Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs
Abstract
:1. Introduction
2. Results and Discussion
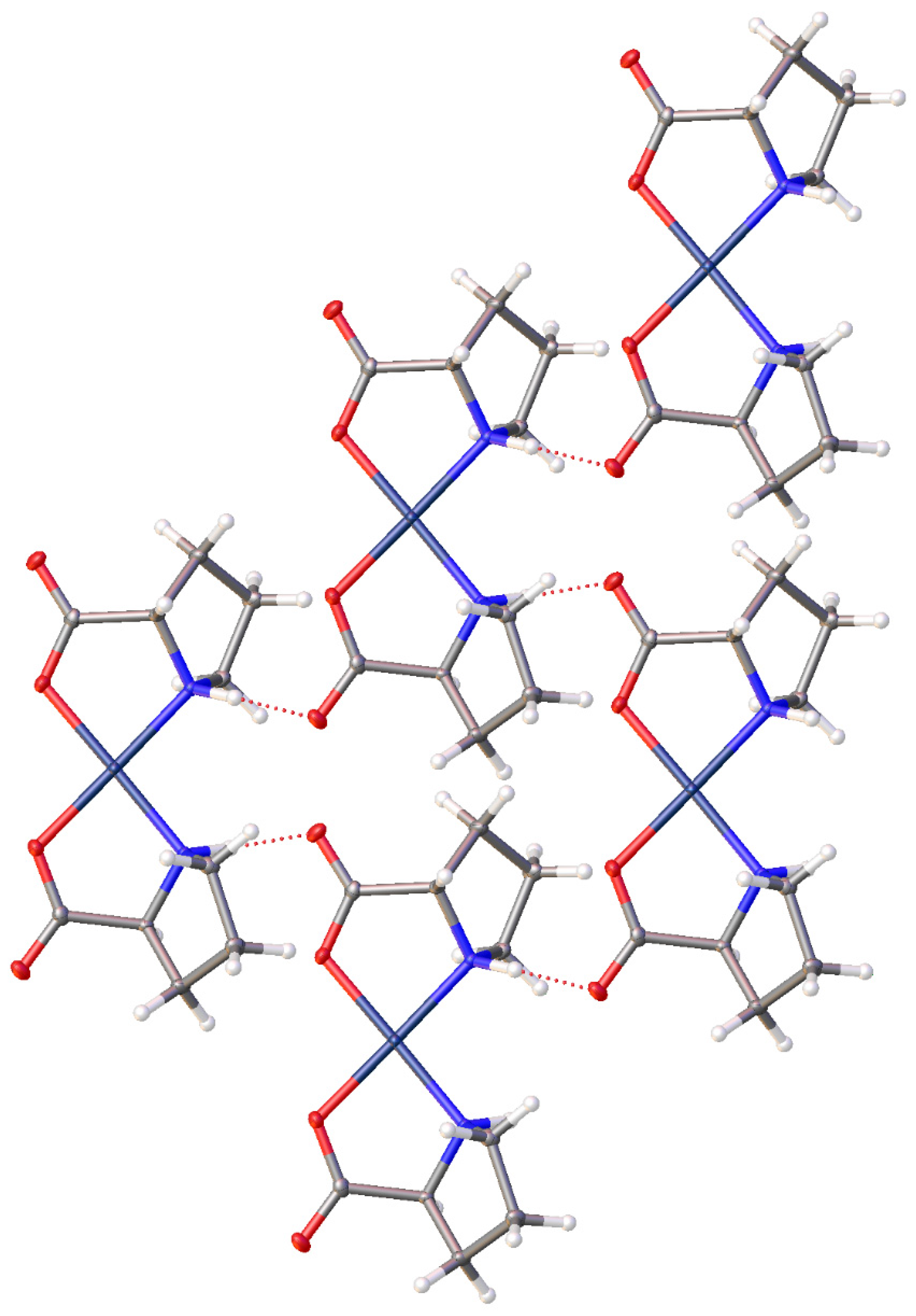
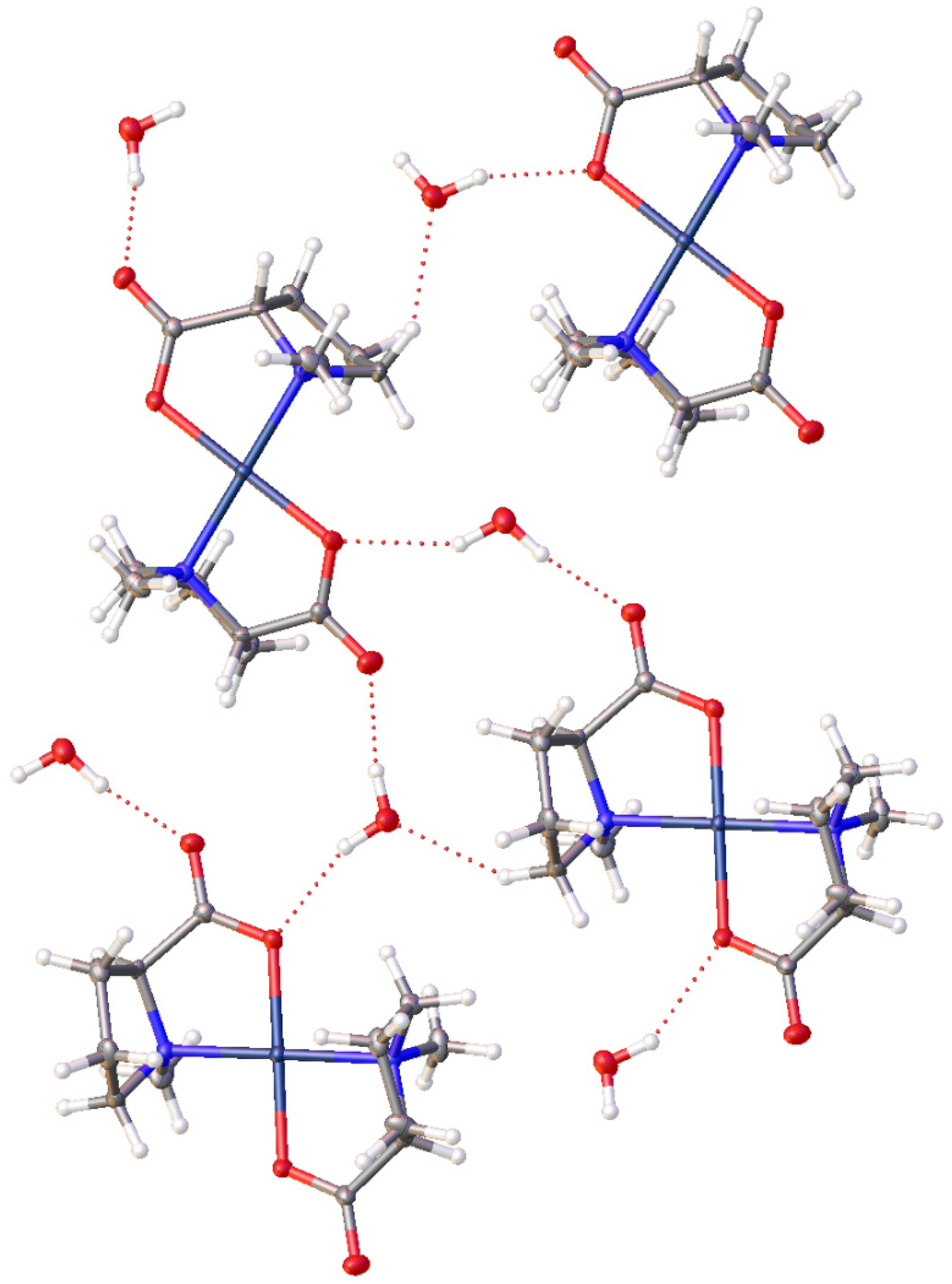
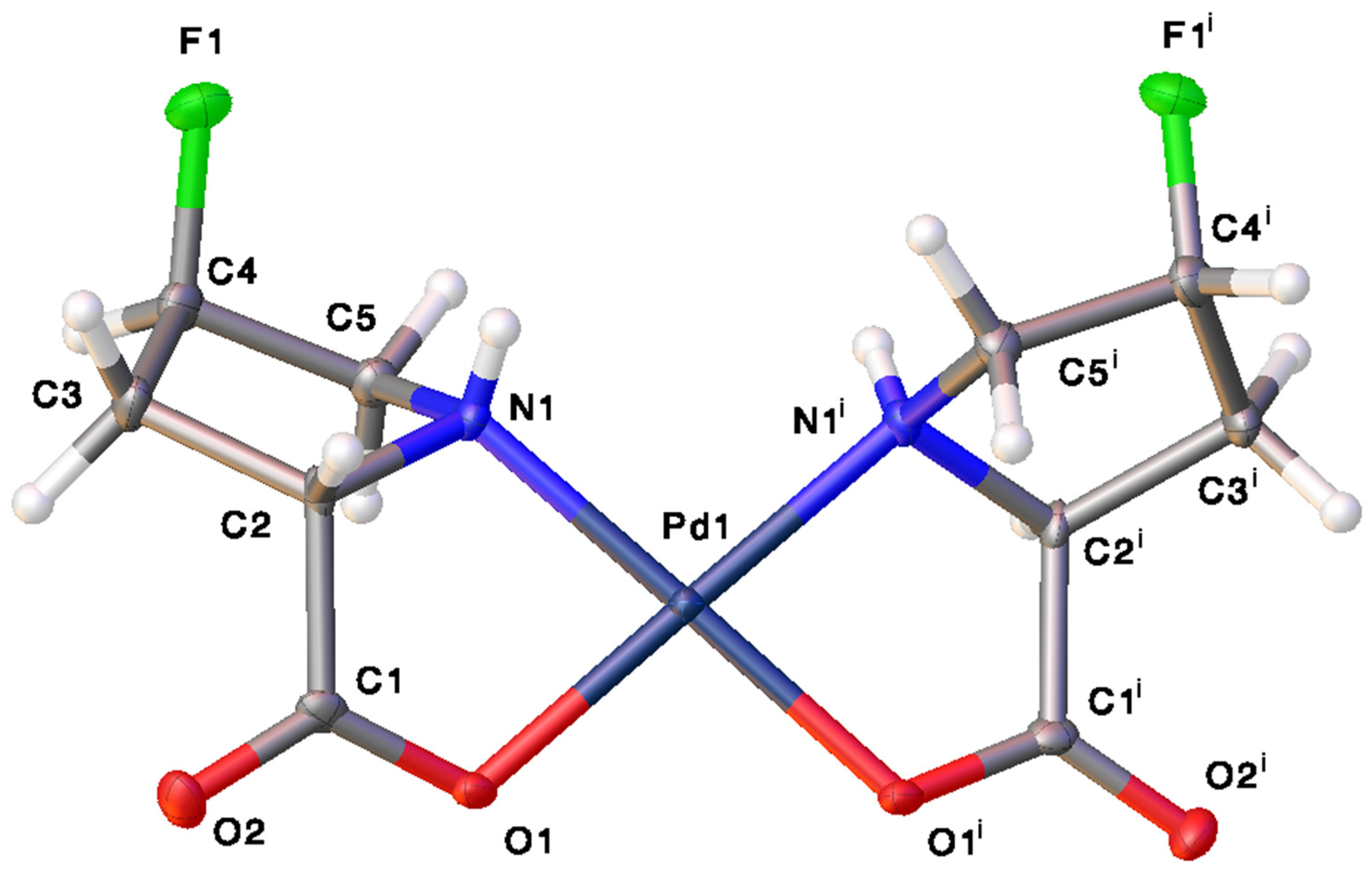
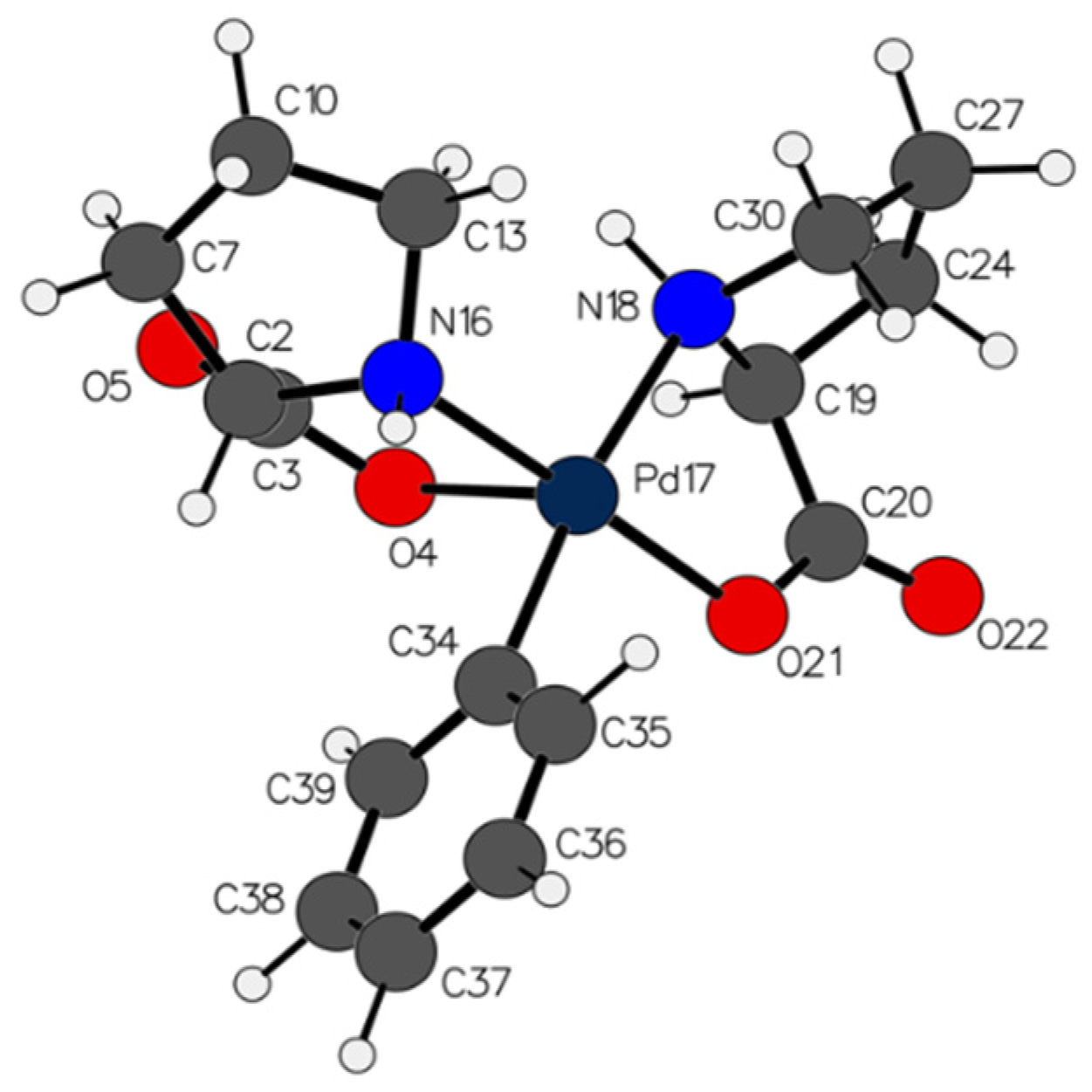
2.1. Characterization and Hydrogen Bonding Interactions
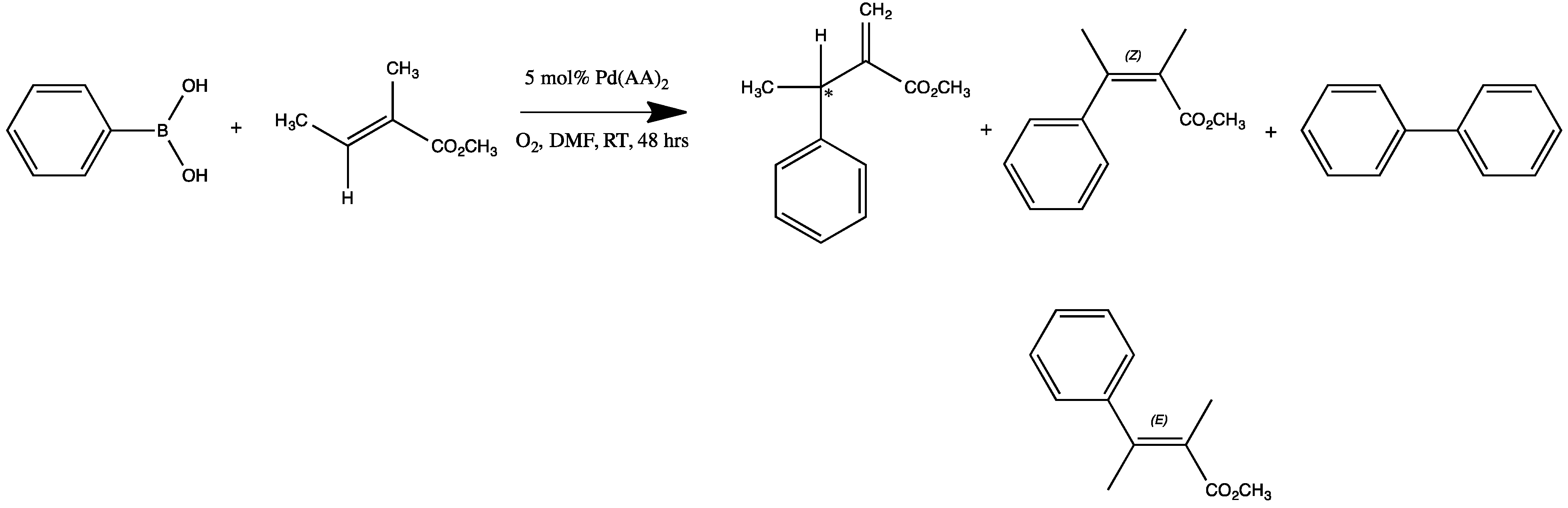
2.2. Catalytic Activity
2.3. Oxidative Coupling of Phenylboronic Acids and Alkenes
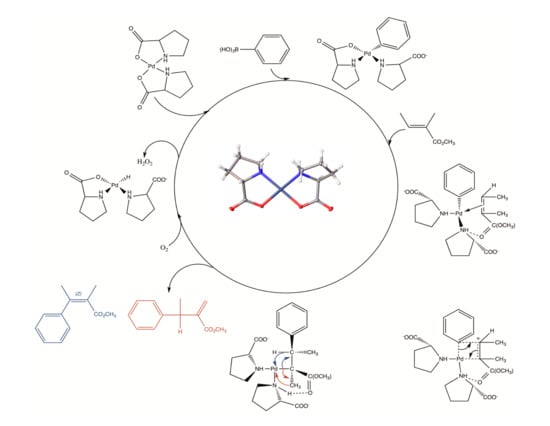
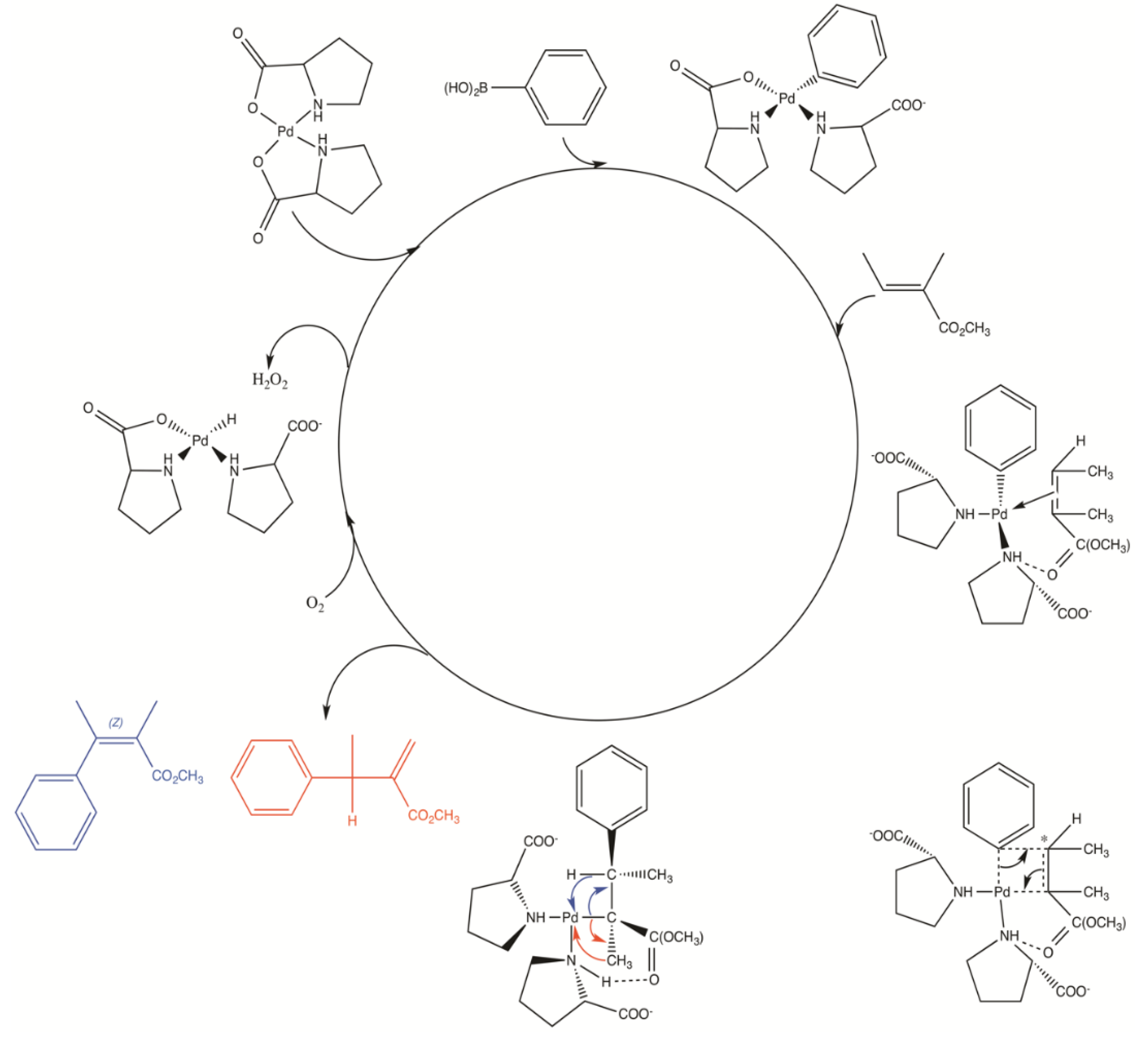
2.4. Proposed Mechanism of Pd‒AA2 Oxidative Coupling
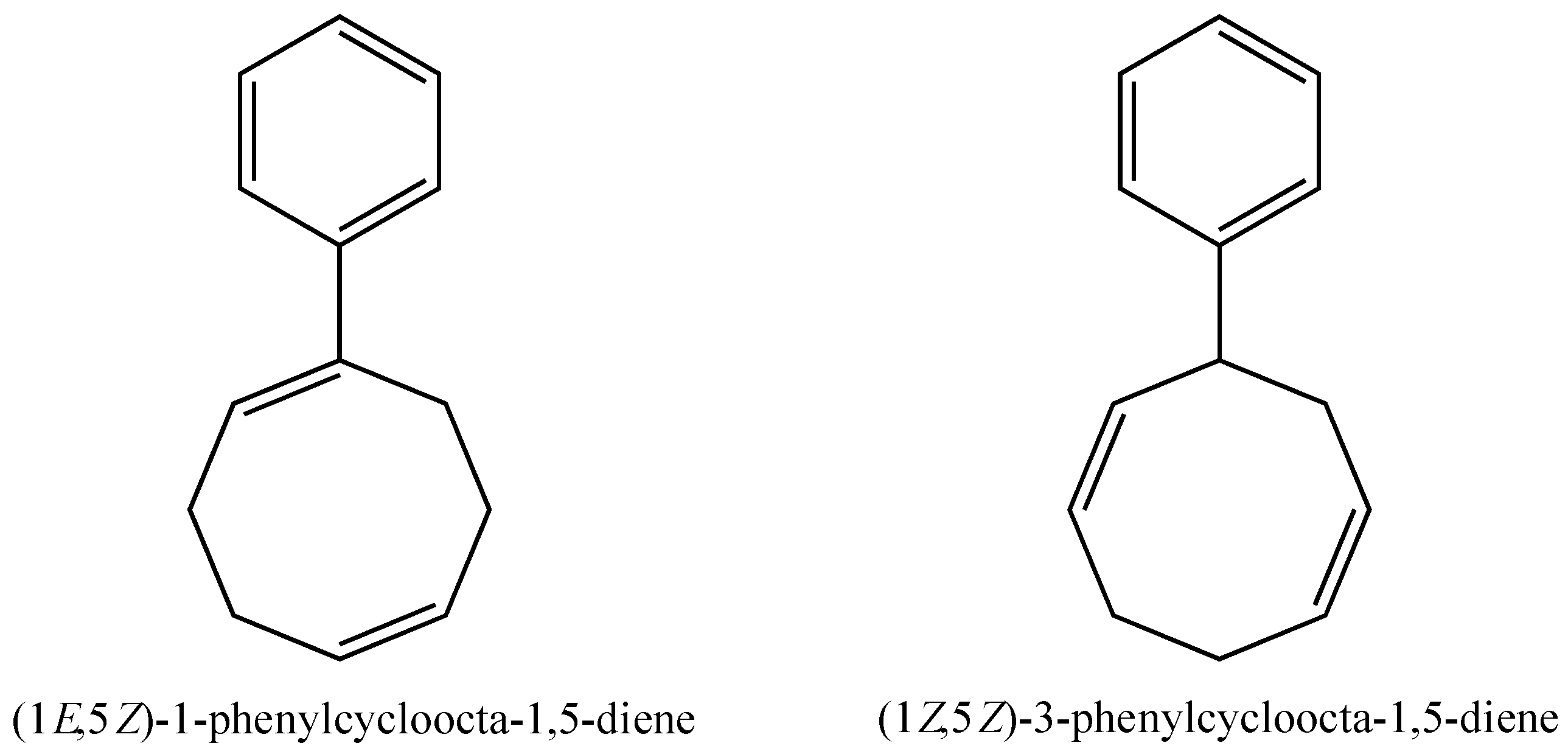
2.5. Biaryl Formation
2.6. Multiple Insertions
2.7. Temperature Effects
2.8. Solvent Effects
2.9. Pd(II)-Amino Acid Complexes as Polymerization Catalysts
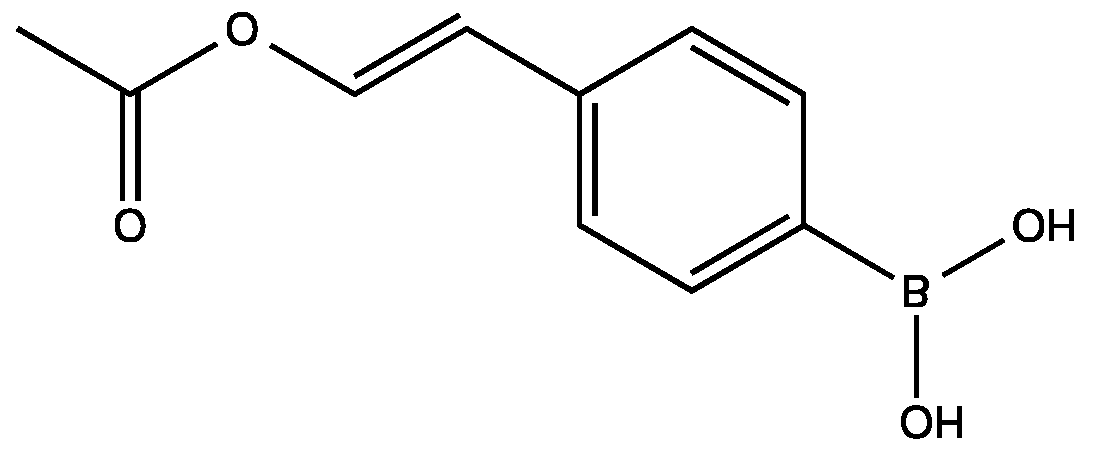
2.10. Other Coupling Substrates
3. Materials and Methods
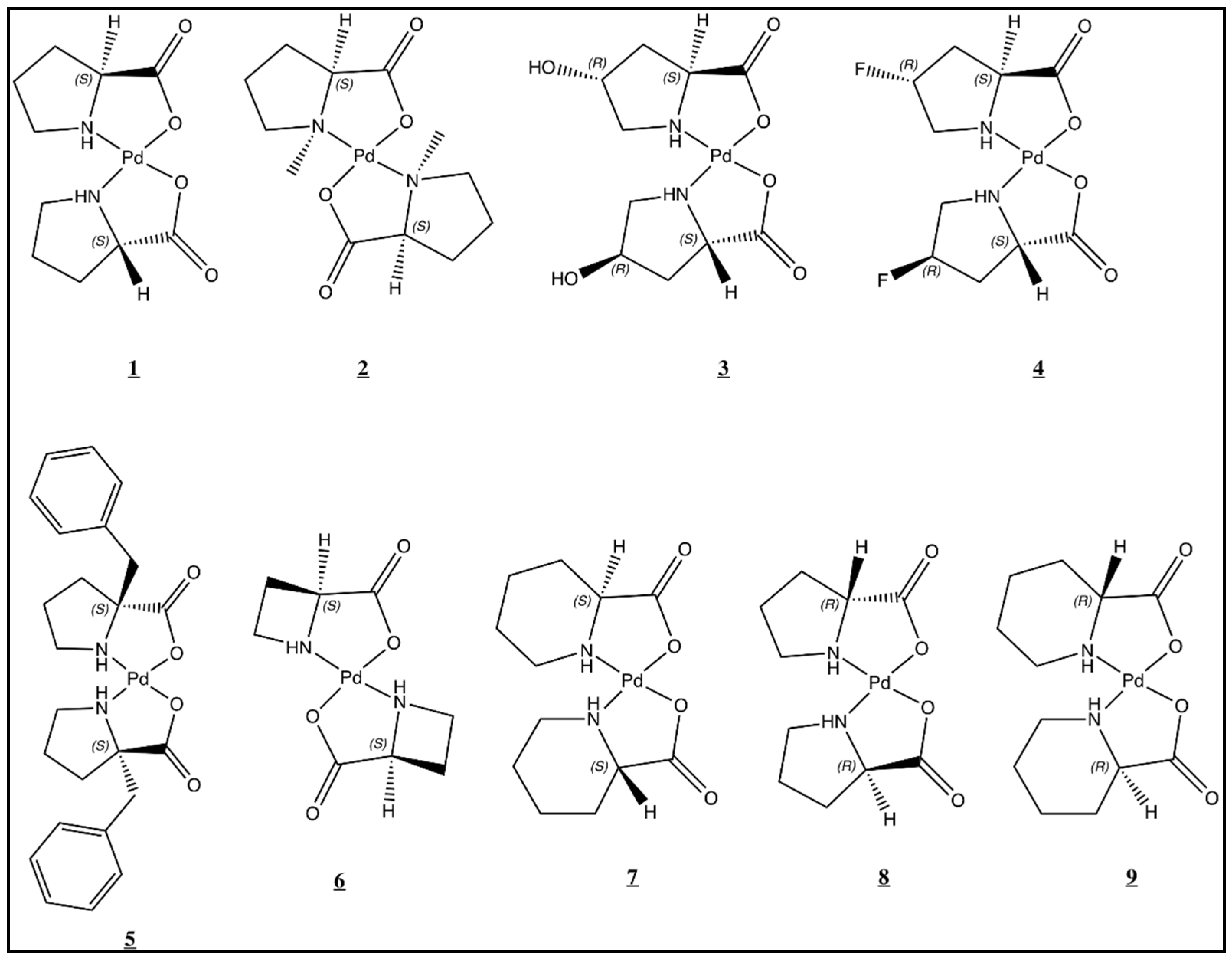
3.1. General Procedure for the Synthesis of Palladium(II) Amino Acid Complexes
3.2. Synthesis of cis-bis-(L-prolinato)palladium(II) (1)
3.3. Synthesis of trans-bis-(N-methyl-L-prolinato)palladium(II) (2)
3.4. Synthesis of cis-bis-(trans-4-hydrox-L-yprolinato)palladium(II) (3)
3.5. Synthesis of cis-bis-(trans-4-fluoro-L-prolinato)palladium(II) (4)
3.6. Synthesis of trans-bis-(2-benzylprolinato)palladium(II) (5)
3.7. Synthesis of trans-bis-(L-azetidine-2-carboxylato)palladium(II) (6)
3.8. Synthesis of cis-bis-(L-pipecolinato)palladium(II) (7)
3.9. Synthesis of cis-bis-(D-prolinato)palladium(II) (8)
3.10. Synthesis of cis-bis-(D-pipecolinato)palladium(II) (9)
3.11. General Procedure for Catalytic Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jin, L.Q.; Lei, A.W. Mechanistic aspects of oxidation of palladium with O2. Sci. China Chem. 2012, 55, 2027–2035. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Martinelli, M.; Sottocornola, S. C-C, C-O, C-N Bond Formation on sp2 Carbon by Palladium(II)-Catalyzed Reactions Involving Oxidant Agents. Chem. Rev. 2007, 107, 5318–5365. [Google Scholar] [CrossRef]
- Obora, Y.; Ishii, Y. Pd(II)/HPMoV-catalyzed direct oxidative coupling reaction of benzene derivatives with olefins. Molecules 2010, 15, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Palladium oxidase catalysis. Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, H. Palladium-Catalyzed Oxidation of Unsaturated Hydrocarbons Using Molecular Oxygen. Acc. Chem. Res. 2012, 45, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium-catalyzed oxidative addition. Chem. Rev. 2006, 106, 4644–4680. