He, C.; Duan, C. Metal-Organic Frameworks: Versatile Materials for Heterogeneous Photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés i Xamena, F.X.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Hausdorf, S.; Wagler, J.; Mossig, R.; Mertens, F.O.R.L. Proton and water activity-controlled structure formation in zinc carboxylate-based metal organic frameworks. J. Phys. Chem. A 2008, 112, 7567–7576. [Google Scholar] [CrossRef] [PubMed]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Luo, S.; Jing, F.; Shen, L.; Qin, N.; Wu, L. Environmental strategy for fabrication of Pd@MIL-100(Fe) nanocomposite as a visible-light-driven photocatalyst for the treatment of pharmaceuticals and personal care products (PPCPs). Appl. Catal. B Environ. 2015, 176–177, 240–248. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.R. Intensification of abamectin pesticide degradation using the combination of ultrasonic cavitation and visible-light driven photocatalytic process: Synergistic effect and optimization study. Ultrason. Sonochem. 2017, 35, 449–457. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhang, J.; Wang, H.; Jiang, L. Photocatalytic Decontamination of Wastewater Containing Organic Dyes by Metal–Organic Frameworks and their Derivatives. ChemCatChem 2017, 9, 41–64. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Gao, J.; Miao, J.; Li, P.Z.; Teng, W.Y.; Yang, L.; Zhao, Y.; Liu, B.; Zhang, Q. A p-type Ti(IV)-based metal-organic framework with visible-light photo-response. Chem. Commun. 2014, 50, 3786–3788. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Bala, S.; Bhattacharya, S.; Goswami, A.; Adhikary, A.; Konar, S.; Mondal, R. Designing functional metal-organic frameworks by imparting a hexanuclear copper-based secondary building unit specific properties: Structural correlation with magnetic and photocatalytic activity. Cryst. Growth Des. 2014, 14, 6391–6398. [Google Scholar] [CrossRef]
- Liu, L.; Ding, J.; Huang, C.; Li, M.; Hou, H.; Fan, Y. Polynuclear CdII polymers: Crystal structures, topologies, and the photodegradation for organic dye contaminants. Cryst. Growth Des. 2014, 14, 3035–3043. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, D.; Zhang, X.; Ma, J.; Liu, L.; Xu, X. Preparation, structure and photocatalysis of metal-organic frameworks derived from aromatic carboxylate and imidazole-based ligands. J. Coord. Chem. 2016, 69, 985–995. [Google Scholar] [CrossRef]
- Jing, H.-P.; Wang, C.-C.; Zhang, Y.-W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, D.; He, X.; Huang, S.; Huang, J.; Zhou, X.; Yang, Z.; Li, J.; Li, H.; Nie, F. Structures, photoluminescence and photocatalytic properties of two novel metal-organic frameworks based on tetrazole derivatives. Cryst. Eng. Comm. 2014, 16, 10485–10491. [Google Scholar] [CrossRef]
- Wang, F.; Li, F.-L.; Xu, M.-M.; Yu, H.; Zhang, J.-G.; Xia, H.-T.; Lang, J.-P. Facile synthesis of a Ag-doped coordination polymer with enhanced catalytic performance in the photodegradation of azo dyes in water. J. Mater. Chem. A 2015, 3, 5908–5916. [Google Scholar] [CrossRef]
- George, P.; Dhabarde, N.R.; Chowdhury, P. Rapid synthesis of Titanium based Metal Organic framework (MIL-125) via microwave route and its performance evaluation in photocatalysis. Mater. Lett. 2017, 186, 151–154. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef]
