On the Importance of H-Bonding Interactions in the Enclathration of Boric Acids in Na(I) Polymers: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Syntheses
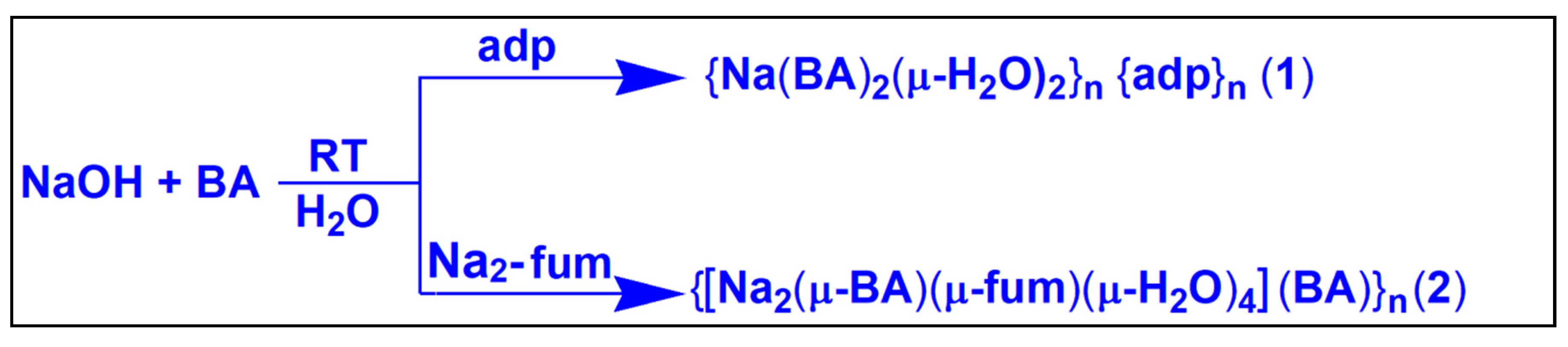
2.2.1. Synthesis of {Na(BA)2(μ-H2O)2}n{adp}n (1)
2.2.2. Synthesis of {[Na2(μ-BA)(μ-fum)(μ-H2O)4](BA)}n (2)
2.3. Crystallographic Data Collection and Refinement
