Degradation of Crystal Violet by Catalytic Wet Peroxide Oxidation (CWPO) with Mixed Mn/Cu Oxides
Abstract
:1. Introduction
2. Results
2.1. Mixed Oxide Catalyst
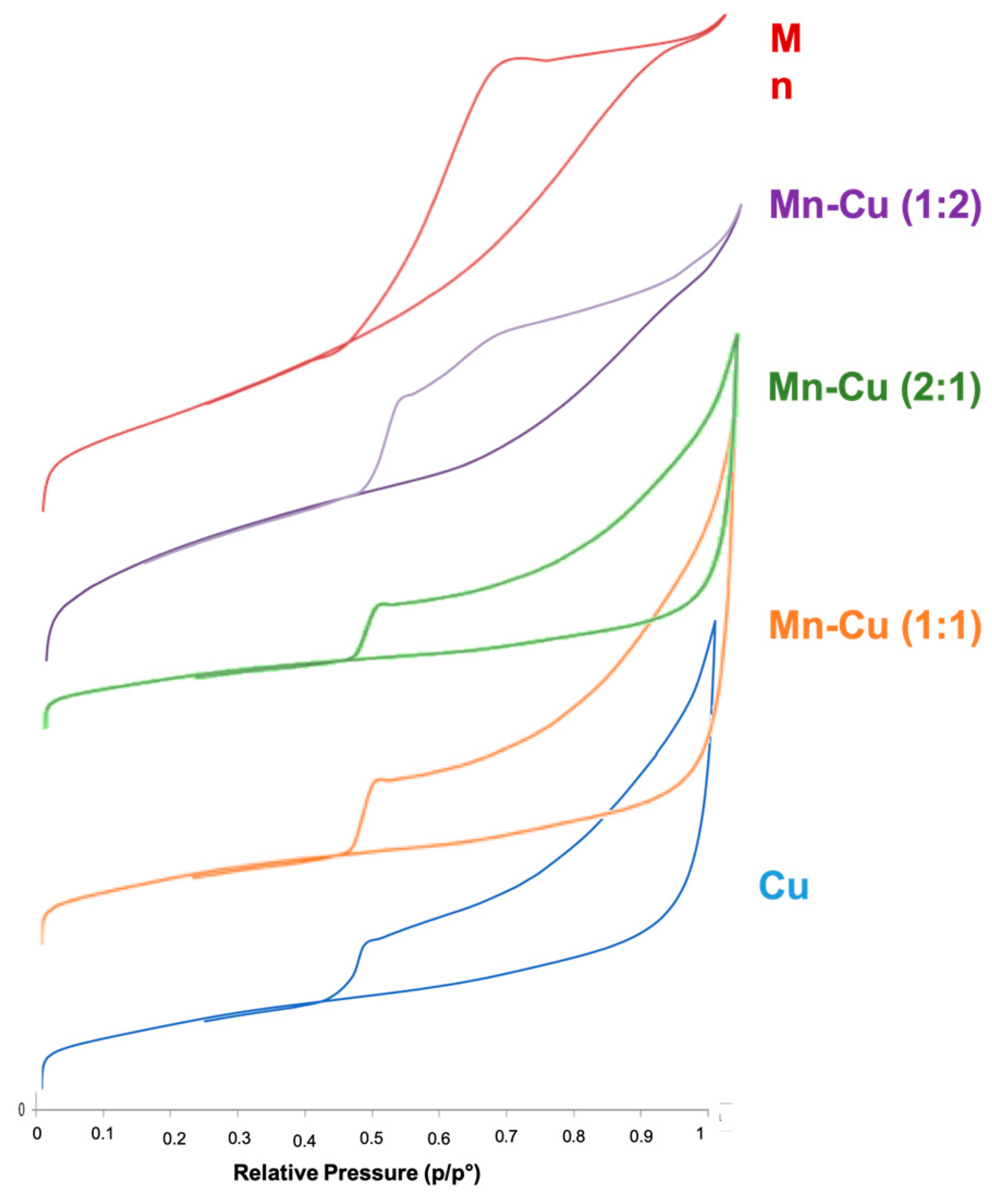
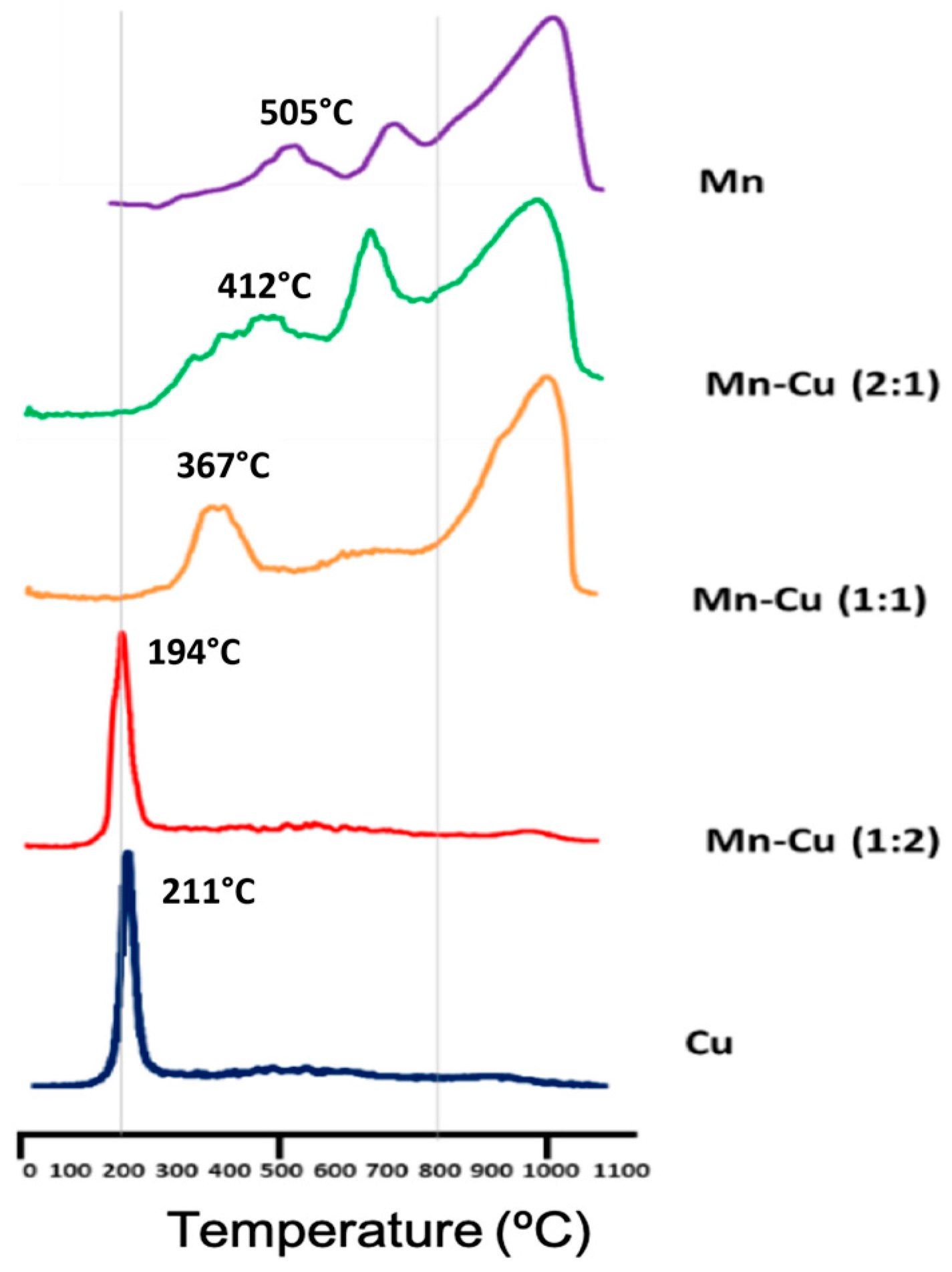
2.2. Characterization of Catalyst
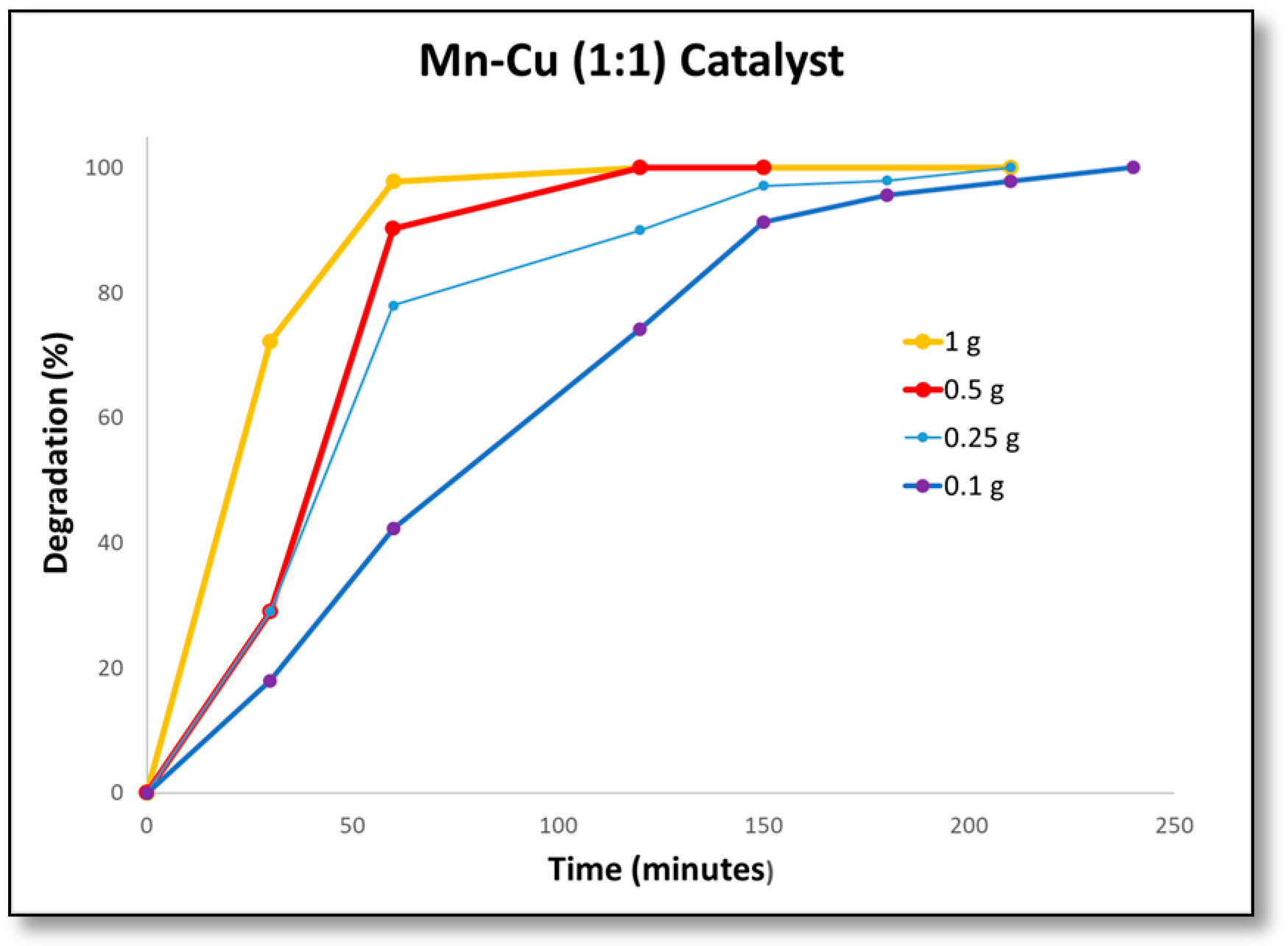
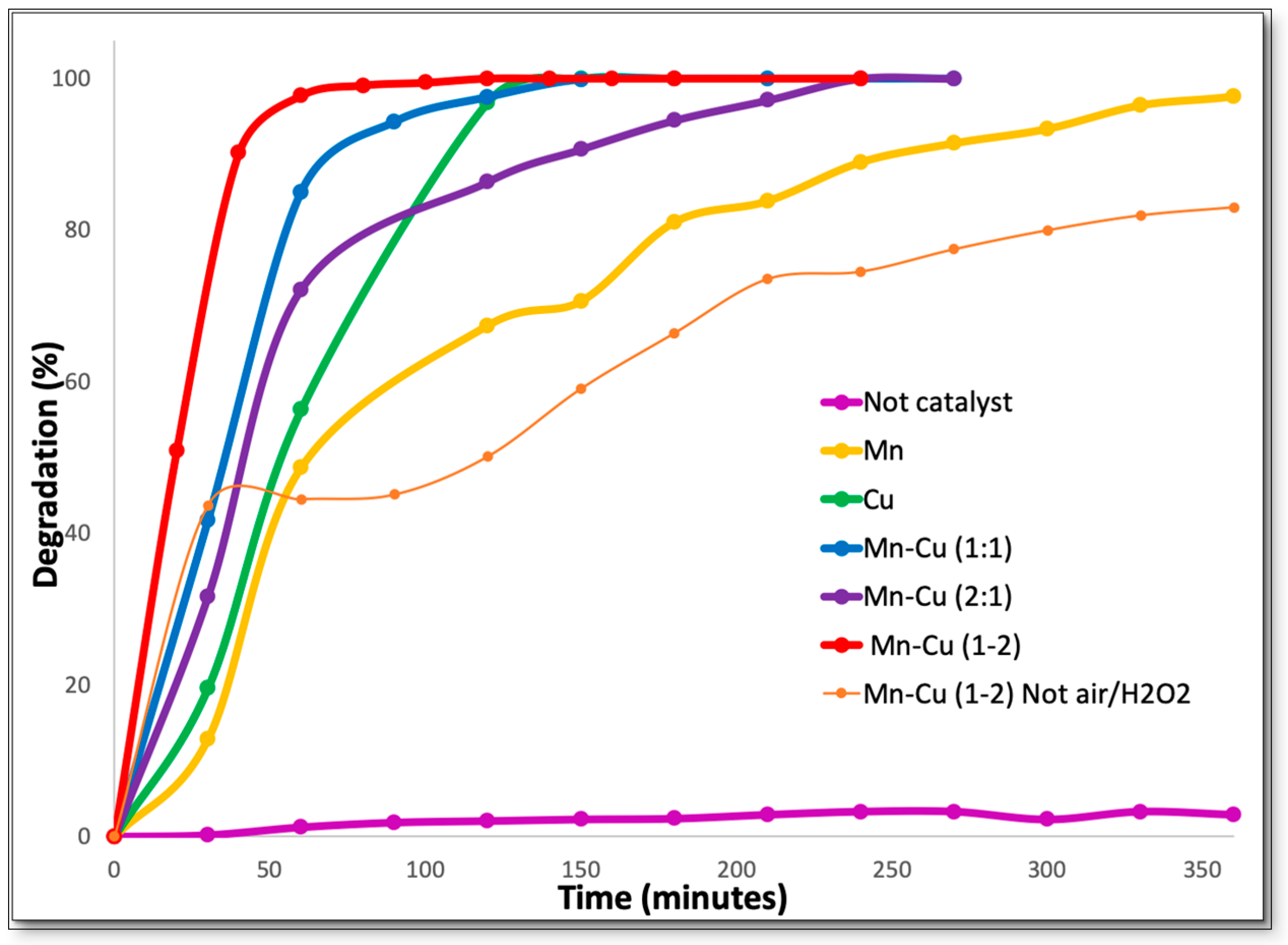
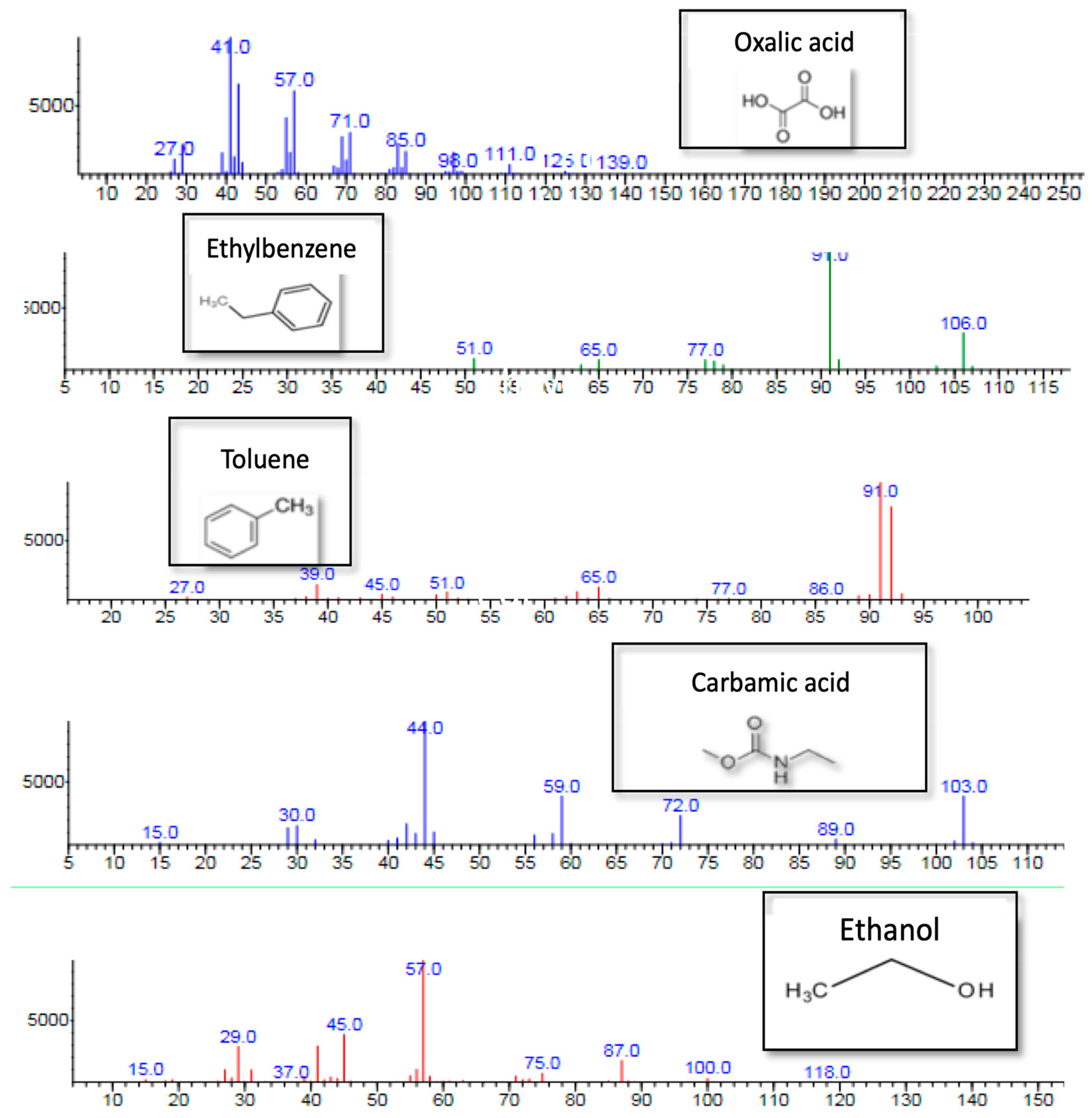
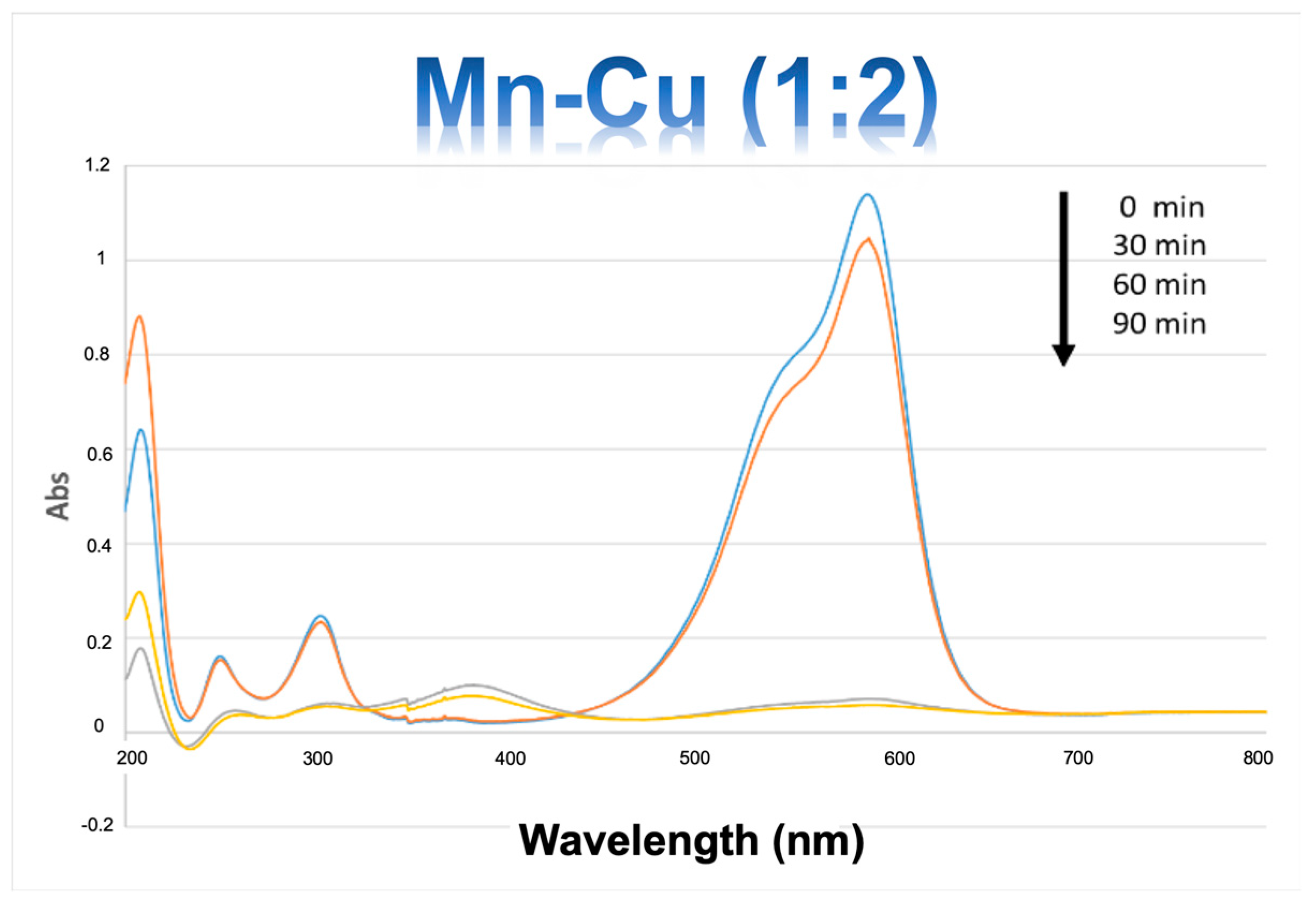
2.3. Catalytic Activity
3. Discussion
Catalytic Activity
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Characterization
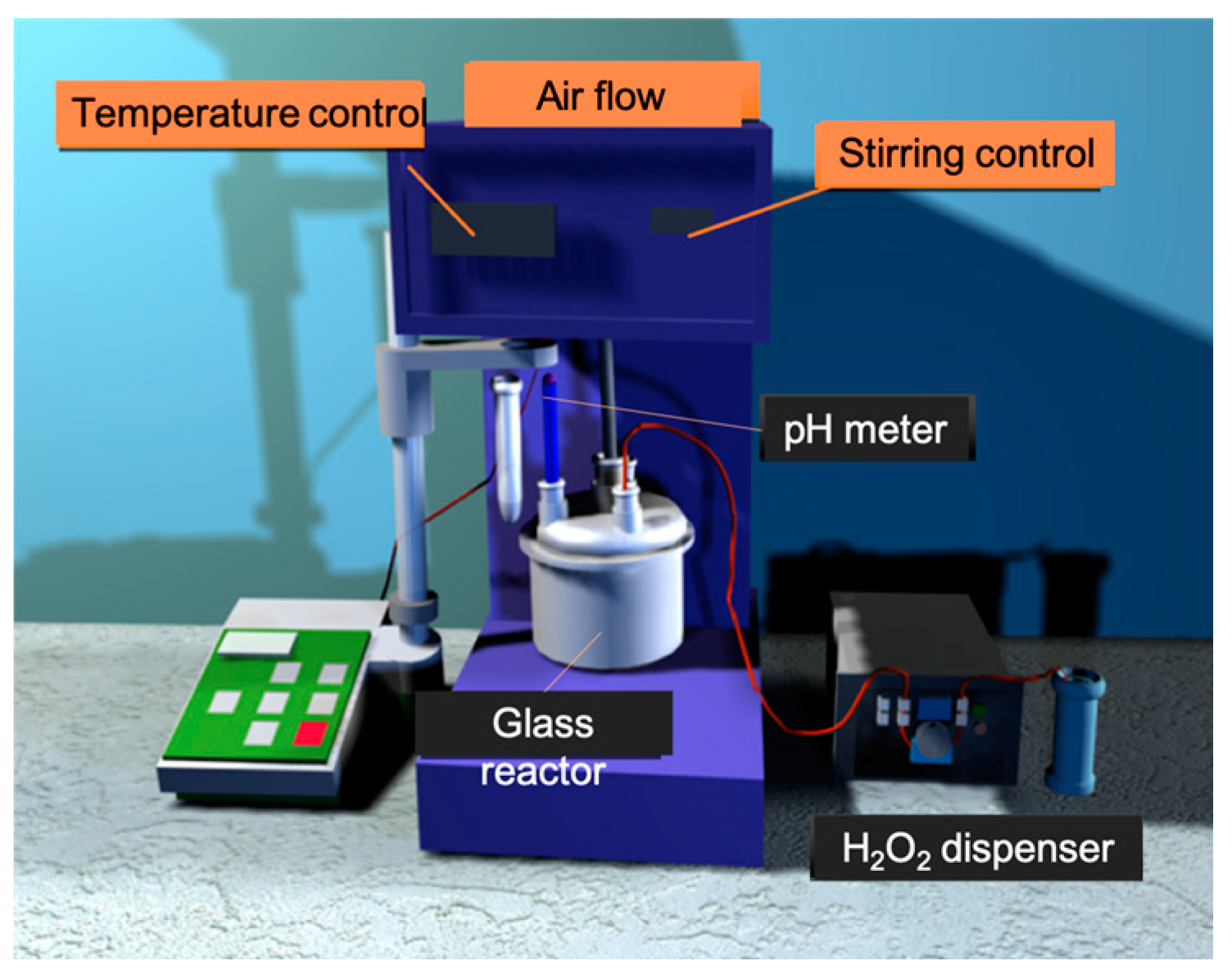
4.3. Catalytic Evaluation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, J.; Cai, J.; Yin, C.; Gao, L.; Zhou, J. Degradation performance of crystal violet over CuO/AC and CeO2-CuO/AC catalysts using microwave catalytic oxidation degradation method. J. Environ. Chem. Eng. 2016, 4, 958–964. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Rezaei, S.; Tahmasbi, H.; Mogharabi, M.; Firuzyar, S.; Ameri, A.; Khoshayand, M.R.; Faramarzi, M.A. Efficient decolorization and detoxification of reactive orange 7 using laccase isolated from Paraconiothyrium variabile, kinetics and energetics. J. Taiwan Inst. Chem. Eng. 2015, 56, 113–121. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; Sánchez, J.G.; López, J.C.; Oller, I.; Malato, S.; Pérez, J.S. Dissolved oxygen concentration: A key parameter in monitoring the photo-Fenton process. Appl. Catal. B Environ. 2011, 104, 316–323. [Google Scholar]
- Inchaurrondo, N.S.; Massa, P.; Fenoglio, R.; Font, J.; Haure, P. Efficient catalytic wet peroxide oxidation of phenol at moderate temperature using a high-load supported copper catalyst. Chem. Eng. J. 2012, 198–199, 426–434. [Google Scholar] [CrossRef]
- Carrillo, A.M.; Carriazo, J.G. Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Deganello, F.; Marcì, G.; Deganello, G. Citrate–nitrate auto-combustion synthesis of perovskite-type nanopowders: A systematic approach. J. Eur. Ceram. Soc. 2009, 29, 439–450. [Google Scholar] [CrossRef]
- Castaño, M.H.; Molina, R.; Moreno, S. Mn–Co–Al–Mg mixed oxides by auto-combustion method and their use as catalysts in the total oxidation of toluene. J. Mol. Catal. A Chem. 2013, 370, 167–174. [Google Scholar] [CrossRef]
- Ovejero, G.; Rodríguez, A.; Vallet, A.; García, J. Ni/Fe-supported over hydrotalcites precursors as catalysts for clean and selective oxidation of Basic Yellow 11: Reaction intermediates determination. Chemosphere 2013, 90, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chen, W.C.; Chiou, M.R.; Chen, S.W.; Chen, Y.Y.; Fan, H.J. Degradation of crystal violet by an FeGAC/H2O2 process. J. Hazard. Mater. 