Catalytic Degradation of Textile Wastewater Effluent by Peroxide Oxidation Assisted by UV Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
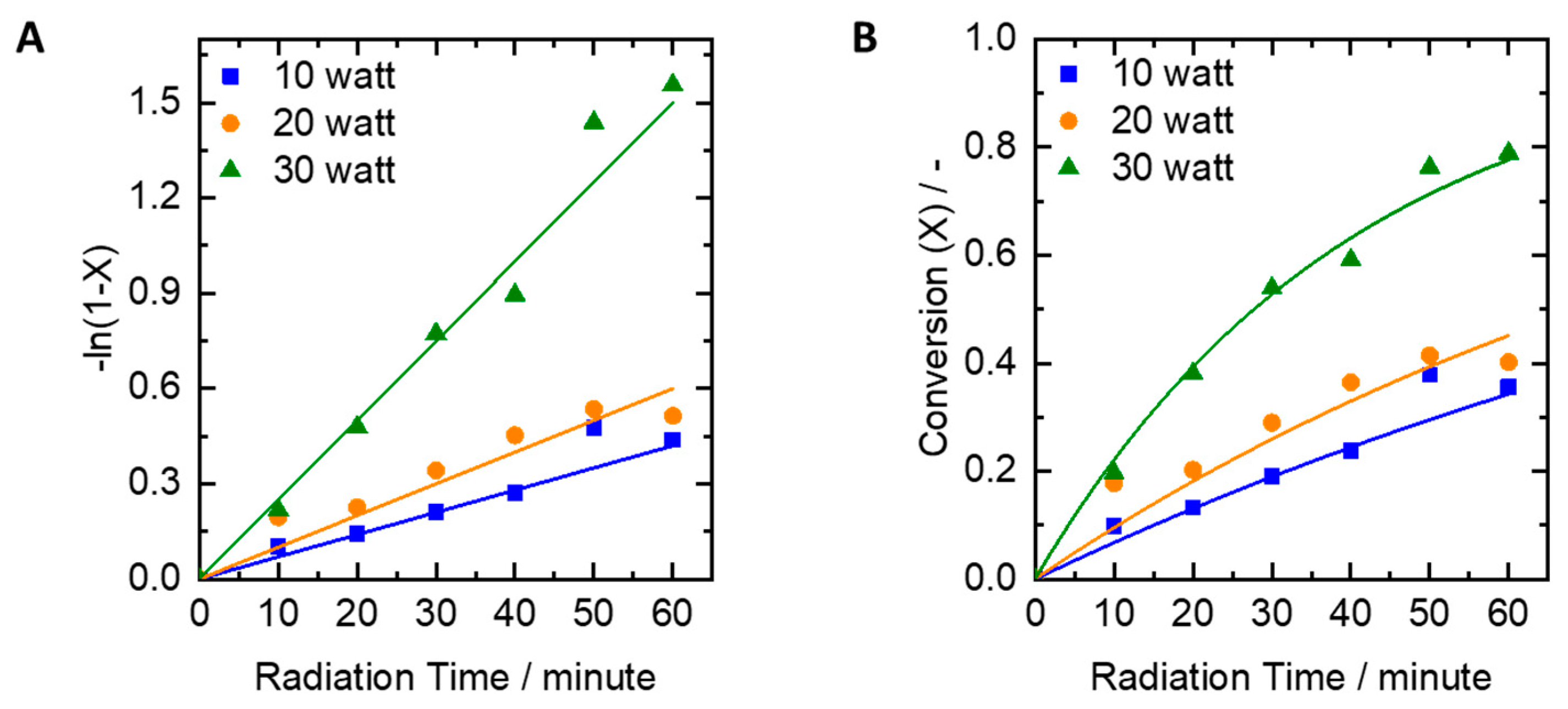
2.1. Influence of UV Lamp Power on the Dye Effluent Degradation
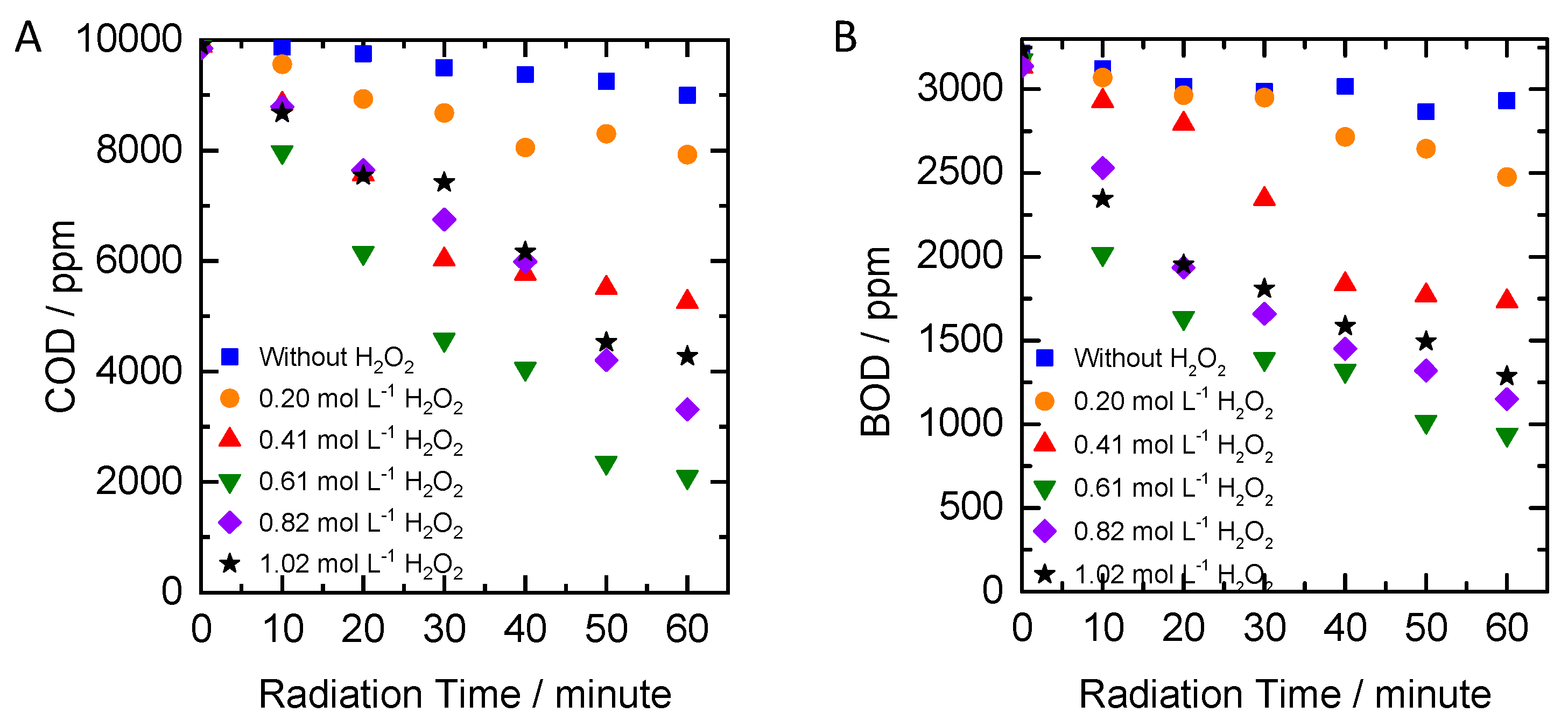
2.2. Influence of Peroxide Concentration
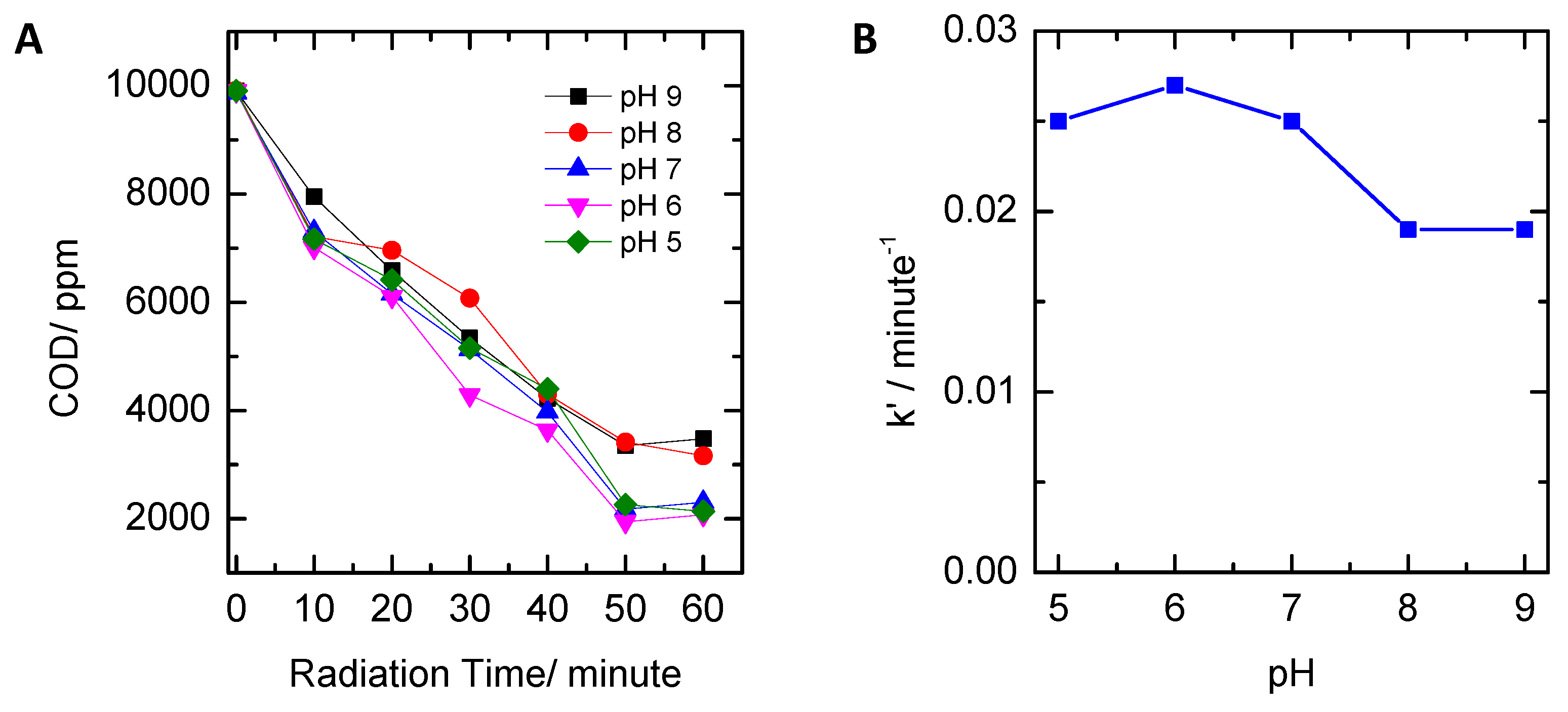
2.3. Influence of pH
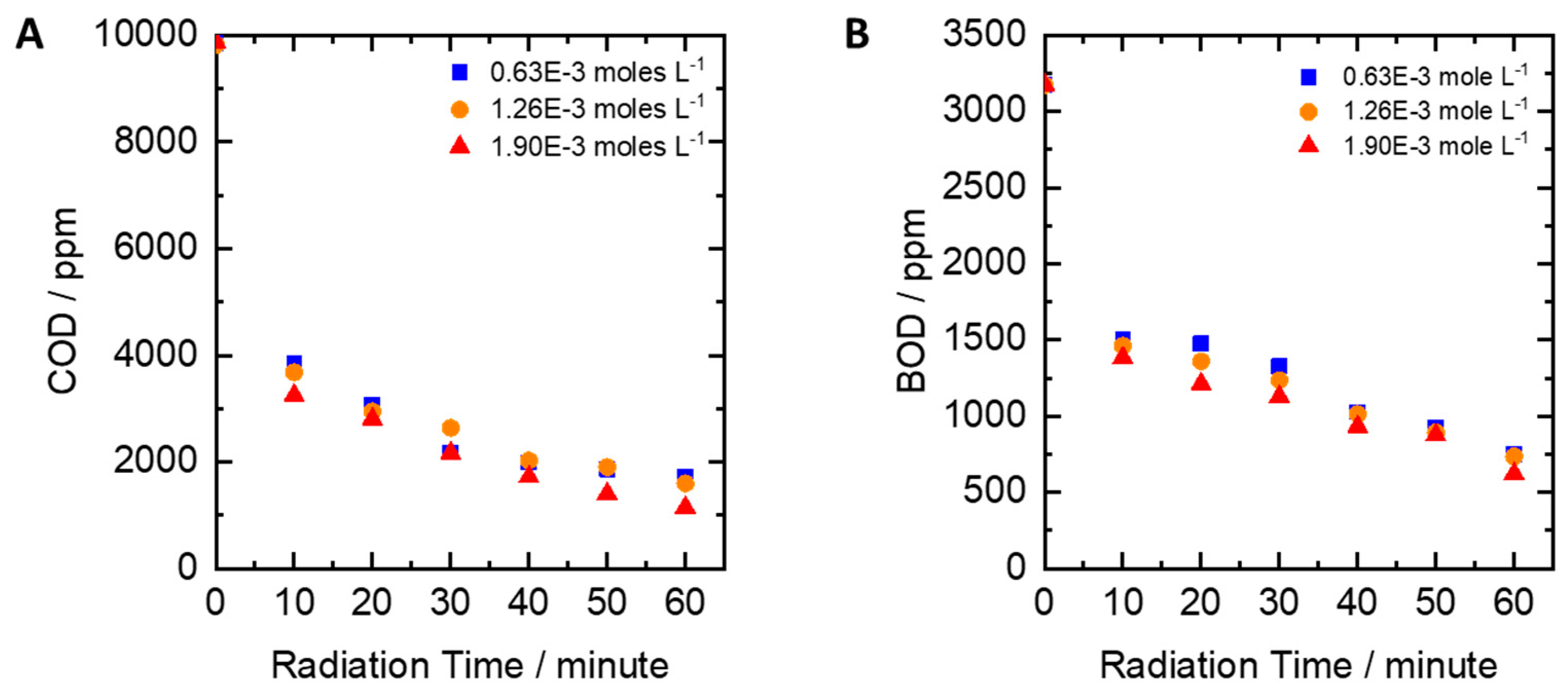
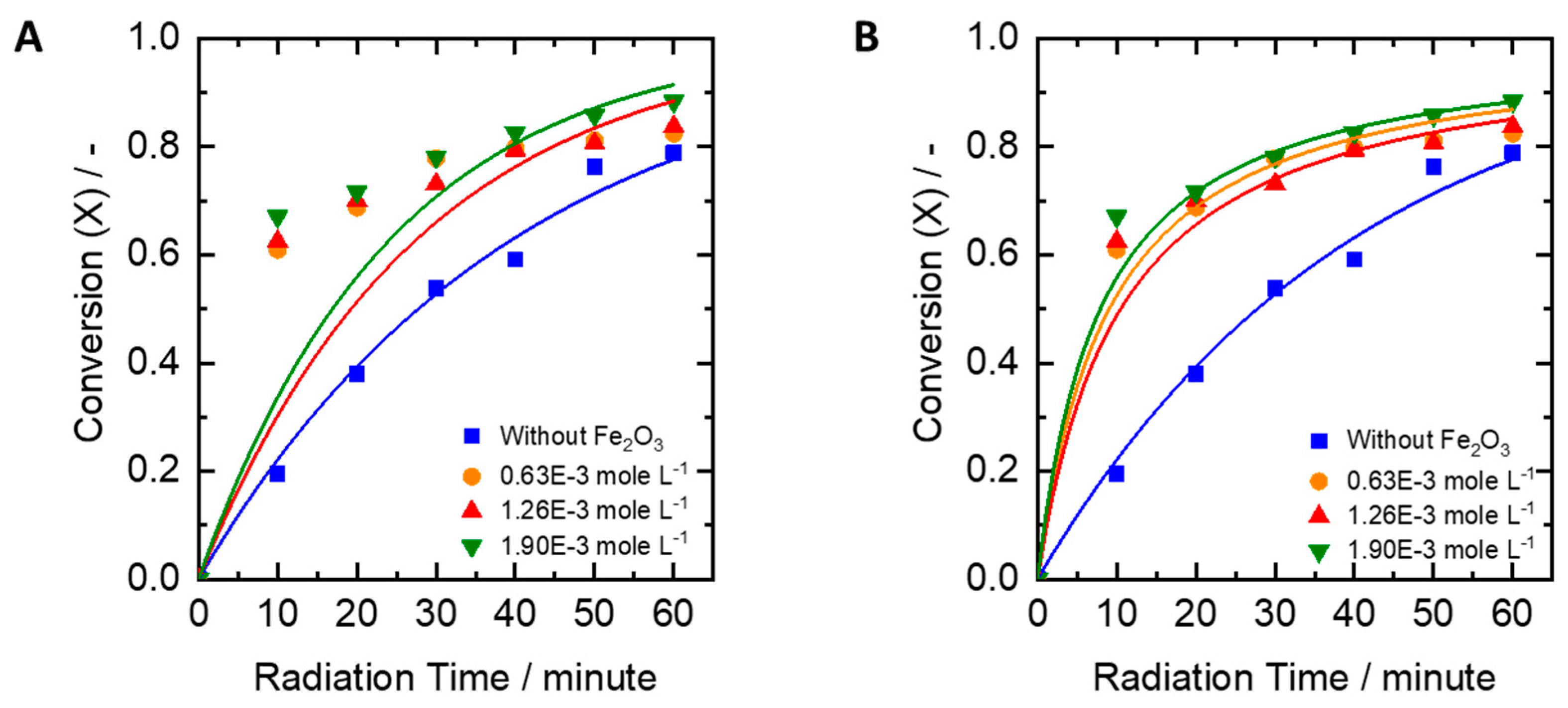
2.4. Influence of Iron Oxide Catalyst
3. Materials and Methods
3.1. Wastewater-Effluent Characteristics
3.2. Fenton Reactions
- (i)
- Hydrogen peroxide (H2O2) concentration variation: concentrations of hydrogen peroxide were set to 0.20; 0.41; 0.61; 0.82; and 1.02 moles/L while UV lamp was set to 30 watts;
- (ii)
- Variation of UV lamp power: the UV lamp power was set to 10, 20, and 30 watts while peroxide concentration employed was 0.61 moles/L;
- (iii)
- Variation of pH: pH was changed in the range of 5–9, while peroxide concentration employed was 0.61 moles/L and UV lamp power was 30 watts. pH was measured using a pH meter (Hanna Instruments, USA);
- (iv)
