Facile Synthesis of P25@Pd Core-Shell Catalyst with Ultrathin Pd Shell and Improved Catalytic Performance in Heterogeneous Enantioselective Hydrogenation of Acetophenone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of the P25@Pd Catalyst
2.2. Structural Properties of the P25@Pd Catalyst
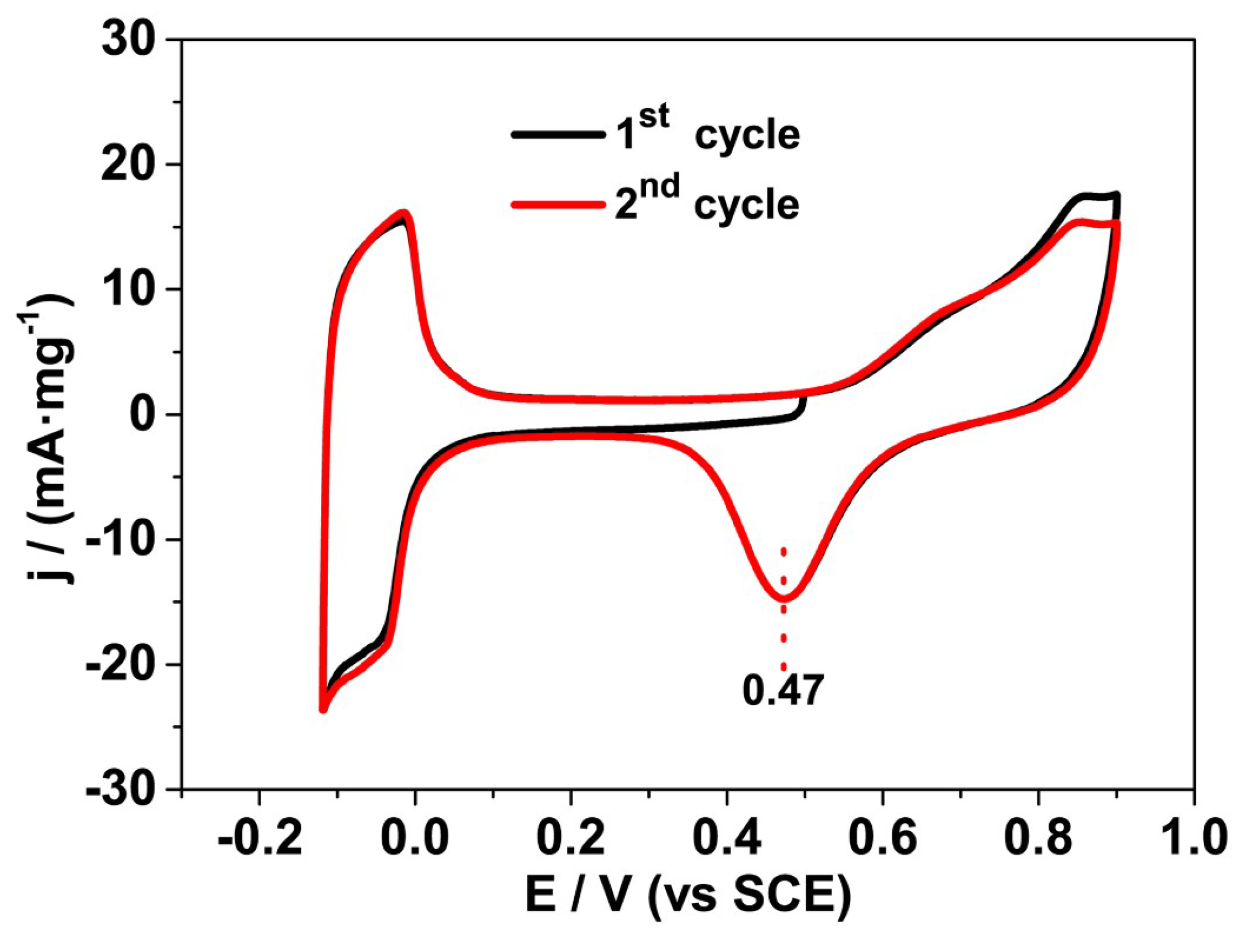
2.3. Study on the Formation of P25@Pd Core-Shell Structure
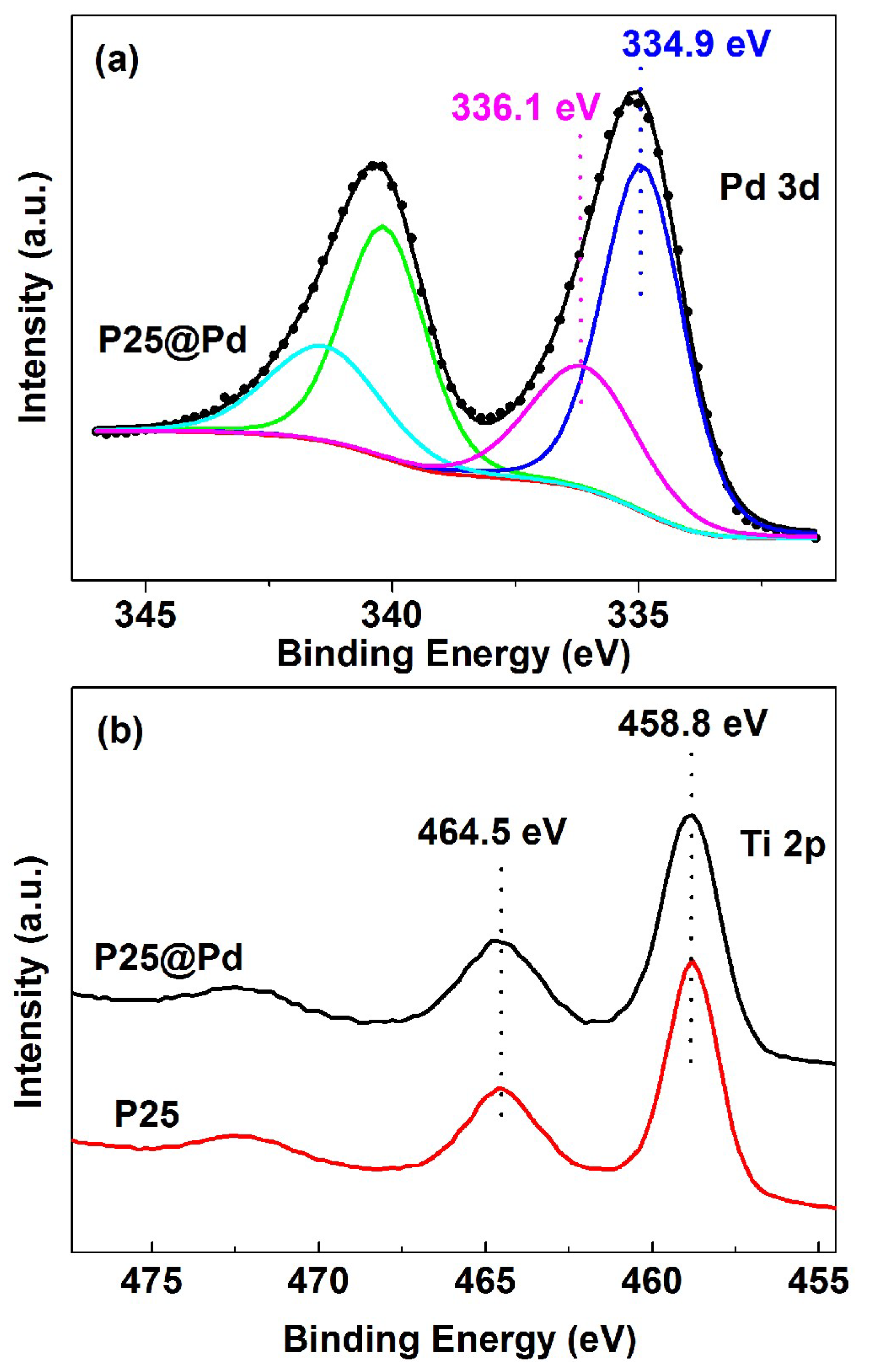
2.4. Electronic Properties of the P25@Pd Catalyst
2.5. Enantioselective Hydrogenation of Acetophenone
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Catalyst Synthesis
3.3. Characterization
3.4. Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blaser, H.U.; Pugin, B. Scope and Limitations of the Application of Heterogeneous Enantioselective Catalysis. In Chiral Reactions in Heterogeneous Catalysis; Jannes, G., Dubois, V., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 33–57. ISBN 978-1-4615-1909-6. [Google Scholar]
- Baiker, A. Crucial Aspects in the Design of Chirally Modified Noble Metal Catalysts for Asymmetric Hydrogenation of Activated Ketones. Chem. Soc. Rev. 2015, 44, 7449–7464. [Google Scholar] [CrossRef] [PubMed]
- Meemken, F.; Baiker, A. Recent Progress in Heterogeneous Asymmetric Hydrogenation of C=O and C=C Bonds on Supported Noble Metal Catalysts. Chem. Rev. 2017, 117, 11522–11569. [Google Scholar] [CrossRef] [PubMed]
- Klabunovskii, E.; Smith, G.V.; Zsigmond, Á. Heterogeneous Enantioselective Hydrogenations-Theory and Practice; Springer: Dordrecht, The Netherlands, 2006; pp. 161–170. ISBN 978-1-4020-4296-6. [Google Scholar]
- Tungler, A.; Tarnai, T.; Mathe, T.; Petro, J. Enantioselective Hydrogenation of Acetophenone in the Presence of S-proline. J. Mol. Catal. 1991, 67, 277–282. [Google Scholar] [CrossRef]
- Hess, R.; Vargas, A.; Mallat, T.; Bürgi, T.; Baiker, A. Inversion of Enantioselectivity in the Platinum-catalyzed Hydrogenation of Substituted Acetophenones. J. Catal. 2004, 222, 117–128. [Google Scholar] [CrossRef]