2011, 196, 420–425. [Google Scholar] [CrossRef]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic decomposition of hydrogen peroxide on transition metal and lanthanide oxides. J. Mol. Catal. A Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Barrault, J.; Bouchoule, C.; Tatibouët, J.M.; Abdellaoui, M.; Majesté, A.; Louloudi, I.; Papayannakos, N.; Gangas, N.H. Catalytic wet peroxide oxidation over mixed (Al-Fe) pillared clays. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 749–754. [Google Scholar]
- Huang, L.; Zhang, F.; Wang, N.; Chen, R.; Hsu, A.T. Nickel-based perovskite catalysts with iron-doping via self-combustion for hydrogen production in auto-thermal reforming of Ethanol. Int. J. Hydrogen Energy 2012, 37, 1272–1279. [Google Scholar] [CrossRef]
- Cesar, M.A. Quimisorción; Universidad de Juarez: Ciudad Juárez, Mexico, 2011. [Google Scholar]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Liu, L.; Song, Y.; Fu, Z.; Ye, Q.; Cheng, S.; Kang, T.; Dai, H. Enhanced catalytic performance of Cu- and/or Mn-loaded Fe-Sep catalysts for the oxidation of CO and ethyl acetate. Chin. J. Chem. Eng. 2017, 25, 1427–1434. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids. 2012. Available online: http://linux0.unsl.edu.ar/~rlopez/cap3new.pdf (accessed on 27 May 2019).
- Wu, J.; Gao, H.; Yao, S.; Chen, L.; Gao, Y.; Zhang, H. Degradation of Crystal Violet by catalytic ozonation using Fe/activated carbon catalyst. Sep. Purif. Technol. 2015, 147, 179–185. [Google Scholar] [CrossRef]
- Han, J.; Zeng, H.; Xu, S.; Chen, C.; Liu, X. Catalytic properties of CuMgAlO catalyst and degradation mechanism in CWPO of methyl orange. Appl. Catal. A Gen. 2016, 527, 72–80. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient photo-Fenton activity in mesoporous MIL-100(Fe) decorated with T ZnO nanosphere for pollutants degradation. Appl. Catal. B Env. 2019, 245, 428–438. [Google Scholar] [CrossRef]
- Lu, H.; Kong, X.; Huang, H.; Zhou, Y.; Chen, Y. Cu–Mn–Ce ternary mixed-oxide catalysts for catalytic combustion of toluene. J. Environ. Sci. 2015, 32, 102–107. [Google Scholar] [CrossRef]
- Chi, H.; Andolina, C.M.; Li, J.; Curnan, M.T.; Saidi, W.A.; Zhou, G.; Yang, J.C.; Veser, G. Dependence of H2 and CO2 selectivity on Cu oxidation state during partial oxidation of methanol on Cu/ZnO. Appl. Catal. A Gen. 2018, 556, 64–72. [Google Scholar] [CrossRef]