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Goldstein, E.L.; Stoltz, B.M. Palladium-Catalyzed Enantioselective Csp3-Csp3 Cross-Coupling for the Synthesis of (Poly)fluorinated Chiral Building Blocks. Org. Lett. 2018, 20, 5657–5660. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Dlugosch, M.; Liu, X.; Banwell, M.G. The Palladium-Catalyzed Ullmann Cross-Coupling Reaction: A Modern Variant on a Time-Honored Process. Acc. Chem. Res. 2018, 51, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, F.; Ward, T.R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2018, 148, 489–511. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent Advances in Palladium-Catalyzed Cross-Coupling Reactions at ppm to ppb Molar Catalyst Loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Devendar, P.; Qu, R.Y.; Kang, W.M.; He, B.; Yang, G.F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef] [Green Version]
- Gligorich, K.M.; Cummings, S.A.; Sigman, M.S. Palladium-catalyzed reductive coupling of styrenes and organostannanes under aerobic conditions. J. Am. Chem. Soc. 2007, 129, 14193–14195. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Amatore, C.; Ciofini, I.; Jutand, A.; Lakmini, H. Mechanism of the Palladium-Catalyzed Homocoupling of Arylboronic Acids: Key Involvement of a Palladium Peroxo Complex. J. Am. Chem. Soc. 2006, 128, 6829–6836. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Chessa, G.; Santo, C.; Levi, C.; Uguagliati, P. Oxidative coupling of activated alkynes with palladium(0) olefin complexes: Side production of the highly symmetric hexamethyl mellitate species under mild conditions at low alkyne/complex molar ratios. Inorg. Chem. Commun. 2006, 9, 388–390. [Google Scholar] [CrossRef] [Green Version]
- Hull, K.L.; Lanni, E.L.; Sanford, M.S. Highly Regioselective Catalytic Oxidative Coupling Reactions: Synthetic and Mechanistic Investigations. J. Am. Chem. Soc. 2006, 128, 14047–14049. [Google Scholar] [CrossRef]
- Hull, K.L.; Sanford, M.S. Determining the mechanism of palladium-catalyzed oxidative coupling reactions. In Proceedings of the 239th ACS National Meeting & Exposition, San Francisco, CA, USA, 21–25 March 2010. [Google Scholar]
- Lu, Y.; Wang, D.-H.; Engle, K.M.; Yu, J.-Q. Pd(II)-Catalyzed Hydroxyl-Directed C-H Olefination Enabled by Monoprotected Amino Acid Ligands. J. Am. Chem. Soc. 2010, 132, 5916–5921. [Google Scholar] [CrossRef]