- Vetere, V. New Approach toward the Synthesis of Asymmetric Heterogeneous Catalysts for Hydrogenation Reactions. J. Catal. 2004, 226, 457–461. [Google Scholar] [CrossRef]
- Mills, P.L.; Ramachandran, P.A.; Chaudhari, R.V. Multiphase Reaction-engineering for Fine Chemicals and Pharmaceuticals. Rev. Chem. Eng. 1992, 8, 1–176. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P.; Selva, M. Multiphase Heterogeneous Catalytic Enantioselective Hydrogenation of Acetophenone over Cinchona-modified Pt/C. J. Mol. Catal. A Chem. 2002, 180, 169–175. [Google Scholar] [CrossRef]
- Mhadgut, S.; Torok, M.; Esquibel, J.; Torok, B. Highly Asymmetric Heterogeneous Catalytic Hydrogenation of Isophorone on Proline Modified Base-supported Palladium Catalysts. J. Catal. 2006, 238, 441–448. [Google Scholar] [CrossRef]
- Kubota, T.; Kubota, H.; Kubota, T.; Moriyasu, E.; Uchida, T.; Nitta, Y.; Sugimura, T.; Okamoto, Y. Enantioselective Hydrogenation of (E)-α-phenylcinnamic Acid over Cinchonidine-modified Pd Catalysts Supported on TiO2 and CeO2. Catal. Lett. 2009, 129, 387–393. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, Z.; Li, M.; Yang, Q.; Li, C. Enhancement of the Performance of a Platinum Nanocatalyst Confined within Carbon Nanotubes for Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2011, 50, 4913–4917. [Google Scholar] [CrossRef]
- Guan, Z.H.; Lu, S.M.; Li, C. Enantioselective Hydrogenation of α,β-unsaturated Carboxylic Acid over Cinchonidine-modified Pd Nanoparticles Confined in Carbon Nanotubes. J. Catal. 2014, 311, 1–5. [Google Scholar] [CrossRef]
- Bui Trung, T.S.; Kim, Y.; Kang, S.; Lee, H.; Kim, S. The Enantioselective Hydrogenation of (E)-α-phenylcinnamic Acid: Role of TiO2 Coated on Al2O3 as a Novel Support for Cinchonidine-modified Pd Catalysts. Catal. Commun. 2015, 66, 21–24. [Google Scholar] [CrossRef]