- Molina, R.; Castaño, M.H.; Moreno, S. Catalizadores de manganeso sintetizados por autocombustión y coprecipitación y su empleo en la oxidación del 2-propanol. Cienc. Químicas 2015, 39, 26–35. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, K.; Zhou, J.; Lin, M.; Sun, Y. Direct synthesis of methyl formate from syngas on CueMn mixed oxide catalyst. Int. J. Hydrogen Eng. 2016, 41, 8819–8828. [Google Scholar] [CrossRef]
- Răciulete, M.; Layrac, G.; Papa, F.; Negrilă, C.; Tichit, D.; Marcu, I.C. Influence of Mn content on the catalytic properties ofCu-(Mn)-Zn-Mg-Al mixed oxides derived from LDH precursors in the total oxidation of methane. Catal. Today 2018, 306, 276–286. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu–Mn and Co–Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wu, X.; Ye, Q.; Xu, X.; Li, B.; Wang, F. Novel synthesis and shape-dependent catalytic performance of Cu–Mn oxides for CO oxidation. Appl. Surf. Sci. 2017, 403, 335–341. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, K.; Dong, F.; Lin, M.; Sun, Y.; Tang, Z. Textual properties of Cu–Mn mixed oxides and application for methyl formate synthesis from syngas. J. Ind. Eng. Chem. 2017, 54, 117–125. [Google Scholar] [CrossRef]
