Advantages of Liquid Nitrogen Quick Freezing Combine Gradient Slow Thawing for Quality Preserving of Blueberry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Blueberry
2.3. Treatments
2.3.1. Freezing Experiments
2.3.2. Thawing Experiments
2.4. Physical and Chemical Analysis
2.4.1. Weight Loss (WL)
2.4.2. Hardness
2.4.3. Distribution of Water States
2.4.4. Soluble Sugar and Pectin Content
2.4.5. Vitamin C (Vc) Ccontent
2.4.6. Polyphenol Content, Anthocyanin Content, Antioxidant Activity, Polyphenol Oxidase (PPO) Activity, and Peroxidase (POD) Activity
2.4.7. Microscopic Observations
2.4.8. Statistical Analysis
3. Results and Discussion
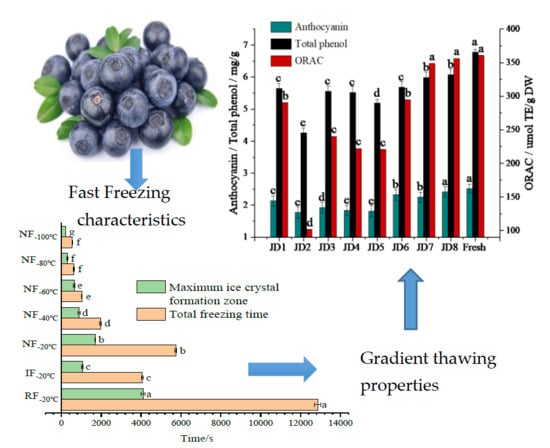
3.1. Effects of Different Freezing Processes on the Quality Changes of Blueberry
3.1.1. Freezing Characteristics
3.1.2. Physical Properties
3.1.3. Functional Components
3.1.4. Microstructure Changes
3.2. Impacts of Different Thawing Method on the Quality Characteristics of Blueberry
3.2.1. Physical Properties
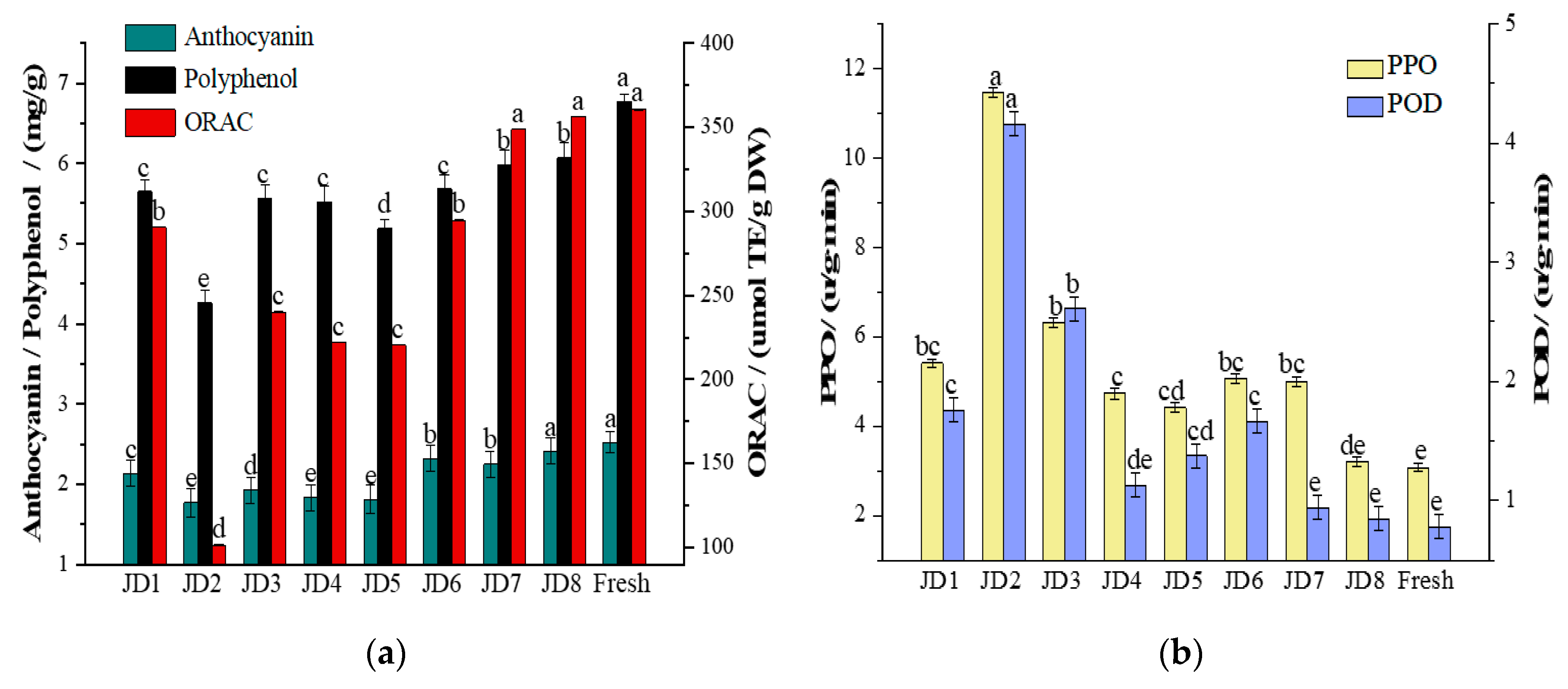
3.2.2. Functional Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zielinska, M.; Sadowski, P.; Błaszczak, W. Freezing/thawing and microwave-assisted drying of blueberries (Vaccinium corymbosum L.). Lwt-Food Sci. Technol. 2015, 62, 555–563. [Google Scholar] [CrossRef]
- Huynh, N.K.; Wilson, M.D.; Eyles, A.; Stanley, R.A. Recent advances in postharvest technologies to extend the shelf life of blueberries (Vaccinium sp.), raspberries (Rubus idaeus L.) and blackberries (Rubus sp.). J. Berry Res. 2019, 9, 687–707. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, W.; Sun, D.-W. Effects of liquid nitrogen quick freezing on polyphenol oxidase and peroxide activities, cell water states and epidermal microstructure of wolfberry. LWT 2020, 120, 108923. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.-W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Yang, F.; Jing, D.; Yu, D.; Xia, W.; Jiang, Q.; Xu, Y.; Yu, P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (Takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Sanchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014, 153, 250–257. [Google Scholar] [CrossRef]
- Lopkulkiaert, W.; Prapatsornwattana, K.; Rungsardthong, V. Effects of sodium bicarbonate containing traces of citric acid in combination with sodium chloride on yield and some properties of white shrimp (Penaeus vannamei) frozen by shelf freezing, air-blast and cryogenic freezing. Lwt-Food Sci. Technol. 2009, 42, 768–776. [Google Scholar] [CrossRef]