- Li, C. Chiral Synthesis on Catalysts Immobilized in Microporous and Mesoporous Materials. Catal. Rev. Sci. Eng. 2004, 46, 419–492. [Google Scholar] [CrossRef]
- Hoxha, F.; Schimmoeller, B.; Cakl, Z.; Urakawa, A.; Mallat, T.; Pratsinis, S.E.; Baiker, A. Influence of Support Acid–base Properties on the Platinum-catalyzed Enantioselective Hydrogenation of Activated Ketones. J. Catal. 2010, 271, 115–124. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, N.; Wang, B.; Liu, C.; Zhu, G. Fabrication of Fe3O4/PAH/PSS@Pd Core-shell Microspheres by Layer-by-layer Assembly and Application in Catalysis. J. Colloid Interface Sci. 2014, 421, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, G.X.; Yang, H.Y.; Wang, X.L.; Liang, J.H.; Liu, P.X.; Chen, M.; Zheng, N.F. Shape-controlled Synthesis of Surface-clean Ultrathin Palladium Nanosheets by Simply Mixing a Dinuclear PdⅠ Carbonyl Chloride Complex with H2O. Angew. Chem. Int. Ed. 2013, 52, 8368–8372. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Tang, S.H.; Mu, X.L.; Dai, Y.; Chen, G.X.; Zhou, Z.Y.; Ruan, F.X.; Yang, Z.L.; Zheng, N.F. Freestanding Palladium Nanosheets with Plasmonic and Catalytic Properties. Nat. Nanotechnol. 2011, 6, 28–32. [Google Scholar] [CrossRef]
- He, M.; Ji, J.; Liu, B.Y.; Huang, H.B. Reduced TiO2 with Tunable Oxygen Vacancies for Catalytic Oxidation of Formaldehyde at Room Temperature. Appl. Surf. Sci. 2019, 473, 934–942. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong Metal-support Interactions. Group 8 Noble Metals Supported on TiO2. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Kovtunov, K.V.; Barskiy, D.A.; Salnikov, O.G.; Burueva, D.B.; Khudorozhkov, A.K.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Bukhtiyarov, V.I.; Koptyug, I.V. Strong Metal-support Interactions for Palladium Supported on TiO2 Catalysts in the Heterogeneous Hydrogenation with Parahydrogen. ChemCatChem 2015, 7, 2581–2584. [Google Scholar] [CrossRef]