2.4. Theoretical Methods
3. Results
3.1. Synthesis and General Aspects
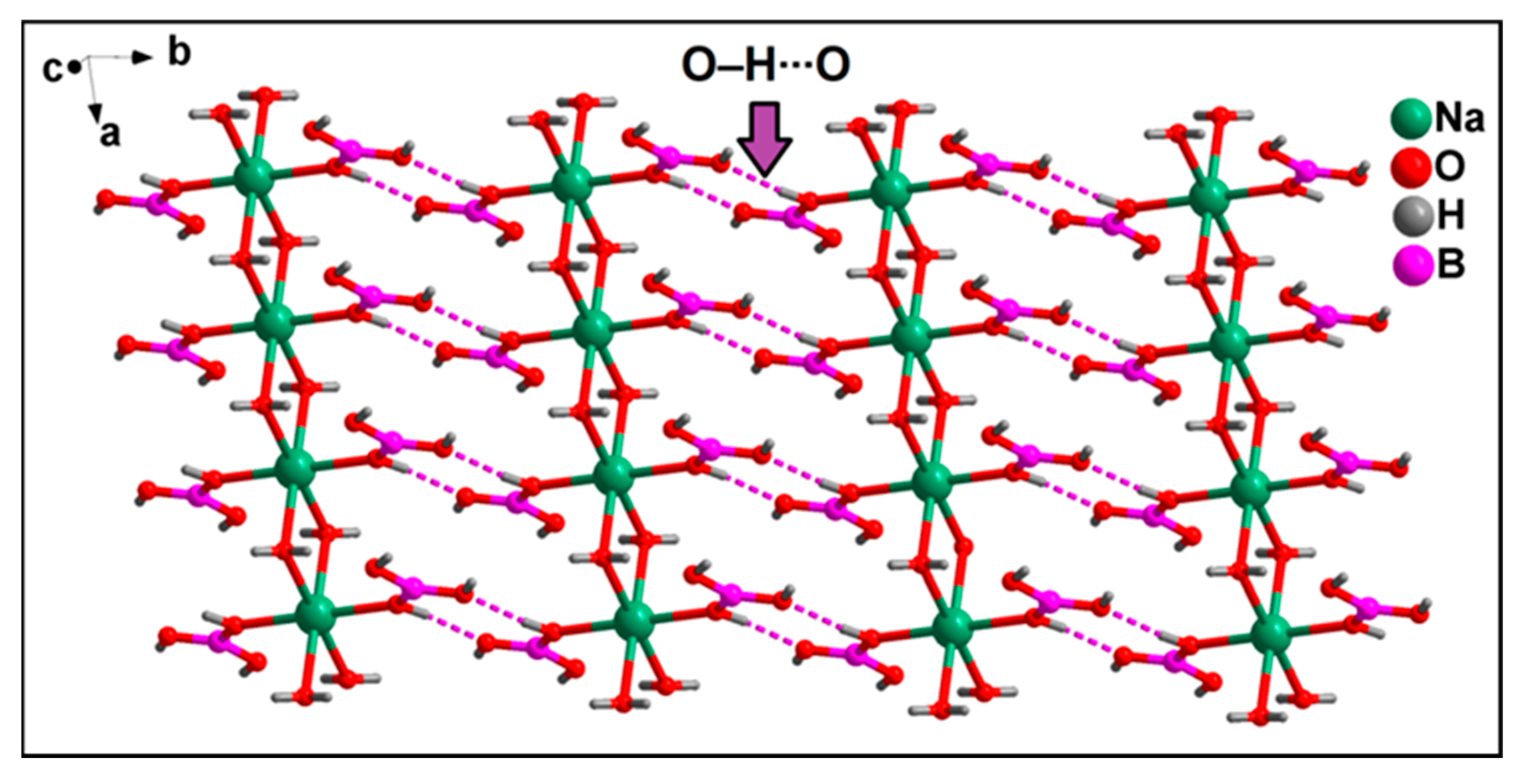
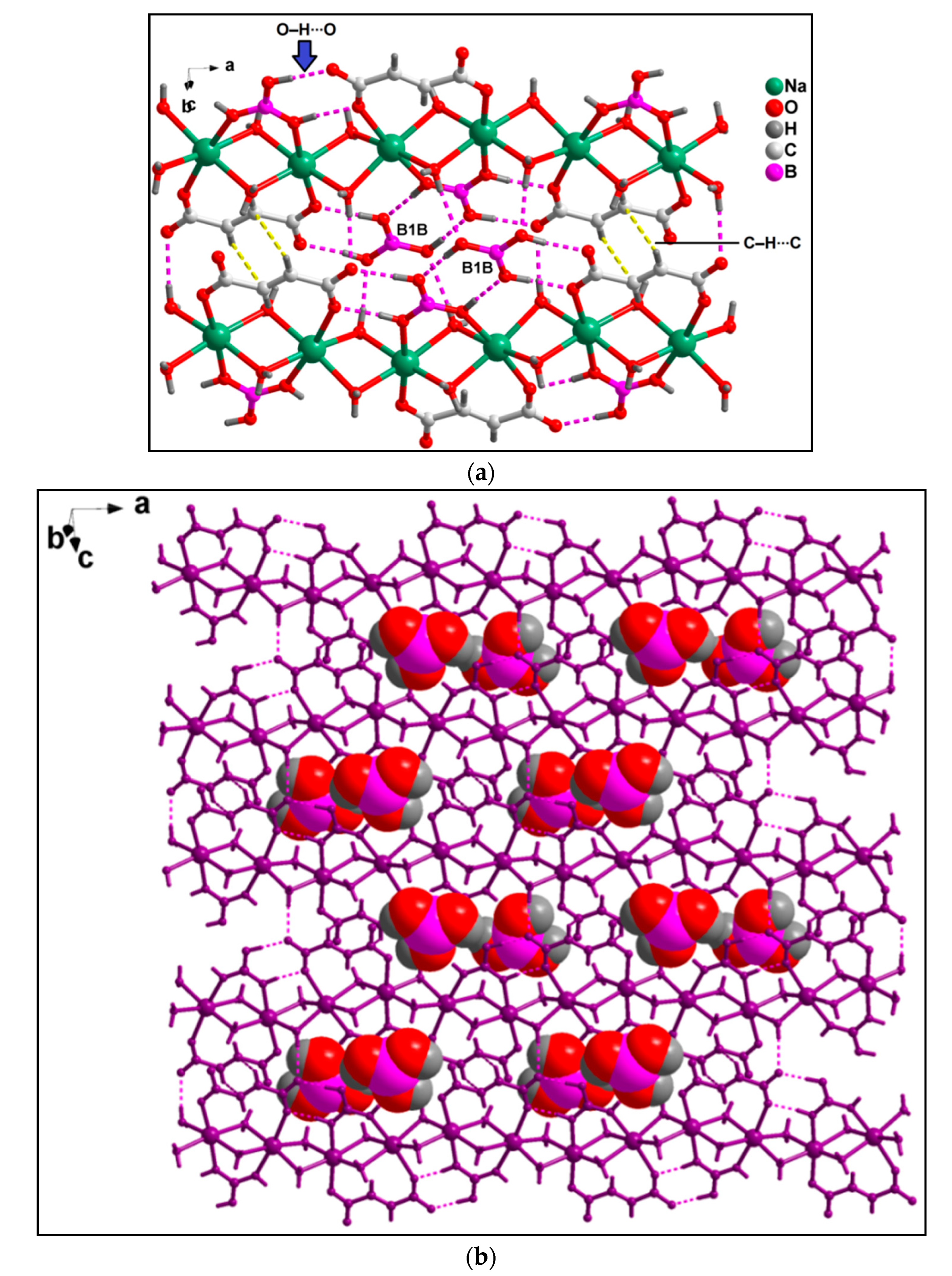