- Muzart, J. Molecular oxygen to regenerate Pd(II) active species. Chem. Asian J. 2006, 1, 508–515. [Google Scholar] [CrossRef]
- Lei, A.; Zhang, X. A novel palladium-catalyzed homocoupling reaction initiated by transmetalation of palladium enolates. Tetrahedron Lett. 2002, 43, 2525–2528. [Google Scholar] [CrossRef]
- Liegault, B.; Fagnou, K. Palladium-Catalyzed Intramolecular Coupling of Arenes and Unactivated Alkanes in Air. Organometallics 2008, 27, 4841–4843. [Google Scholar] [CrossRef]
- Liu, C.; Jin, L.; Lei, A. Transition-metal-catalyzed oxidative cross-coupling reactions. Synlett 2010, 2010, 2527–2536. [Google Scholar]
- Martinez, C.; Alvarez, R.; Aurrecoechea, J.M. Palladium-Catalyzed Sequential Oxidative Cyclization/Coupling of 2-Alkynylphenols and Alkenes: A Direct Entry into 3-Alkenylbenzofurans. Org. Lett. 2009, 11, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Prateeptongkum, S.; Driller, K.M.; Jackstell, R.; Spannenberg, A.; Beller, M. Efficient Synthesis of Biologically Interesting 3,4-Diaryl-Substituted Succinimides and Maleimides: Application of Iron-Catalyzed Carbonylations. Chem. Eur. J. 2010, 16, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Van Aeken, S.; Verbeeck, S.; Deblander, J.; Maes, B.U.W.; Tehrani, K.A. Synthesis of 3-substituted benzo[g]isoquinoline-5,10-diones: A convenient one-pot Sonogashira coupling/iminoannulation procedure. Tetrahedron 2011, 67, 2269–2278. [Google Scholar] [CrossRef]
- Venkatraman, S.; Huang, T.; Li, C.-J. Carbon-carbon bond formation via palladium-catalyzed reductive coupling of aryl halides in air and water. Adv. Synth. Catal. 2002, 344, 399–405. [Google Scholar] [CrossRef]
- Wakioka, M.; Mutoh, Y.; Takita, R.; Ozawa, F. A highly selective catalytic system for the cross-coupling of (E)-styryl bromide with benzene boronic acid: Application to the synthesis of all-trans poly(arylenevinylene)s. Bull. Chem. Soc. Jpn. 2009, 82, 1292–1298. [Google Scholar] [CrossRef]
- Yoo, K.S.; O’Neill, J.; Sakaguchi, S.; Giles, R.; Lee, J.H.; Jung, K.W. Asymmetric Intermolecular Boron Heck-Type Reactions via Oxidative Palladium(II) Catalysis with Chiral Tridentate NHC-Amidate-Alkoxide Ligands. J. Org. Chem. 2010, 75, 95–101. [Google Scholar] [CrossRef]
- Yoo, K.S.; Park, C.P.; Yoon, C.H.; Sakaguchi, S.; O’Neill, J.; Jung, K.W. Asymmetric Intermolecular Heck-Type Reaction of Acyclic Alkenes via Oxidative Palladium(II) Catalysis. Org. Lett. 2007, 9, 3933–3935. [Google Scholar] [CrossRef]