- Du, J.J.; Yuan, Y.P.; Sun, J.X.; Peng, F.M.; Jiang, X.; Qiu, L.G.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. New photocatalysts based on MIL-53 metal-organic frameworks for the decolorization of methylene blue dye. J. Hazard. Mater. 2011, 190, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.T.; Ma, L.; Ke, F.; Peng, F.M.; Xu, G.S.; Shen, Y.H.; Zhu, J.F.; Qiu, L.G.; Yuan, Y.P. Metal-organic frameworks MIL-88A hexagonal microrods as a new photocatalyst for efficient decolorization of methylene blue dye. Dalton Trans. 2014, 43, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhou, Y.F.; Guan, W.S.; Liu, L.H.; Liu, B.; Che, G.B.; Liu, C.B.; Lin, X.; Zhu, E.W. Syntheses, structures, and photocatalytic properties of two new one-dimensional chain transition metal complexes with mixed N,O-donor ligands. Inorg. Chim. Acta 2017, 466, 291–297. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. High efficiency of mechanosynthesized Zn-based metal–organic frameworks in photodegradation of congo red under UV and visible light. RSC Adv. 2016, 6, 13272–13277. [Google Scholar] [CrossRef]
- Pu, S.; Xu, L.; Sun, L.; Du, H. Tuning the optical properties of the zirconium–UiO-66 metal–organic framework for photocatalytic degradation of methyl orange. Inorg. Chem. Commun. 2015, 52, 50–52. [Google Scholar] [CrossRef]
- Xu, X.Y.; Chen, Q.C.; Yu, Y.D.; Huang, X.C. Ligand Induced Anionic Cuprous Cyanide Framework for Cupric Ion Turn on Luminescence Sensing and Photocatalytic Degradation of Organic Dyes. Inorg. Chem. 2016, 55, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Liu, D.-N.; Lin, H.-Y.; Liu, G.-C.; Rong, X. Different Anderson-Type Polyoxometalate-Directed Metal–Organic Complexes Based on 2-Pyrazine Carboxylic Acid: Assembly, Structures and Properties. J. Clust. Sci. 2016, 27, 169–181. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, Y.; Wang, Z.; Chen, M.; Sun, L.; Liu, X. Titanium incorporated with UiO-66(Zr)-type Metal-Organic Framework (MOF) for photocatalytic application. RSC Adv. 2016, 6, 3671–3679. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal-Organic Framework (MOF) Compounds: Photocatalysts for Redox Reactions and Solar Fuel Production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Cai, S.-L.; Xie, B.; Li, B.-H.; Man, J.-H.; He, Y.-X.; Singh, A.; Kumar, A. Efficient photocatalytic degradation of methyl violet with two metall–organic frameworks. J. Coord. Chem. 2017, 70, 3409–3421. [Google Scholar] [CrossRef]
- Mu, X.; Jiang, J.; Chao, F.; Lou, Y.; Chen, J. Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation. Dalton Trans. 2018, 47, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Bedia, J.; Gómez, A.; Peñas-Garzón, M.; Rodriguez, J.J. Semiconductor Photocatalysis for water purification. In Nanoscale Materials in Water Purification; Thomas, S., Pasiquin, D., Leu, S.-Y., Gopakumar, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, R.; Ren, X.; Ma, H.; Feng, X.; Lin, Z.; Li, X.; Hu, C.; Wang, B. Nickel-substituted zeolitic imidazolate frameworks for time-resolved alcohol sensing and photocatalysis under visible light. J. Mater. Chem. A 2014, 2, 5724–5729. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Simões, M.M.Q.; Silva, A.M.S.; Rocha, J. Enhanced Photocatalytic Activity of MIL-125 by Post-Synthetic Modification with CrIIIand Ag Nanoparticles. Chem. Eur. J. 2015, 21, 11072–11081. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Peng, Y.; Li, X.; Zhou, C.; Xu, R.; Zhang, Y. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Tabatabaei, N.; Dashtian, K.; Ghaedi, M.; Sabzehmeidani, M.M.; Ameri, E. Novel visible light-driven Cu-based MOFs/Ag2O composite photocatalysts with enhanced photocatalytic activity toward the degradation of orange G: Their photocatalytic mechanism and optimization study. New J. Chem. 2018, 42, 9720–9734. [Google Scholar] [CrossRef]