- Shen, W.J.; Okumura, M.; Matsumura, Y.; Haruta, M. The Influence of the Support on the Activity and Selectivity of Pd in CO Hydrogenation. Appl. Catal. A Gen. 2001, 213, 225–232. [Google Scholar] [CrossRef]
- Tapin, B.; Epron, F.; Especel, C.; Ly, B.K.; Pinel, C.; Besson, M. Study of Monometallic Pd/TiO2 Catalysts for the Hydrogenation of Succinic Acid in Aqueous Phase. ACS Catal. 2013, 3, 2327–2335. [Google Scholar] [CrossRef]
- Lu, M.H.; Du, H.; Wei, B.; Zhu, J.; Li, M.S.; Shan, Y.H.; Song, C.S. Catalytic Hydrodeoxygenation of Guaiacol over Palladium Catalyst on Different Titania Supports. Energy Fuels 2017, 31, 10858–10865. [Google Scholar] [CrossRef]
- Xiang, Q.; Lv, K.; Yu, J. Pivotal Role of Fluorine in Enhanced Photocatalytic Activity of Anatase TiO2 Nanosheets with Dominant (001) Facets for the Photocatalytic Degradation of Acetone in Air. Appl. Catal. B Environ. 2010, 96, 557–564. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3333. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, C.B.; Ma, J.Z.; Chen, M.; Deng, H.; He, H. High Temperature Reduction Dramatically Promotes Pd/TiO2 Catalyst for Ambient Formaldehyde Oxidation. Appl. Catal. B Environ. 2017, 217, 560–569. [Google Scholar] [CrossRef]
- Masson, J.; Cividino, P.; Court, J. Selective Hydrogenation of Acetophenone on Chromium Promoted Raney Nickel Catalysts. Ⅲ. The Influence of the Nature of the Solvent. Appl. Catal. A Gen. 1997, 161, 191–197. [Google Scholar] [CrossRef]
- Bergault, I.; Fouilloux, P.; Joly-Vuillemin, C.; Delmas, H. Kinetics and Intraparticle Diffusion Modelling of a Complex Multistep Reaction: Hydrogenation of Acetophenone over a Rhodium Catalyst. J. Catal. 1998, 175, 328–337. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Onodera, Y.; Yoshida, H.; Arai, M. Selective Hydrogenation of Acetophenone with Supported Pd and Rh Catalysts in Water, Organic Solvents, and CO2-dissolved Expanded Liquids. Green Chem. 2016, 18, 4934–4940. [Google Scholar] [CrossRef]