- Chen, Q.; Li, C. Activation of the Vinylic C-Cl Bond by Complexation of Fe(CO)3: Palladium-Catalyzed Coupling Reactions of (η4-Chlorodiene)tricarbonyliron Complexes. Organometallics 2007, 26, 223–229. [Google Scholar] [CrossRef]
- Yoo, K.S.; Yoon, C.H.; Jung, K.W. Oxidative Palladium(II) Catalysis: A Highly Efficient and Chemoselective Cross-Coupling Method for Carbon-Carbon Bond Formation under Base-Free and Nitrogenous-Ligand Conditions. J. Am. Chem. Soc. 2006, 128, 16384–16393. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.F.; Nolley, J., Jr. P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Herr, R.J.; Dowling, M.S.; Scampini, A.C.; Smith, T.M. Iridium- and Palladium-Catalyzed Syntheses of (S)(+) and (R)(-) Coniine from Enantiopure Allylic Alcohols. In Proceedings of the 35th Northeast Regional Meeting of the American Chemical Society, Burlington, VT, USA, 29 June–2 July 2008. [Google Scholar]
- Horiguchi, H.; Tsurugi, H.; Satoh, T.; Miura, M. Palladium/phosphite or phosphate catalyzed oxidative coupling of arylboronic acids with alkynes to produce 1,4-diaryl-1,3-butadienes. Adv. Synth. Catal. 2008, 350, 509–514. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, Y.; Wang, H.; Lei, A. Palladium-catalyzed R(sp3)-Zn/R(sp)-SnBu3 oxidative cross-coupling. Synthesis 2008, 2008, 649–654. [Google Scholar]
- Johnson, T.; Lautens, M. Palladium(II)-Catalyzed Enantioselective Synthesis of α-(Trifluoromethyl)arylmethylamines. Org. Lett. 2013, 15, 4043–4045. [Google Scholar] [CrossRef]
- Jordan-Hore, J.A.; Sanderson, J.N.; Lee, A.-L. Mild and Ligand-Free Pd(II)-Catalyzed Conjugate Additions to Hindered γ-Substituted Cyclohexenones. Org. Lett. 2012, 14, 2508–2511. [Google Scholar] [CrossRef] [PubMed]
- Khabibulin, V.R.; Kulik, A.V.; Oshanina, I.V.; Bruk, L.G.; Temkin, O.N.; Nosova, V.M.; Ustynyuk, Y.A.; Bel’skii, V.K.; Stash, A.I.; Lysenko, K.A.; et al. Mechanism of the Oxidative Carbonylation of Terminal Alkynes at the C-H Bond in Solutions of Palladium Complexes. Kinet. Catal. 2007, 48, 228–244. [Google Scholar] [CrossRef]
- Alvarez, R.; Martinez, C.; Madich, Y.; Denis, J.G.; Aurrecoechea Jose, M.; de Lera Angel, R. A general synthesis of alkenyl-substituted benzofurans, indoles, and isoquinolones by cascade palladium-catalyzed heterocyclization/oxidative Heck coupling. Chemistry 2010, 16, 12746–12753. [Google Scholar] [CrossRef]