- The effect of the reaction with Fe2O3 catalyst (phase: α-Fe2O3, particle size <5 µm, pro-analysis, from Merck, Germany): pH, peroxide concentration, and UV lamp power were set to 6, 0.61 moles/L, and 30 watts, respectively. Iron (III) oxide was added at the concentrations of 0.00063 moles/L, 0.00126 moles/L, and 0.00190 moles/L.
3.3. Sample Analyses
3.3.1. Determination of COD Values
3.3.2. Determination of BOD Values
3.4. Kinetic Models
- −rCOD = Oxidation of COD reaction rate, (mg/L.minute)
- K = Reaction rate constant, mg/(L.minute)/(mg/L)α+ β
- CCOD = COD concentration, (mg/L)
- C*OH = Hydroxy radical concentration, (mg/L)
- α = COD concentration order reaction
- β = Hydroxy ion concentration order reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doble, M.; Kumar, A. Biotreatment of Industrial Effluents; Elsevier Science: Amsterdam, Netherlands, 2005; ISBN 9780080456218. [Google Scholar]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783906390239. [Google Scholar]
- Weber, E.J.; Stickney, V.C. Hydrolysis kinetics of Reactive Blue 19-Vinyl Sulfone. Water Res. 1993, 27, 63–67. [Google Scholar] [CrossRef]