- Jabłońska, M.; Chmielarz, L.; Węgrzyn, A.; Góra-Marek, K.; Piwowarska, Z.; Witkowski, S.; Bidzińska, E.; Kuśtrowski, P.; Wach, A.; Majda, D. Hydrotalcite derived (Cu, Mn)–Mg–Al metal oxide systems doped with palladium as catalysts for low-temperature methanol incineration. Appl. Clay Sci. 2015, 114, 273–282. [Google Scholar] [CrossRef]
- Perdomo, C.; Perez, A.; Molina, R.; Moreno, S. Storage capacity and oxygen mobility in mixed oxides from transition metals promoted by cerium. Appl. Surf. Sci. 2016, 383, 42–48. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, S.T.; Chung, W.H.; Jan, J.L.; Lin, W.Y.; Chen, C.C. Degradation pathways of crystal violet by Fenton and Fenton-like systems: Condition optimization and intermediate separation and identification. J. Hazard. Mater. 2009, 171, 1032–1044. [Google Scholar] [CrossRef]
- Fida, H.; Zhang, G.; Guo, S.; Naeem, A. Heterogeneous Fenton degradation of organic dyes in batch and fixed bed using La-Fe montmorillonite as catalyst. J. Colloid Interface Sci. 2017, 490, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-C.; Tsai, J.S.; Huang, T.H.; Peng, C.H.; Chen, S.Y. Combustion synthesis of Ni–Zn ferrite powder—influence of oxygen balance value. J. Solid State Chem. 2005, 178, 382–389. [Google Scholar] [CrossRef]