- Aouf, C.; Thiery, E.; Le Bras, J.; Muzart, J. Palladium-Catalyzed Dehydrogenative Coupling of Furans with Styrenes. Org. Lett. 2009, 11, 4096–4099. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Borsini, E.; Broggini, G.; Rigamonti, M.; Sottocornola, S. Intramolecular palladium-catalyzed oxidative coupling on thiophene and furan rings. Determinant role of the electronic availability of the heterocycle. Synlett 2008, 2008, 1053–1057. [Google Scholar]
- Maehara, A.; Satoh, T.; Miura, M. Palladium-catalyzed direct oxidative vinylation of thiophenes and furans under weakly basic conditions. Tetrahedron 2008, 64, 5982–5986. [Google Scholar] [CrossRef]
- Thiery, E.; Harakat, D.; Le Bras, J.; Muzart, J. Palladium-Catalyzed Oxidative Coupling of 2-Alkylfurans with Olefins through C-H Activation: Synthesis of Difurylalkanes. Organometallics 2008, 27, 3996–4004. [Google Scholar] [CrossRef]
- Xi, P.; Yang, F.; Qin, S.; Zhao, D.; Lan, J.; Gao, G.; Hu, C.; You, J. Palladium(II)-Catalyzed Oxidative C-H/C-H Cross-Coupling of Heteroarenes. J. Am. Chem. Soc. 2010, 132, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of Condensed Heteroaromatic Compounds by Palladium-Catalyzed Oxidative Coupling of Heteroarene Carboxylic Acids with Alkynes. Org. Lett. 2009, 11, 2337–2340. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-D.; Sun, C.-L.; Fang, Z.; Li, B.-J.; Li, Y.-Z.; Shi, Z.-J. Palladium-catalyzed direct arylation of (hetero)arenes with aryl boronic acids. Angew. Chem. Int. Ed. 2008, 47, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, S.; Wacharasindhu, S.; Wan, Z.-K.; Mansour, T.S. Heteroaryl ethers by oxidative palladium catalysis of pyridotriazol-1-yloxy pyrimidines with arylboronic acids. Org. Lett. 2009, 11, 2511–2514. [Google Scholar] [CrossRef]
- Belitsky, J.M. Palladium catalyzed homocoupling of indole and aryl boronic acids. In Proceedings of the 236th ACS National Meeting, Philadelphia, PA, USA, 17–21 August 2008. [Google Scholar]
- Belitsky, J.M. Palladium Catalyzed Homocoupling of Indole and Aryl Boronic Acids. In Proceedings of the Abstract Central Cent. Regional Meeting of the American Chemical Society, Cleveland, OH, USA, 20–23 May 2009. [Google Scholar]
- Clawson, R.W.; Deavers, R.E.; Akhmedov, N.G.; Soederberg, B.C.G. Palladium-catalyzed synthesis of 3-alkoxysubstituted indoles. Tetrahedron 2006, 62, 10829–10834. [Google Scholar] [CrossRef]