- Babaei, A.A.; Kakavandi, B.; Rafiee, M.; Kalantarhormizi, F.; Purkaram, I.; Ahmadi, E.; Esmaeili, S. Comparative treatment of textile wastewater by adsorption, Fenton, UV-Fenton and US-Fenton using magnetic nanoparticles-functionalized carbon (MNPs@C). J. Ind. Eng. Chem. 2017, 56, 163–174. [Google Scholar] [CrossRef]
- Ariyanto, T.; Kurniasari, M.; Laksmana, W.T.; Prasetyo, I. Pore size control of polymer-derived carbon adsorbent and its application for dye removal. Int. J. Environ. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vlad, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4 during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Vial, C.; Barkaoui, M. Studies on the decolorization of textile dye wastewater by continuous electrocoagulation process. Chem. Eng. J. 2009, 149, 207–214. [Google Scholar] [CrossRef]
- Hu, T.-L. Removal of reactive dyes from aqueous solution by different bacterial genera. Water Sci. Technol. 1996, 34, 89–95. [Google Scholar]
- Martins, A.F. Advanced oxidation processes applied to effluent streams from an agrochemical industry. Pure Appl. Chem. 1998, 70, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Munter, R. Advanced oxidation processes current status and prospect. Proc. Est. Acad. Sci. Chem 2001, 50, 59–80. [Google Scholar]