- James, C.; Purnell, G.; James, S.J. A Review of Novel and Innovative Food Freezing Technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- James, S.J.; James, C. Food Technologies: Freezing. Encycl. Food Saf. 2014, 3, 187–195. [Google Scholar] [CrossRef]
- Li, Y.; Jia, W.; Zhang, C.H.; Li, X.; Wang, J.Z.; Mu, G.F. Fluctuated low temperature combined with high-humidity thawing to reduce physicochemical quality deterioration of beef. Food Bioprocess Technol. 2014, 7, 3370–3380. [Google Scholar] [CrossRef]
- Roiha, I.S.; Tveit, G.M.; Backi, C.J.; Jónsson, Á.; Karlsdóttir, M.; Lunestad, B.T. Effects of controlled thawing media temperatures on quality and safety of pre-rigor frozen Atlantic cod (Gadus morhua). Lwt 2018, 90, 138–144. [Google Scholar] [CrossRef]
- Alfaro, L.; Siramard, S.; Chouljenko, A.; Sathivel, S. Effects of liquid nitrogen pretreatment on the osmotic dehydration and quality of cryogenically frozen blueberries ( Vaccinium angustifolium Ait.). Food Biosci. 2018, 22, 165–169. [Google Scholar] [CrossRef]
- Ketata, M.; Desjardins, Y.; Ratti, C. Effect of liquid nitrogen pretreatments on osmotic dehydration of blueberries. J. Food Eng. 2013, 116, 202–212. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, F.; Zhao, D.; Zhu, D.; Li, J. Effects of freezing conditions on quality changes in blueberries. J. Sci. Food Agric. 2018, 98, 4673–4679. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Yang, B.; Prasad, K.N.; Jiang, Y. Structure identification of a polysaccharide purified from litchi (Litchi chinensis Sonn.) pulp. Carbohydr. Polym. 2016, 137, 570–575. [Google Scholar] [CrossRef]
- Chen, H.; Cao, S.; Fang, X.; Mu, H.; Yang, H.; Wang, X.; Xu, Q.; Gao, H. Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Sci. Hortic. 2015, 188, 44–48. [Google Scholar] [CrossRef]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry ( Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.M.; Forney, C.F.; Carbyn, S.E.; Nicholas, K.U.K.G. Microstructural Indicators of Quality-related Characteristics of Blueberries—An Integrated Approach. Lwt-Food Sci. Technol. 2001, 34, 23–32. [Google Scholar] [CrossRef]
- Jo, H.-J.; Kim, J.-E.; Yu, M.-J.; Lee, W.-H.; Song, K.B.; Kim, H.-Y.; Hwang, I.G.; Yoo, S.M.; Han, G.J.; Park, J.-T. Effect of Freezing Temperature on Blueberry Quality. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1906–1912. [Google Scholar] [CrossRef]
- Bonat Celli, G.; Ghanem, A.; Su-Ling Brooks, M. Influence of freezing process and frozen storage on the quality of fruits and fruit products. Food Rev. Int. 2016, 32, 280–304. [Google Scholar] [CrossRef]