- Djakovitch, L.; Rouge, P. New homogeneously and heterogeneously [Pd/Cu]-catalysed C3-alkenylation of free NH-indoles. J. Mol. Catal. A Chem. 2007, 273, 230–239. [Google Scholar] [CrossRef]
- Gong, X.; Song, G.; Zhang, H.; Li, X. Palladium-Catalyzed Oxidative Cross-Coupling between Pyridine N-Oxides and Indoles. Org. Lett. 2011, 13, 1766–1769. [Google Scholar] [CrossRef]
- He, C.-Y.; Fan, S.; Zhang, X. Pd-catalyzed oxidative cross-coupling of perfluoroarenes with aromatic heterocycles. J. Am. Chem. Soc. 2010, 132, 12850–12852. [Google Scholar] [CrossRef]
- Wang, Z.; Li, K.; Zhao, D.; Lan, J.; You, J. Palladium-Catalyzed Oxidative C-H/C-H Cross-Coupling of Indoles and Pyrroles with Heteroarenes. Angew. Chem. Int. Ed. 2011, 50, 5365–5369. [Google Scholar] [CrossRef] [PubMed]
- Henke, A.; Srogl, J. Pd2+ and Cu2+ catalyzed oxidative cross-coupling of mercaptoacetylenes and arylboronic acids. Chem. Commun. 2011, 47, 4282–4284. [Google Scholar] [CrossRef] [PubMed]
- Kirchberg, S.; Tani, S.; Ueda, K.; Yamaguchi, J.; Studer, A.; Itami, K. Oxidative biaryl coupling of thiophenes and thiazoles with arylboronic acids through palladium catalysis: Otherwise difficult C4-selective C-H arylation enabled by boronic acids. Angew. Chem. Int. Ed. 2011, 50, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Schwan, A.L. Palladium catalyzed cross-coupling reactions for phosphorus-carbon bond formation. Chem. Soc. Rev. 2004, 33, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Schuh, B.; Beck, W. Metal Complexes of Biologically Important Ligands, CLXXVII. Dichlorido Platinum(II) and Palladium(II) Complexes with Long Chain Amino Acids and Amino Acid Amides. Z. Anorg. Allg. Chem. 2017, 643, 632–635. [Google Scholar] [CrossRef]
- Liu, R.R.; Li, B.L.; Lu, J.; Shen, C.; Gao, J.R.; Jia, Y.X. Palladium/l-Proline-Catalyzed Enantioselective α-Arylative Desymmetrization of Cyclohexanones. J. Am. Chem. Soc. 2016, 138, 5198–5201. [Google Scholar] [CrossRef]
- Tsvelikhovsky, D.; Popov, I.; Gutkin, V.; Rozin, A.; Shvartsman, A.; Blum, J. On the involvement of palladium nanoparticles in the Heck and Suzuki reactions. European J. Org. Chem. 2009, 98–102. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki-Miyaura Cross-Coupling Reaction in Water. Catal. Letters 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Klaerner, C.; Greiner, A. Synthesis of polybenzyls by Suzuki Pd-catalyzed crosscoupling of boronic acids and benzyl bromides. Model reactions and polyreactions. Macromol. Rapid Commun. 1998, 19, 605–608. [Google Scholar] [CrossRef]