- Munoz, M.; Mora, F.J.; de Pedro, Z.M.; Alvarez-Torrellas, S.; Casas, J.A.; Rodriguez, J.J. Application of CWPO to the treatment of pharmaceutical emerging pollutants in different water matrices with a ferromagnetic catalyst. J. Hazard. Mater. 2017, 331, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Schrank, S.G.; dos Santos, J.N.R.; Souza, D.S.; Souza, E.E.S. Decolourisation effects of Vat Green 01 textile dye and textile wastewater using H2O2/UV process. J. Photochem. Photobiol. A Chem. 2007, 186, 125–129. [Google Scholar] [CrossRef]
- Wang, Q.; Pang, W.; Mao, Y.; Sun, Q.; Zhang, P.; Ke, Q.; Yu, H.; Dai, C.; Zhao, M. Study of the degradation of trimethoprim using photo-Fenton oxidation technology. Water (Switzerland) 2019, 11, 207. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 3, 557–572. [Google Scholar] [CrossRef]
- Shu, H.Y.; Hunag, C.R.; Chang, M.C. Decolorization of mono-azo dyes in wastewater by advanced oxidation process: a case study of acid red 1 and acid yellow 23. Chemosphere 1994, 29, 2597–2607. [Google Scholar] [CrossRef]