- Hua, Z.; Cao, Z.; Deng, Y.; Jiang, Y.; Yang, S. Sol–gel autocombustion synthesis of Co–Ni alloy powder. Mater. Chem. Phys. 2011, 126, 542–545. [Google Scholar] [CrossRef]


| Catalyst | Mn/Cu | Al | Mg | Glycine |
|---|---|---|---|---|
| Mn–Cu (1:1) | 1 | 20 | 30 | 100 |
| Mn–Cu (1:2) | 0.5 | 20 | 30 | 100 |
| Mn–Cu (2:1) | 2 | 20 | 30 | 100 |
| Mn | 0 | 20 | 30 | 100 |
| Cu | 5 | 20 | 30 | 100 |
| Catalyst | Crystaline Phase | FWHM | 2θ | Crystal Size (nm) * | Ratios | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Cu | Mn | Cu | Cu/Al | Mn/Al | Mg/Al | ||||
| Mn | Mn3O4 | 0.2525 | - | 44.4 | - | 6.59 | - | - | 0.4 | 1.8 |
| Cu | Cu2O | - | 0.8762 | - | 42.5 | - | 1.89 | 0.4 | - | 1.8 |
| Mn–Cu (1:1) | Mn3O4; Cu2O | 0.2800 | 0.3244 | 44.4 | 42.5 | 5.51 | 5.09 | 0.2 | 0.2 | 1.7 |
| Mn–Cu (2:1) | Mn3O4; Cu2O | 0.9153 | 0.8840 | 44.4 | 42.5 | 1.82 | 1.87 | 0.1 | 0.3 | 1.8 |
| Mn–Cu (1:2) | Mn3O4; Cu2O | 1.2066 | 1.2212 | 44.4 | 42.5 | 1.38 | 1.35 | 0.3 | 0.1 | 1.8 |
| Catalyst | T Max (°C) TPR-H2 | |
|---|---|---|
| Mn | 71 | 505 |
| Cu | 112 | 211 |
| Mn–Cu (1:2) | 92 | 367 |
| Mn–Cu (1:1) | 55 | 194 |
| Mn–Cu (2:1) | 62 | 412 |
| Catalyst | Mn | Cu | Mn–Cu | ||