- Wu, N.; Li, X.; Xu, X.; Wang, Y.; Xu, Y.; Chen, X. Homocoupling reaction of aryl boronic acids catalyzed by Pd(OAc)2/K2CO3 in water under air atmosphere. Lett. Org. Chem. 2010, 7, 11–14. [Google Scholar] [CrossRef]
- Xu, Z.; Mao, J.; Zhang, Y. Pd(OAc)2-catalyzed room temperature homocoupling reaction of arylboronic acids under air without ligand. Catal. Commun. 2007, 9, 97–100. [Google Scholar] [CrossRef]
- Yamamoto, Y. Homocoupling of arylboronic acids with a catalyst system consisting of a palladium(II) N-heterocyclic carbene complex and p-benzoquinone. Synlett 2007, 1913–1916. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Q.X.; Jiang, H.F. Palladium-catalyzed homo-coupling of boronic acids with supported reagents in supercritical carbon dioxide. Chin. Chem. Lett. 2007, 18, 1043–1046. [Google Scholar] [CrossRef]
- Morris, D.M.D.M.; McGeagh, M.; De Peña, D.; Merola, J.S. Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl(alkyl or aryl)cyclopentadienes (Cp∗R), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. [Google Scholar] [CrossRef]
- Hobart, D.B.; Berg, M.A.G.G.; Merola, J.S. Bis-glycinato complexes of palladium(II): Synthesis, structural determination, and hydrogen bonding interactions. Inorg. Chim. Acta 2014, 423, 21–30. [Google Scholar] [CrossRef]
- Ito, T.; Marumo, F.; Saito, Y. The crystal structure of bis-(l-prolinato)palladium(II). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 2002, 27, 1062–1066. [Google Scholar] [CrossRef]
- Chernova, N.N.; Strukov, V.V.; Avetikyan, G.B.; Chernonozhkin, V.N. Synthesis and structure of complex palladium(II) bis(histidinates). Zh. Neorg. Khim. 1980, 25, 1569–1574. [Google Scholar]
- Jarzab, T.C.; Hare, C.R.; Langs, D.A. cis-Bis(l-tyrosinato)palladium(II) hemihydrate, C36H42N4O13Pd2. Cryst. Struct. Commun. 1973, 2, 399–403. [Google Scholar]
- Jarzab, T.C.; Hare, C.R.; Langs, D.A. cis-Bis(l-valinato)palladium(II) monohydrate, C10H22N2O5Pd. Cryst. Struct. Commun. 1973, 2, 395–398. [Google Scholar]
- Komorita, T.; Hidaka, J.; Shimura, Y. Metal complexes with amino acid amides. III. Geometrical structures and electronic spectra of bis(α-amino acid-amidato)palladium(II), -nickel(II), and -copper(II). Bull. Chem. Soc. Jpn. 1971, 44, 3353–3363. [Google Scholar] [CrossRef]