- Santagapita, P.; Laghi, L.; Panarese, V.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Modification of Transverse NMR Relaxation Times and Water Diffusion Coefficients of Kiwifruit Pericarp Tissue Subjected to Osmotic Dehydration. Food Bioprocess Technol. 2012, 6, 1434–1443. [Google Scholar] [CrossRef]
- Li, J.; Kang, J.; Wang, L.; Li, Z.; Wang, R.; Chen, Z.X.; Hou, G.G. Effect of Water Migration between Arabinoxylans and Gluten on Baking Quality of Whole Wheat Bread Detected by Magnetic Resonance Imaging (MRI). J. Agric. Food Chem. 2012, 60, 6507–6514. [Google Scholar] [CrossRef]
- Qiao, S.; Tian, Y.; Song, P.; He, K.; Song, S. Analysis and detection of decayed blueberry by low field nuclear magnetic resonance and imaging. Postharvest Biol. Technol. 2019, 156, 110951. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Meng, X.; Liu, S.; Mu, J.; Ning, C. Comparison of polyphenol, anthocyanin and antioxidant capacity in four varieties of Lonicera caerulea berry extracts. Food Chem. 2016, 197, 522–529. [Google Scholar] [CrossRef]
- Tomaz, I.; Šeparović, M.; Štambuk, P.; Preiner, D.; Maletić, E.; Karoglan Kontić, J. Effect of freezing and different thawing methods on the content of polyphenolic compounds of red grape skins. J. Food Process. Preserv. 2018, 42, e13550. [Google Scholar] [CrossRef]
- Jha, P.K.; Xanthakis, E.; Chevallier, S.; Jury, V.; Le-Bail, A. Assessment of freeze damage in fruits and vegetables. Food Res. Int. 2019, 121, 479–496. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D.-W. Effects of freezing on cell structure of fresh cellular food materials: A review. Trends Food Sci. Technol. 2018, 75, 46–55. [Google Scholar] [CrossRef]
- Fava, J.; Alzamora, S.M.; Castro, M.A. Structure and Nanostructure of the Outer Tangential Epidermal Cell Wall in Vaccinium corymbosum L. (Blueberry) Fruits by Blanching, Freezing-Thawing and Ultrasound. Food Sci. Technol. Int. 2016, 12, 241–251. [Google Scholar] [CrossRef]
- Charoenrein, S.; Owcharoen, K. Effect of freezing rates and freeze-thaw cycles on the texture, microstructure and pectic substances of mango. Int. Food Res. J. 2016, 23, 613. [Google Scholar]
