High-Efficiency Visible Light Responsive Sulfide KSb5S8 Photocatalyst with a Layered Crystal Structure
Abstract
:1. Introduction
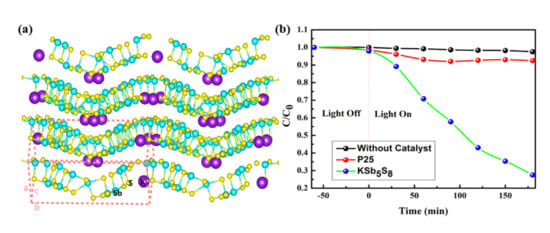
2. Results and Discussion
3. Experimental and Theoretical Methods
3.1. Synthesis of KSb5S8
3.2. Characterizations
3.3. Photocatalytic Performance
3.4. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.C.; Ma, W.H.; Zhao, J.C. Semiconductor-mediated photo-degradation of pollutants under visible light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Gao, Y.J.; Gao, D.Q.; Wang, Y.H. Effect of Oxide Defect on Photocatalytic Properties of MSnO3 (M = Ca, Sr and Ba) Photo-catalysts. Appl. Catal. B Environ. 2019, 243, 428–437. [Google Scholar] [CrossRef]
- Kim, D.H.; Yeo, H.C.; Shin, D.B.; Choi, H.; Kim, S.; Park, N.; Han, S.S. Dissimilar Anisotropy of Electron Versus Hole Bulk Transport in Anatase TiO2: Implications for Photo-catalysis. Phys. Rev. B 2017, 95, 045209–045214. [Google Scholar] [CrossRef]
- Hussain, H.; Tocci, G.; Woolcot, T.; Torrelles, X.; Pang, C.L.; Humphrey, D.S.; Yim, C.M.; Grinter, D.C.; Cabailh, G.; Bikondoa, O.; et al. Structure of a Model TiO2 Photocatalytic Interface. Nat. Mater. 2017, 16, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Stecher, T.; Reuter, K.; Oberhofer, H. First Principles Free Energy Barriers for Photo-electrochemical Surface Reactions: Proton Abstraction at TiO2(110). Phys. Rev. Lett. 2016, 117, 276001–276007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L.; Jiang, X.; Jiang, X.X.; Xiao, K.; Guo, Y.W.; Huang, H.W.; Lin, Z.S.; Yao, J.Y.; Tung, C.H.; Wu, L.Z.; et al. Wu, BaAu2S2: A Au-Based Intrinsic Photo-catalyst for High Performance Visible Light Photo-catalysis. Inorg. Chem. 2017, 56, 5173–5181. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, H.; Li, Z.; Chen, Y.; Ngo, H.T.; Bhethanabotla, V.R.; Joseph, H.; Ma, S.Q.; Schlaf, R.; Takshi, A. Toward a Visible Light-Driven Photo-catalyst: The Effect of Midgap-States-Induced Energy Gap of Undoped TiO2 Nanoparticles. ACS Catal. 2015, 5, 327–335. [Google Scholar] [CrossRef]
- Wen, B.; Yin, W.J.; Selloni, A.; Liu, L.M. Defects, Adsorbates and Photo-activity of Rutile TiO2 (110): Insight by First Principles Calculations. J. Phys. Chem. Lett. 2018, 9, 5281–5287. [Google Scholar] [CrossRef]
- Liu, L.C.; Ji, Z.Y.; Zou, W.X.; Gu, X.R.; Deng, Y.; Gao, F.; Tang, C.J.; Dong, L. In Situ Loading Transition Metal Oxide Clusters on TiO2 Nano-sheets As Co-catalysts for Exceptional High Photo-activity. ACS Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- David, S.; Mahadik, M.A.; Chung, H.S.; Ryu, J.H.; Jang, J.S. Facile Hydrothermally Synthesized a Novel CdS Nanoflower/Rutile-TiO2 Nanorod Heterojunction Photoanode Used for Photo-electro-catalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2017, 5, 7537–7548. [Google Scholar] [CrossRef]