- Amelia, S.; Sediawan, W.B.; Prasetyo, I.; Munoz, M.; Ariyanto, T. Role of the pore structure of Fe/C catalysts on heterogeneous Fenton oxidation. J. Environ. Chem. Eng. 2019, 102921. (In Press) [Google Scholar] [CrossRef]
- Natalija Koprivance, H.K. AOP as an effective tool for the mineralization of hazardous organic pollutants in colored wastewater; chemical and photochemical processes. In Hazardous Materials and Wastewater: Treatment, Removal and Analysis; Lewinsky, A.A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 149–199. ISBN 1-60021-257-3. [Google Scholar]
- Tanyong, I.B. Penurunan kadar chemical oxygen demand limbah rhodamin B. Bachelor Thesis, Universitas Gadjah Mada, Yogyakarya, Indonesia, 31 January 2012. [Google Scholar]
- Paesal, P. Studi degradasi limbah cair industri tekstil dengan menggunakan metode proses oksidasi lanjut. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 22 August 2008. [Google Scholar]



| UV Lamp Power, watt | k’, min−1 |
|---|---|
| 10 | 0.0070 |
| 20 | 0.0100 |
| 30 | 0.0250 |
| Hydrogen Peroxide Concentration, moles/L | k’, min−1 |
|---|---|
| 0 | 0.0010 |
| 0.2 | 0.0040 |
| 0.41 | 0.0120 |
| 0.61 | 0.0250 |
| 0.82 | 0.0160 |
| 1.02 | 0.0130 |