- Xu, Y.T.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Miano, A.C.; Rojas, M.L.; Augusto, P.E.D. Structural changes caused by ultrasound pretreatment: Direct and indirect demonstration in potato cylinders. Ultrason. Sonochem. 2019, 52, 176–183. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Yang, X.-H.; Wang, J.; Zhang, Q.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. High humidity hot air impingement blanching (HHAIB) enhances drying rate and softens texture of apricot via cell wall pectin polysaccharides degradation and ultrastructure modification. Food Chem. 2018, 261, 292–300. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Conde, J.; Pagan, J. Optimisation of steam blanching on enzymatic activity, color and protein degradation of alfalfa (Medicago sativa) to improve some quality characteristics of its edible protein. Food Chem. 2019, 276, 591–598. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, L.; Li, D.; Liu, C.; Ma, R.; Song, J.; Zhao, J. Low intensity ultrasound as a pretreatment to drying of daylilies: Impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrason. Sonochem. 2017, 36, 50–58. [Google Scholar] [CrossRef]
- Cano, M.P.; de Ancos, B.A.; Lobo, G. Peroxidase and Polyphenoloxidase Activities in Papaya During Postharvest Ripening and After Freezing/Thawing. J. Food Sci. 1995, 60, 815–817. [Google Scholar] [CrossRef]





| Group | Process | Equipment Model |
|---|---|---|
| JD1 | Microwave at 500 w | NN-GF362M, Shanghai matsushita microwave oven co., LTD., Shanghai, China |
| JD2 | Room temperature at 25 °C | |
| JD3 | Static-water thawing at 25 °C | |
| JD4 | Ultrasound thawing at 28 kHZ | Dl-800b, Shanghai zhixin instrument co., Ltd., Shanghai, China |
| JD5 | Ultrasound thawing at 47 kHZ | |
| JD6 | Low temperature thawing at 4 °C | In a refrigerator (BCD-239WTGM, Hefei midea refrigerator co., LTD., Hefei, China |
| JD7 | Gradient thawing (−20 °C→0 °C→4 °C) | |
| JD8 | Gradient thawing(−20 °C→−5 °C→4 °C) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Wu, W.; An, K.; Xu, Y.; Yu, Y.; Wen, J.; Wu, J.; Zou, Y.; Liu, H.; Zhu, J.; et al. Advantages of Liquid Nitrogen Quick Freezing Combine Gradient Slow Thawing for Quality Preserving of Blueberry. Crystals 2020, 10, 368. https://doi.org/10.3390/cryst10050368
Cheng L, Wu W, An K, Xu Y, Yu Y, Wen J, Wu J, Zou Y, Liu H, Zhu J, et al. Advantages of Liquid Nitrogen Quick Freezing Combine Gradient Slow Thawing for Quality Preserving of Blueberry. Crystals. 2020; 10(5):368. https://doi.org/10.3390/cryst10050368
Chicago/Turabian StyleCheng, Lina, Weijun Wu, Kejing An, Yujuan Xu, Yuanshan Yu, Jing Wen, Jijun Wu, Ying Zou, Haocheng Liu, Jieli Zhu, and et al. 2020. "Advantages of Liquid Nitrogen Quick Freezing Combine Gradient Slow Thawing for Quality Preserving of Blueberry" Crystals 10, no. 5: 368. https://doi.org/10.3390/cryst10050368