- Sabat, M.; Jezowska, M.; Kozlowski, H. X-Ray Evidence of the Metal-Ion Tyrosine Aromatic Ring Interaction in Bis(l-Tyrosinato)Palladium(Ii). Inorg. Chim. Acta 1979, 37, L511–L512. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals radii of elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Bhowmick, S.; Bhowmick, K.C. Catalytic asymmetric carbon-carbon bond-forming reactions in aqueous media. Tetrahedron Asymmetry 2011, 22, 1945–1979. [Google Scholar] [CrossRef]
- Zhang, G.; Luan, Y.; Han, X.; Wang, Y.; Wen, X.; Ding, C. Pd(l-proline)2 complex: An efficient catalyst for Suzuki-Miyaura coupling reaction in neat water. Appl. Organomet. Chem. 2014, 28, 332–336. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction CrysAlisPro Software System. 2018. Available online: https://www.rigakuxrayforum.com/forumdisplay.php?fid=57 (accessed on 2 June 2019).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 9 Citation. Available online: https://gaussian.com/g03citation/ (accessed on 4 May 2016).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Abinitio Pseudopotentials for the 2nd and 3rd Row Transition-Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]








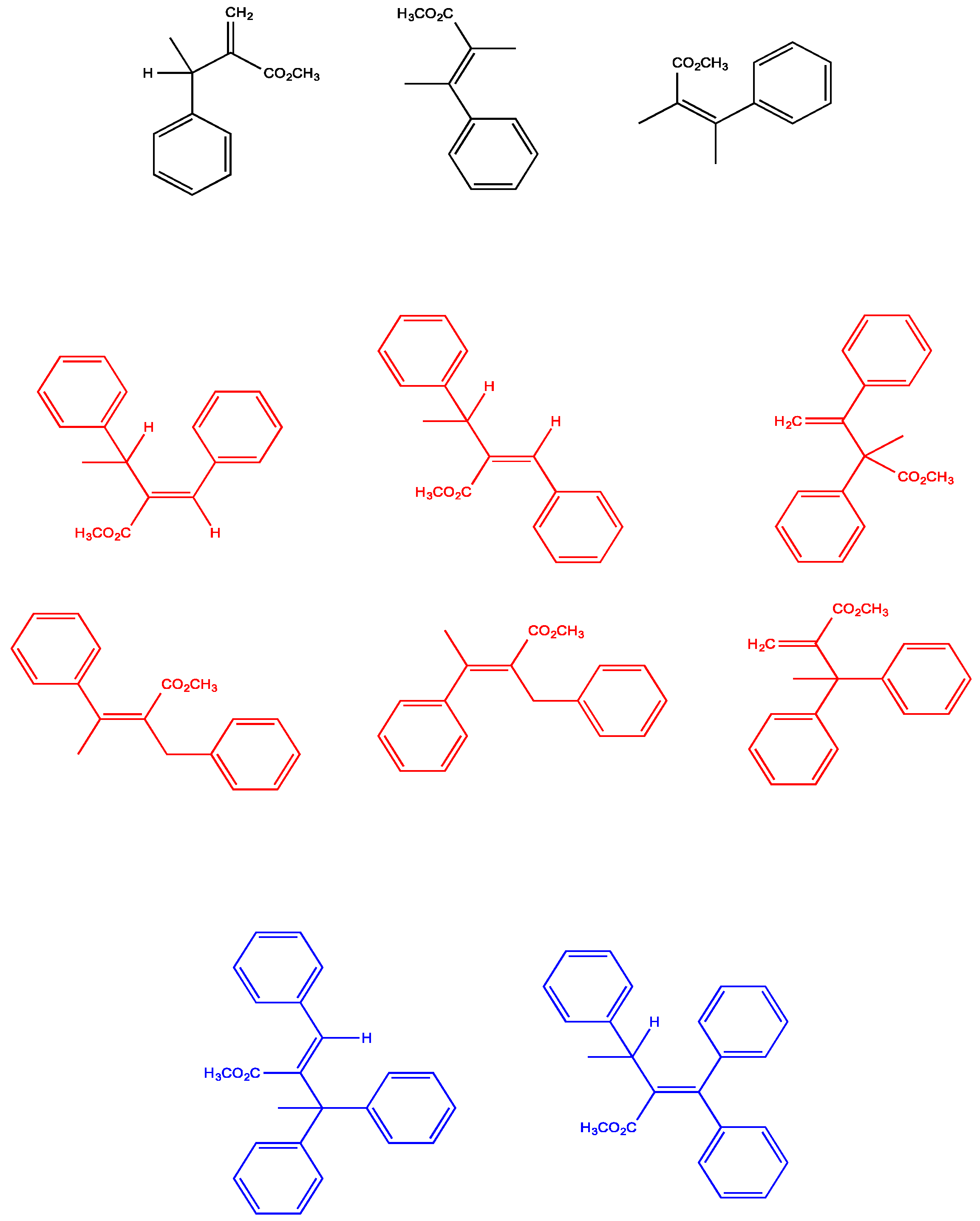

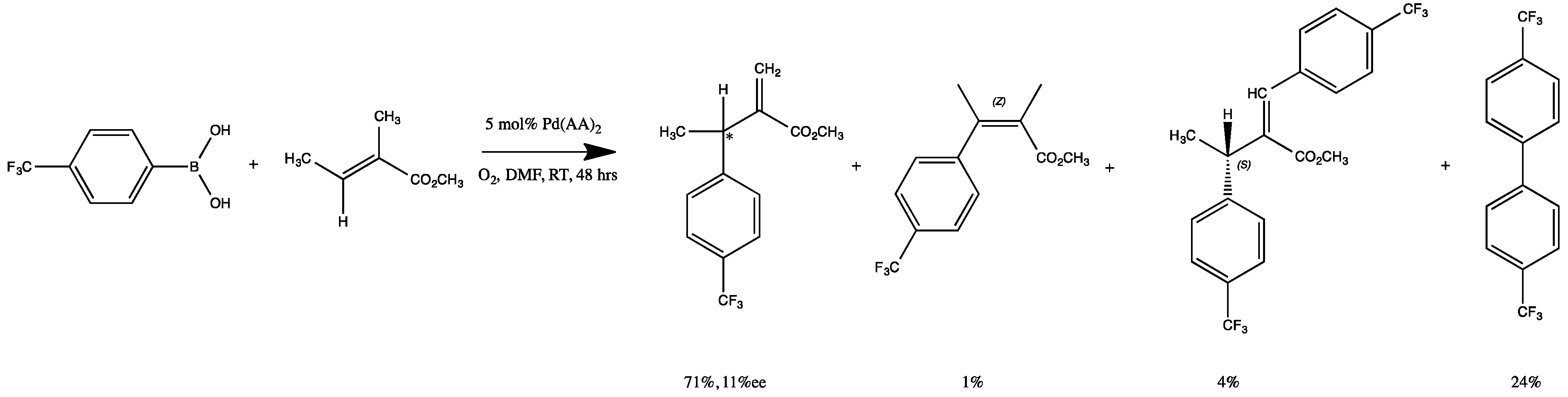



| Complex | R/S Yield, % | % ee | E/Z Yield, % | Biaryl, % |
|---|---|---|---|---|
 1 1 | 42 | 24 | 28 | 30 |
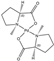 2 2 | No Reaction Observed | |||
 3 3 | 91 | 14 | 9 | 0 |
 4 4 | 94 | 11 | 6 | 0 |
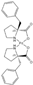 5 5 | 33 | 2 | 67 | 0 |
 6 6 | 66 | 11 | 30 | 4 |
 7 7 | 97 | 1 | 2 | 1 |
| Reaction Temperature, °C | %ee |
|---|---|
| 65 | ~1 |
| 25 | 20 |
| 0 | 41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobart, D.B., Jr.; Merola, J.S.; Rogers, H.M.; Sahgal, S.; Mitchell, J.; Florio, J.; Merola, J.W. Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs. Catalysts 2019, 9, 515. https://doi.org/10.3390/catal9060515
Hobart DB Jr., Merola JS, Rogers HM, Sahgal S, Mitchell J, Florio J, Merola JW. Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs. Catalysts. 2019; 9(6):515. https://doi.org/10.3390/catal9060515
Chicago/Turabian StyleHobart, David B., Jr., Joseph S. Merola, Hannah M. Rogers, Sonia Sahgal, James Mitchell, Jacqueline Florio, and Jeffrey W. Merola. 2019. "Synthesis, Structure, and Catalytic Reactivity of Pd(II) Complexes of Proline and Proline Homologs" Catalysts 9, no. 6: 515. https://doi.org/10.3390/catal9060515