- Yan, T.J.; Li, L.P.; Li, G.S.; Wang, Y.J.; Hu, W.B.; Guan, X.F. Porous SnIn4S8 Microspheres in a New Polymorph that Promotes Dyes Degradation under Visible Light Irradiation. J. Hazard. Mater. 2011, 186, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, H.; Yang, D.F.; Gao, W.L.; Cong, R.H.; Yang, T. ZnGa2-xInxS4(0 < x < 0.4) and Zn1-2y(CuGa)yGa1.7In0.3S4(0.1 < y < 0.2): Optimize Visible Light Photocatalytic H2 Evolution by Fine Modulation of Band Structures. Inorg. Chem. 2015, 54, 2467–2473. [Google Scholar] [PubMed]
- Zhao, Q.Y.; Guo, Y.H.; Zhou, Y.X.; Yao, Z.H.; Ren, Z.Y.; Bai, J.T.; Xu, X.L. Band Alignments and Hetero-structures of Monolayer Transition Metal Trichalcogenides MX3 (M = Zr, Hf; X = S, Se) and Di-chalcogenides MX2 (M = Tc, Re; X = S, Se) for Solar Applications. Nanoscale 2018, 10, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, K.; Crovetto, A.; Pandey, M.; Thygesen, K.S.; Seger, B.; Vesborg, P.C.K.; Hansen, O.; Chorkendorff, I.K.; Jacobsen, W. Sulfide Perovskites for Solar Energy Conversion Applications: Computational Screening and Synthesis of the Selected Compound LaYS3. Energy Environ. Sci. 2017, 10, 2579–2593. [Google Scholar] [CrossRef]
- Mir, S.H.; Chakraborty, S.; Warna, J.; Narayan, S.; Jha, P.C.; Jha, P.K.; Ahuja, R. A Comparative Study of Hydrogen Evolution Reaction on Pseudo Monolayer WS2 and PtS2: Insights Based on the Density Functional Theory. Catal. Sci. Technol. 2017, 7, 687–692. [Google Scholar] [CrossRef]
- Zhu, X.X.; Luo, X.K.; Yuan, H.K.; Chen, H.; Tian, C.L. Band Gap Engineering of SnS2 Nanosheets by Anion-Anion Co-doping for Visible Light Photo-catalysis. RSC Adv. 2018, 8, 3304–3313. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.D.; Wu, D.H.; Jing, Y.; Zhou, Z. MnPSe3 Monolayer: A Promising 2D Visible Light Photohydrolytic Catalyst with High Carrier Mobility. Adv. Sci. 2016, 3, 1600062–1600066. [Google Scholar] [CrossRef] [PubMed]
- Campbell, Q.; Fisher, D.; Dabo, I. Voltage-dependent Reconstruction of Layered Bi2WO6 and Bi2MoO6 Photocatalysts and Its Influence on Charge Separation for Water Splitting. Phys. Rev. Mater. 2019, 3, 015404. [Google Scholar] [CrossRef]
- Xiong, T.; Cen, W.L.; Zhang, Y.X.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photo-catalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Li, Z.J.; Qu, Y.; Hu, K.; Humayun, M.; Chen, S.Y.; Jing, L.Q. Improved Photo-electrocatalytic Activities of BiOCl with High Stability for Water Oxidation and MO degradation by Coupling RGO and Modifying Phosphate Groups to Prolong Carrier Lifetime. Appl. Catal. B Environ. 2017, 203, 355–362. [Google Scholar] [CrossRef]
- Wachter, J.B.; Chrissafis, K.; Petkov, V.; Malliakas, C.D.; Bilc, D.; Kyratsi, T.; Paraskevopoulos, K.M.; Mahanti, S.D.; Torbrugge, T.; Eckert, H.; et al. Local Structure and Influence of Bonding on the Phase-Change Behavior of the Chalcogenide Compounds K1-XRbxSb5S8. J. Solid State Chem. 2007, 180, 420–431. [Google Scholar] [CrossRef]