- Binh, N.T.; Thu, P.T.; Le, N.T.H.; Tien, D.M.; Khuyen, H.T.; Giang, L.T.K.; Huong, N.T.; Lam, T.D. Study on preparation and properties of a novel photo-catalytic material based on copper-centred metal-organic frameworks (Cu-MOF) and titanium dioxide. Int. J. Nanotechnol. 2015, 12, 447–455. [Google Scholar] [CrossRef]
- Liu, X.; Dang, R.; Dong, W.; Huang, X.; Tang, J.; Gao, H.; Wang, G. A sandwich-like heterostructure of TiO2nanosheets with MIL-100(Fe): A platform for efficient visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 209, 506–513. [Google Scholar] [CrossRef]
- Feng, X.; Chen, H.; Jiang, F. In-situ ethylenediamine-assisted synthesis of a magnetic iron-based metal-organic framework MIL-53(Fe) for visible light photocatalysis. J. Colloid Interface Sci. 2017, 494, 32–37. [Google Scholar] [CrossRef]
- Sha, Z.; Sun, J.; On Chan, H.S.; Jaenicke, S.; Wu, J. Bismuth tungstate incorporated zirconium metal-organic framework composite with enhanced visible-light photocatalytic performance. RSC Adv. 2014, 4, 64977–64984. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Z.; He, C.; Tong, X.; Li, Y.; Niu, X.; Zhang, H. UiO-66(Zr) coupled with Bi2MoO6 as photocatalyst for visible-light promoted dye degradation. J. Colloid Interface Sci. 2017, 497, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Niu, X.; An, S.; Chen, W.; Wang, J.; Liu, W. Facile synthesis of Bi2MoO6-MIL-100(Fe) metal-organic framework composites with enhanced photocatalytic performance. RSC Adv. 2017, 7, 2943–2952. [Google Scholar] [CrossRef]
- Sha, Z.; Wu, J. Enhanced visible-light photocatalytic performance of BiOBr/UiO-66(Zr) composite for dye degradation with the assistance of UiO-66. RSC Adv. 2015, 5, 39592–39600. [Google Scholar] [CrossRef]
- Zhu, S.-R.; Liu, P.-F.; Wu, M.-K.; Zhao, W.-N.; Li, G.-C.; Tao, K.; Yi, F.-Y.; Han, L. Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light. Dalton Trans. 2016, 45, 17521–17529. [Google Scholar] [CrossRef]
- Sha, Z.; Chan, H.S.O.; Wu, J. Ag2CO3/UiO-66(Zr) composite with enhanced visible-light promoted photocatalytic activity for dye degradation. J. Hazard. Mater. 2015, 299, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Sun, J.; Chan, H.S.O.; Jaenicke, S.; Wu, J. Enhanced Photocatalytic Activity of the AgI/UiO-66(Zr) Composite for Rhodamine B Degradation under Visible-Light Irradiation. Chempluschem 2015, 80, 1321–1328. [Google Scholar] [CrossRef]
- Han, Y.; Shi, H.; Bai, C.; Zhang, L.; Wu, J.; Meng, H.; Xu, Y.; Zhang, X. Ag3PO4-MIL-53(Fe) Composites with Visible-Light-Enhanced Photocatalytic Activities for Rhodamine B Degradation. ChemistrySelect 2018, 3, 8045–8050. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. B Environ. 2015, 174, 445–454. [Google Scholar] [CrossRef]
- Hong, J.; Chen, C.; Bedoya, F.E.; Kelsall, G.H.; O’Hare, D.; Petit, C. Carbon nitride nanosheet/metal–organic framework nanocomposites with synergistic photocatalytic activities. Catal. Sci. Technol. 2016, 6, 5042–5051. [Google Scholar] [CrossRef]