- Su, N.; Gao, X.Y.; Chen, X.Y.; Yue, B.; He, H.Y. The Enantioselective Hydrogenation of Acetophenone over Pd Concave Tetrahedron Nanocrystals Affected by the Residual Adsorbed Capping Agent Polyvinylpyrrolidone (PVP). J. Catal. 2018, 367, 244–251. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Gomez, J.F.; Herrera, A.; Jimenez, C.; Marinas, J.M. Reduction of Acetophenones over Pd/AlPO4 Catalysts. Linear Free Energy Relationship (LFER). J. Catal. 1993, 140, 335–343. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Waksmundzka, A.; Makowski, W.; Sobczak, J.W.; Krol, A.; Zieba, A. Acetophenone Hydrogenation on Polymer-palladium Catalysts. The Effect of Polymer Matrix. Catal. Lett. 2004, 94, 143–156. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]





| Entry | Catalyst | Reaction Rate (mmol h−1 gPd−1) | e.e. (%) | Dominant Enantiomer |
|---|---|---|---|---|
| 1 | P25@Pd | 36.5 | 30 | R |
| 2 | Pd/P25 | 1.00 | 20 | R |
| 3 | Pd black | 1.21 | 13 | R |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; He, L.; Xu, J.; Chen, X.; He, H. Facile Synthesis of P25@Pd Core-Shell Catalyst with Ultrathin Pd Shell and Improved Catalytic Performance in Heterogeneous Enantioselective Hydrogenation of Acetophenone. Catalysts 2019, 9, 513. https://doi.org/10.3390/catal9060513
Gao X, He L, Xu J, Chen X, He H. Facile Synthesis of P25@Pd Core-Shell Catalyst with Ultrathin Pd Shell and Improved Catalytic Performance in Heterogeneous Enantioselective Hydrogenation of Acetophenone. Catalysts. 2019; 9(6):513. https://doi.org/10.3390/catal9060513
Chicago/Turabian StyleGao, Xiuyun, Lulu He, Juntong Xu, Xueying Chen, and Heyong He. 2019. "Facile Synthesis of P25@Pd Core-Shell Catalyst with Ultrathin Pd Shell and Improved Catalytic Performance in Heterogeneous Enantioselective Hydrogenation of Acetophenone" Catalysts 9, no. 6: 513. https://doi.org/10.3390/catal9060513