- Kyratsi, T.; Chrissafis, K.; Wachter, J.; Paraskevopoulos, K.M.; Kanatzidis, M.G. KSb5S8: A Wide Bandgap Phase-Change Material for Ultra High Density Rewritable Information Storage. Adv. Mater. 2003, 15, 1428–1430. [Google Scholar] [CrossRef]
- Berlepsch, P.; Miletich, R.; Armbruster, T. The Crystal Structures of Synthetic KSb5S8 and (Tl0.598, K0.402) Sb5S8 and their relation to parapierrotite (TlSb5S8). Z Krist. 1999, 214, 57–63. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.Y.; Zhu, Y.Q.; Zhao, J.; Jiang, F.X.; Chen, S.; Sun, H.; Xu, J.K.; Deng, W.Y.; Wang, C.Y. Efficient Photo-dechlorination of Chlorophenols on Polarzied MZnB5O10(M = Na and K) Nonlinear Optical Materials. Appl. Catal. B Environ. 2016, 181, 436–444. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Theoretical Perspective of Photocatalytic Porperties of Single Layer SnS2. Phys. Rev. B 2013, 88, 115314–115318. [Google Scholar] [CrossRef]
- Fan, X.Y.; Wu, Z.P.; Wang, L.C.; Wang, C.Y. Exploring the Origin of High Dechlorination Activity in Polar Materials M2B5O9Cl (M = Ca, Sr, Ba and Pb) with Built-in Electric Field. Chem. Mater. 2017, 29, 639–647. [Google Scholar] [CrossRef]
- Li, B.S.; Lai, C.; Zeng, G.M.; Qin, L.; Yi, H.; Huang, D.L.; Zhou, C.Y.; Liu, X.G.; Cheng, M.; Xu, P.; et al. Facile Hydrothermal Synthesis of Z-scheme Bi2Fe4O9/Bi2WO6 Heterojunction Photo-catalyst with Enhanced Visible Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 18824–18836. [Google Scholar] [CrossRef]
- Harb, M.; Cavallo, L. Suitable Fundamental Properties of Ta0.75V0.25ON Material for Visible Light Driven Photo-catalysis: A DFT Study. ACS Omega 2016, 1, 1041–1048. [Google Scholar] [CrossRef]
- Ma, Z.J.; Yi, Z.G.; Sun, J.; Wu, K.C. Electronic and Photocatalytic Properties of Ag3PC4 (C = O, S, Se): A Systemic Hybrid DFT Study. J. Phys. Chem. C 2012, 116, 25074–25080. [Google Scholar] [CrossRef]
- Yan, T.J.; Tian, J.; Guan, W.F.; Qiao, Z.; Li, W.J.; You, J.M.; Huang, B.B. Ultra-low Loading of Ag3PO4 on Hierarchical In2S3 Microspheres to Improve the Photo-catalytic Performance: The Cocatalytic Effect of Ag and Ag3PO4. Appl. Catal. B Environ. 2017, 202, 84–94. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, M.; Wang, Q.; Wang, K.; Zhang, H.; Quan, X.; Zhang, B.; Yang, D. High-Efficiency Visible Light Responsive Sulfide KSb5S8 Photocatalyst with a Layered Crystal Structure. Catalysts 2019, 9, 529. https://doi.org/10.3390/catal9060529
Li Y, Wu M, Wang Q, Wang K, Zhang H, Quan X, Zhang B, Yang D. High-Efficiency Visible Light Responsive Sulfide KSb5S8 Photocatalyst with a Layered Crystal Structure. Catalysts. 2019; 9(6):529. https://doi.org/10.3390/catal9060529
Chicago/Turabian StyleLi, Yuanyuan, Meijun Wu, Qiang Wang, Kun Wang, He Zhang, Xuejun Quan, Bin Zhang, and Dingfeng Yang. 2019. "High-Efficiency Visible Light Responsive Sulfide KSb5S8 Photocatalyst with a Layered Crystal Structure" Catalysts 9, no. 6: 529. https://doi.org/10.3390/catal9060529