- Guo, D.; Wen, R.; Liu, M.; Guo, H.; Chen, J.; Weng, W. Facile fabrication of g-C3N4/MIL-53(Al) composite with enhanced photocatalytic activities under visible-light irradiation. Appl. Organomet. Chem. 2015, 29, 690–697. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B. Synthesis of a novel and stable reduced graphene oxide/MOF hybrid nanocomposite and photocatalytic performance for the degradation of dyes. RSC Adv. 2016, 6, 17873–17879. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H. Synthesis of iron(iii)-based metal-organic framework/graphene oxide composites with increased photocatalytic performance for dye degradation. RSC Adv. 2014, 4, 40435–40438. [Google Scholar] [CrossRef]
- Fazaeli, R.; Aliyan, H.; Banavandi, R.S. Sunlight assisted photodecolorization of malachite green catalyzed by MIL-101/graphene oxide composites. Russ. J. Appl. Chem. 2015, 88, 169–177. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, C.; Ai, L.; Hao, Z.; Jiang, J. Boosting visible light photoreactivity of photoactive metal-organic framework: Designed plasmonic Z-scheme Ag/AgCl@MIL-53-Fe. Appl. Catal. B Environ. 2018, 224, 38–45. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Wang, Y.; Wang, Y.; Zhang, X.; Fan, C. The active roles of ZIF-8 on the enhanced visible photocatalytic activity of Ag/AgCl: Generation of superoxide radical and adsorption. J. Alloys Compd. 2017, 693, 543–549. [Google Scholar] [CrossRef]
- Gao, S.T.; Liu, W.H.; Shang, N.Z.; Feng, C.; Wu, Q.H.; Wang, Z.; Wang, C. Integration of a plasmonic semiconductor with a metal-organic framework: A case of Ag/AgCl@ZIF-8 with enhanced visible light photocatalytic activity. RSC Adv. 2014, 4, 61736–61742. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, G.; Zhang, H.; Yang, H.; Ma, P.; Li, X.; Qiu, X.; Liu, G. Highly efficient visible-light-driven photocatalytic degradation of rhodamine B by a novel Z-scheme Ag3PO4/MIL-101/NiFe2O4 composite. Catal. Sci. Technol. 2018, 8, 2402–2416. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, H.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Li, H. One-pot self-assembly and photoreduction synthesis of silver nanoparticle-decorated reduced graphene oxide/MIL-125(Ti) photocatalyst with improved visible light photocatalytic activity. Appl. Organomet. Chem. 2016, 30, 289–296. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Zhang, L. Pd nanoparticles supported on MIL-101/reduced graphene oxide photocatalyst: An efficient and recyclable photocatalyst for triphenylmethane dye degradation. Environ. Sci. Pollut. Res. 2015, 22, 17238–17243. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Guo, W.; Dang, Z.; Hu, Q.; Wu, F.; Feng, C.; Zhao, X.; Meng, W.; Xing, B.; Giesy, J.P. Refocusing on Nonpriority Toxic Metals in the Aquatic Environment in China. Environ. Sci. Technol. 2017, 51, 3117–3118. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.C.; Sharma, V.K. Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Liang, R.; Xiong, J.; Chen, R.; Zhang, S.; Li, Y.; Wu, L. MIL-68(Fe) as an efficient visible-light-driven photocatalyst for the treatment of a simulated waste-water contain Cr(VI) and Malachite Green. Appl. Catal. B Environ. 2017, 206, 9–15. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Wu, Z.; Jiang, L.; Li, H. Facile synthesis of amino-functionalized titanium metal-organic frameworks and their superior visible-light photocatalytic activity for Cr(VI) reduction. J. Hazard. Mater. 2015, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, W.; Liang, R.; Lin, R.; Wu, L. Highly dispersed palladium nanoparticles anchored on UiO-66(NH2) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst. Nanoscale 2013, 5, 9374–9382. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal organic framework g-C3N4/MIL-53(Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-reduced graphene oxide nanocomposites by a simple self-assembly strategy for increasing interfacial contact: Efficient visible-light photocatalysts. ACS Appl. Mater. Interfaces 2015, 7, 9507–9515. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Garnaud, S.; Rocher, V.; Moilleron, R. Priority pollutants in wastewater and combined sewer overflow. Sci. Total Environ. 2008, 407, 263–272. [Google Scholar] [CrossRef]
- Surib, N.A.; Sim, L.C.; Leong, K.H.; Kuila, A.; Saravanan, P.; Lo, K.M.; Ibrahim, S.; Bahnemann, D.; Jang, M. Ag+, Fe3+ and Zn2+-intercalated cadmium(II)-metal-organic frameworks for enhanced daylight photocatalysis. RSC Adv. 2017, 7, 51272–51280. [Google Scholar] [CrossRef]
- la Farré, M.; Pérez, S.; Kantiani, L. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Huang, W.; Jing, C.; Zhang, X.; Tang, M.; Tang, L.; Wu, M.; Liu, N. Integration of plasmonic effect into spindle-shaped MIL-88A(Fe): Steering charge flow for enhanced visible-light photocatalytic degradation of ibuprofen. Chem. Eng. J. 2018, 349, 603–612. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, X.; Luo, J.; Peng, H.; Lin, H.; Bao, M.; Hong, L.; Zhou, J. Rapid synthesis of Ag/AgCl@ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light. Chem. Eng. J. 2018, 351, 782–790. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core–shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Panneri, S.; Thomas, M.; Ganguly, P.; Nair, B.N.; Mohamed, A.P.; Warrier, K.G.K.; Hareesh, U.S. C3N4 anchored ZIF 8 composites: Photo-regenerable, high capacity sorbents as adsorptive photocatalysts for the effective removal of tetracycline from water. Catal. Sci. Technol. 2017, 7, 2118–2128. [Google Scholar] [CrossRef]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Araya, T.; Jia, M.; Yang, J.; Zhao, P.; Cai, K.; Ma, W.; Huang, Y. Resin modified MIL-53 (Fe) MOF for improvement of photocatalytic performance. Appl. Catal. B Environ. 2017, 203, 768–777. [Google Scholar] [CrossRef]
- Hu, P.; Liang, X.; Yaseen, M.; Sun, X.; Tong, Z.; Zhao, Z.; Zhao, Z. Preparation of highly-hydrophobic novel N-coordinated UiO-66(Zr) with dopamine via fast mechano-chemical method for (CHO-/Cl-)-VOCs competitive adsorption in humid environment. Chem. Eng. J. 2018, 332, 608–618. [Google Scholar] [CrossRef]









| Photocatalyst Type | Examples | Ref. |
|---|---|---|
| Metal oxides | TiO2, ZnO, WO3, CeO2, Fe2O3, In2O3 or Bi2O3 | [35,36,37] |
| Metal sulfides | ZnS, CdS, MoS2, Bi2S3 or CuS/ZnS | [38,39,40] |
| Ternary compounds | Titanates (BaTiO3, SrTiO3 or La2Ti2O7) | [41,42,43] |
| Tungstates (Bi2WO6 or ZnWO4) | [44,45] | |
| Metalates [AxByOz] (such as BiVO4) | [46,47] | |
| Non-metal semiconductors | g-C3N4, graphene | [48,49,50] |
| Multicomponent materials | Bi2S3/Bi2O3/Bi2O2CO3 | |
| Bi2O2CO3/Bi2O4 | [51,52,53] | |
| BiVO4/Bi2O2CO3 | ||
| g-C3N4-heterojunctions | [49] | |
| Graphene-heterojunctions | [54] |
| MOFs | Dye Target 1 | Light Source | Reference |
|---|---|---|---|
| Cu(II)-pyrazole MOFs | MB, MO | UV (+H2O2) | [176] |
| Cd(II)-imidazole MOFs | MB, MO | UV | [177] |
| MO | [178] | ||
| Zn(II)-imidazole MOFs | MO | UV | [178] |
| ZIF-8 (Zn-imidazole MOF) | MB | UV | [179] |
| Ag(I)-MOFs | R6G | UV | [180] |