| Iron (III) Oxide Concentration, moles/L | First Order k’, min−1 | Second Order k’, L. (mg·min)−1 |
|---|---|---|
| 6 × 10−4 | 0.0254 | 1.12 × 10−5 |
| 1 × 10−3 | 0.0255 | 9.71 × 10−6 |
| 2 × 10−3 | 0.0410 | 1.28 × 10−5 |
| No. | Parameter | Value |
|---|---|---|
| 1 | Color (TCU) | 47,000 |
| 2 | COD (mg/L) | 9906 |
| 3 | BOD5 (mg/L) | 3175 |
| 4 | pH | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarto, S.; Paesal, P.; Tanyong, I.B.; Laksmana, W.T.; Prasetya, A.; Ariyanto, T. Catalytic Degradation of Textile Wastewater Effluent by Peroxide Oxidation Assisted by UV Light Irradiation. Catalysts 2019, 9, 509. https://doi.org/10.3390/catal9060509
Sarto S, Paesal P, Tanyong IB, Laksmana WT, Prasetya A, Ariyanto T. Catalytic Degradation of Textile Wastewater Effluent by Peroxide Oxidation Assisted by UV Light Irradiation. Catalysts. 2019; 9(6):509. https://doi.org/10.3390/catal9060509
Chicago/Turabian StyleSarto, Sarto, Paesal Paesal, Irine Bellina Tanyong, William Teja Laksmana, Agus Prasetya, and Teguh Ariyanto. 2019. "Catalytic Degradation of Textile Wastewater Effluent by Peroxide Oxidation Assisted by UV Light Irradiation" Catalysts 9, no. 6: 509. https://doi.org/10.3390/catal9060509