|---|---|---|---|---|---|
| 1:1 | 1:2 | 2:1 | |||
| Removal time 100% (min) | 360 | 150 | 150 | 60 | 240 |
| %TOC 1 (±2) | 49 | 68 | 70 | 74 | 52 |
| %COD 2 (±1) | 52 | 60 | 59 | 71 | 50 |
| Solid | Temperature °C | Reduction Signal |
|---|---|---|
| Mn | <300 | Mn5O8 to Mn2O3 (Mn+4 -> Mn+3) |
| 350–450 | Mn2O3 to Mn3O4 (Mn+3 -> Mn+2) | |
| 500–600 | Mn3O4 a MnO | |
| >600 | Mn spinel phase |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, A.M.; Riaño, P.F.; Lugo, D.L.; Barriga, J.A.; Celis, C.A.; Moreno, S.; Pérez, A. Degradation of Crystal Violet by Catalytic Wet Peroxide Oxidation (CWPO) with Mixed Mn/Cu Oxides. Catalysts 2019, 9, 530. https://doi.org/10.3390/catal9060530
Campos AM, Riaño PF, Lugo DL, Barriga JA, Celis CA, Moreno S, Pérez A. Degradation of Crystal Violet by Catalytic Wet Peroxide Oxidation (CWPO) with Mixed Mn/Cu Oxides. Catalysts. 2019; 9(6):530. https://doi.org/10.3390/catal9060530
Chicago/Turabian StyleCampos, Ana María, Paula Fernanda Riaño, Diana Lorena Lugo, Jenny Alejandra Barriga, Crispín Astolfo Celis, Sonia Moreno, and Alejandro Pérez. 2019. "Degradation of Crystal Violet by Catalytic Wet Peroxide Oxidation (CWPO) with Mixed Mn/Cu Oxides" Catalysts 9, no. 6: 530. https://doi.org/10.3390/catal9060530