| 12 azo dyes (among them MO, AO7, CR and EBT) | [181] | ||
| MIL-125 (Ti-MOF) | MB | UV | [182] |
| MIL-88B, MIL-100, MIL-101 (Fe-MOFs) | R6G | visible | [167] |
| MIL-53 (Fe-MOF) | MG, RhB | visible | [183] |
| MB | [184] | ||
| MIL-88A | MB | visible | [185] |
| NTU-9 (Ti-MOF) | RhB, MB | visible | [174] |
| Cu(II)-imidazole MOFsZn(II)-imidazole MOFs | MB | visible | [174] |
| TMU (Zn-MOF) | CR | UV and visible | [186] |
| UiO-66 (Zr-MOF) | MB | visible | [187] |
| Cu (II)-imidazole MOFs | RhB, MB | natural light | [188] |
| Cu (II)-pyrazine MOFs | MB | sunlight | [189] |
| UiO-66 (Zr-Ti MOFs) | MB | sunlight | [190] |
| MOFs | Dye Target 1 | Degradation Rate | Light Source | Reference |
|---|---|---|---|---|
| Fe2O3/MIL-53(Fe) | MB | 70%–240 min | visible | [204] |
| BiVO4/UiO-66 | RhB | 100%–140 min | visible | [160] |
| Bi2WO6/UiO-66 | RhB | 100%–180 min | visible | [205] |
| Bi2MoO6/UiO-66 | RhB | 95%–120 min | visible | [206] |
| Bi2MoO6/MIL-100 | RhB | 90%–90 min | visible | [207] |
| BiOBr/UiO-66 | RhB | 100%–15 min | visible | [208] |
| BiOBr/NH2-MIL-125 | RhB | 100%–100 min | visible | [209] |
| Ag2CO3/UiO-66(Zr) | RhB | 100%–120 min | visible | [210] |
| AgI/UiO-66(Zr) | RhB | 100%–60 min | visible | [211] |
| Ag3PO4/MIL-53(Fe) | RhB | 100%–90 min | visible | [212] |
| g-C3N4/MIL-125 | RhB | 100%–60 min | visible | [213] |
| g-C3N4/MIL-100 | RhB | 100%–240 min | visible | [214] |
| g-C3N4/MIL-53(Al) | RhB | 100%–75 min | visible | [215] |
| rGO/NH2-MIL-125 | MB | 100%–30 min | visible | [216] |
| rGO/MIL-88(Fe) | RhB, MB | 100%–20 min | sunlight | [217] |
| GO/MIL-101(Cr) | MG | 92%–60 min | sunlight | [218] |
| MOFs | Dye Target 1 | Degradation Rate | Light Source | Reference |
|---|---|---|---|---|
| Ag/AgCl/ZIF-8 | RhB | 70%–60 min | visible | [220] |
| 98%–16 min | sunlight | [221] | ||
| Ag/AgCl/MIL-53 | RhB, MB | 100%–50 min | visible | [219] |
| Ag/Ag3PO4/HKUST-1 | PBS | 80%–90 min | visible | [141] |
| Ag3PO4/MIL-101/NiFe2O4 | RhB | 95%–30 min | visible | [222] |
| Ag/GO/MIL-125 | RhB | 95%–50 min | visible | [223] |
| Pd/GO/MIL-101(Cr) | brilliant green and acid fuchsi | 100%–15 min | visible | [224] |
| MOFs | Degradation Rate | Reference |
|---|---|---|
| MIL-68 | 100%–5 min (pH = 3) | [228] |
| NH2-MIL-88, NH2-MIL-101 | 100%–40/60 min | [50] |
| NH2-MIL-125 | 80%–60 min | [229] |
| Au, Pd, Pt@MIL-100 | 100%–8 min | [134] |
| Pd@UiO-66-NH2 | 100%–100 min | [230] |
| rGO/UiO-66-NH2 | 100%–100 min | [140] |
| g-C3N4/MIL-53(Fe) | 100%–180 min | [231] |
| rGO/MIL-53(Fe) | 100%–80 min | [232] |
| Ag/AgCl/MIL-53 | 100%–240 min | [219] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts 2019, 9, 52. https://doi.org/10.3390/catal9010052
Bedia J, Muelas-Ramos V, Peñas-Garzón M, Gómez-Avilés A, Rodríguez JJ, Belver C. A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts. 2019; 9(1):52. https://doi.org/10.3390/catal9010052
Chicago/Turabian StyleBedia, Jorge, Virginia Muelas-Ramos, Manuel Peñas-Garzón, Almudena Gómez-Avilés, Juan J. Rodríguez, and Carolina Belver. 2019. "A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification" Catalysts 9, no. 1: 52. https://doi.org/10.3390/catal9010052
APA StyleBedia, J., Muelas-Ramos, V., Peñas-Garzón, M., Gómez-Avilés, A., Rodríguez, J. J., & Belver, C. (2019). A Review on the Synthesis and Characterization of Metal Organic Frameworks for Photocatalytic Water Purification. Catalysts, 9(1), 52. https://doi.org/10.3390/catal9010052