3.2. Crystal Structure Analysis
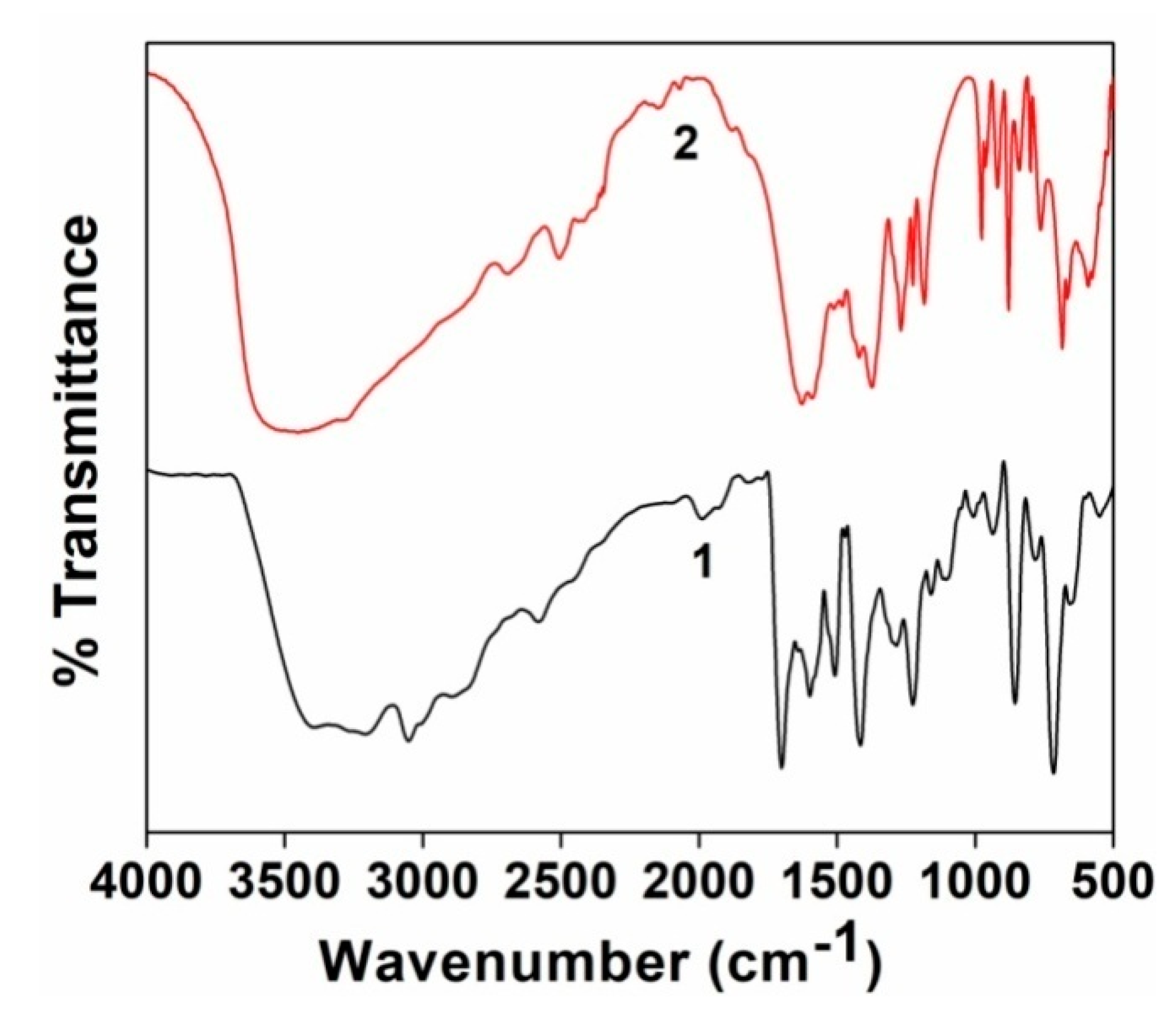
3.3. FT-IR Spectroscopy
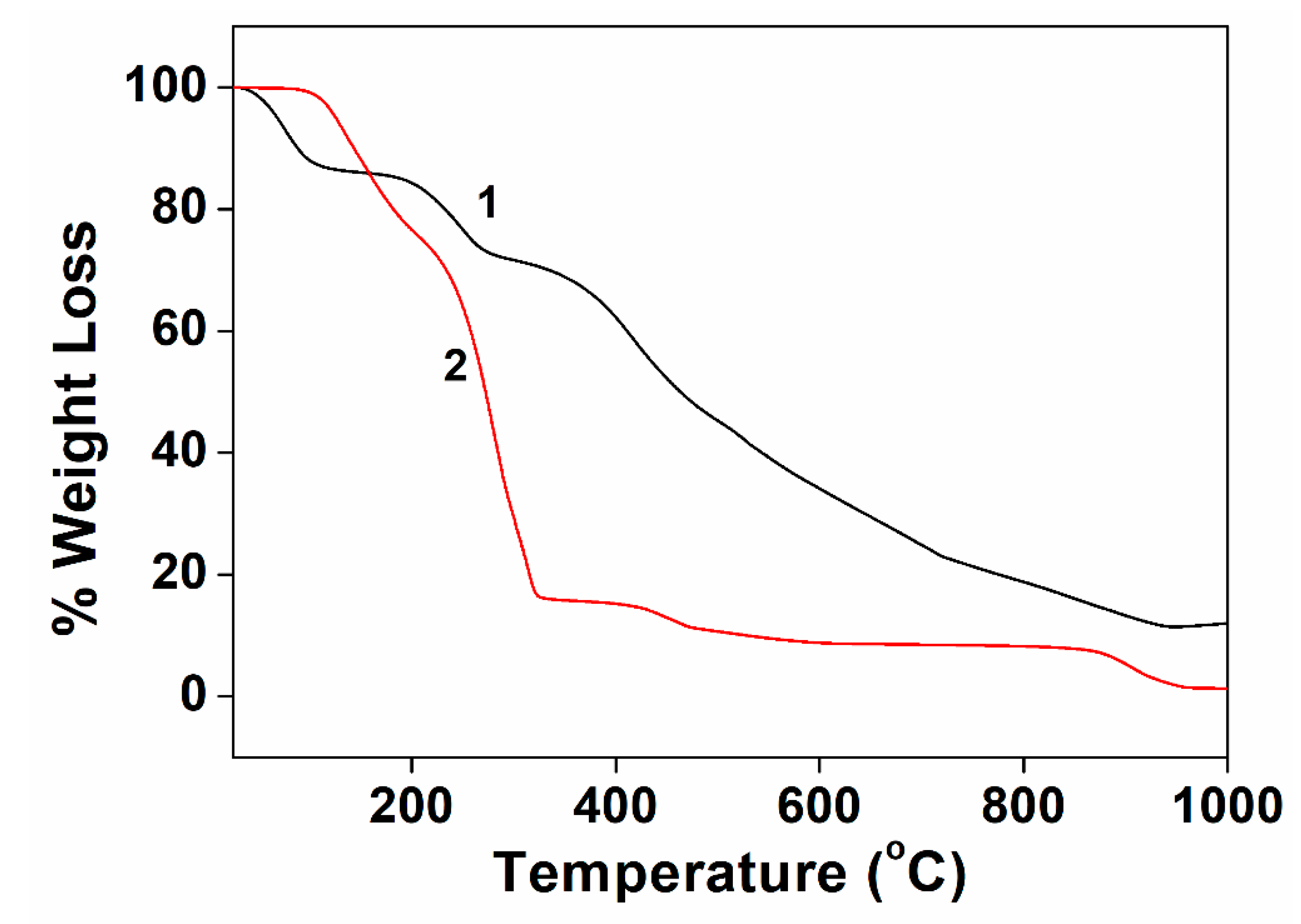
3.4. Thermogravimetric Analysis
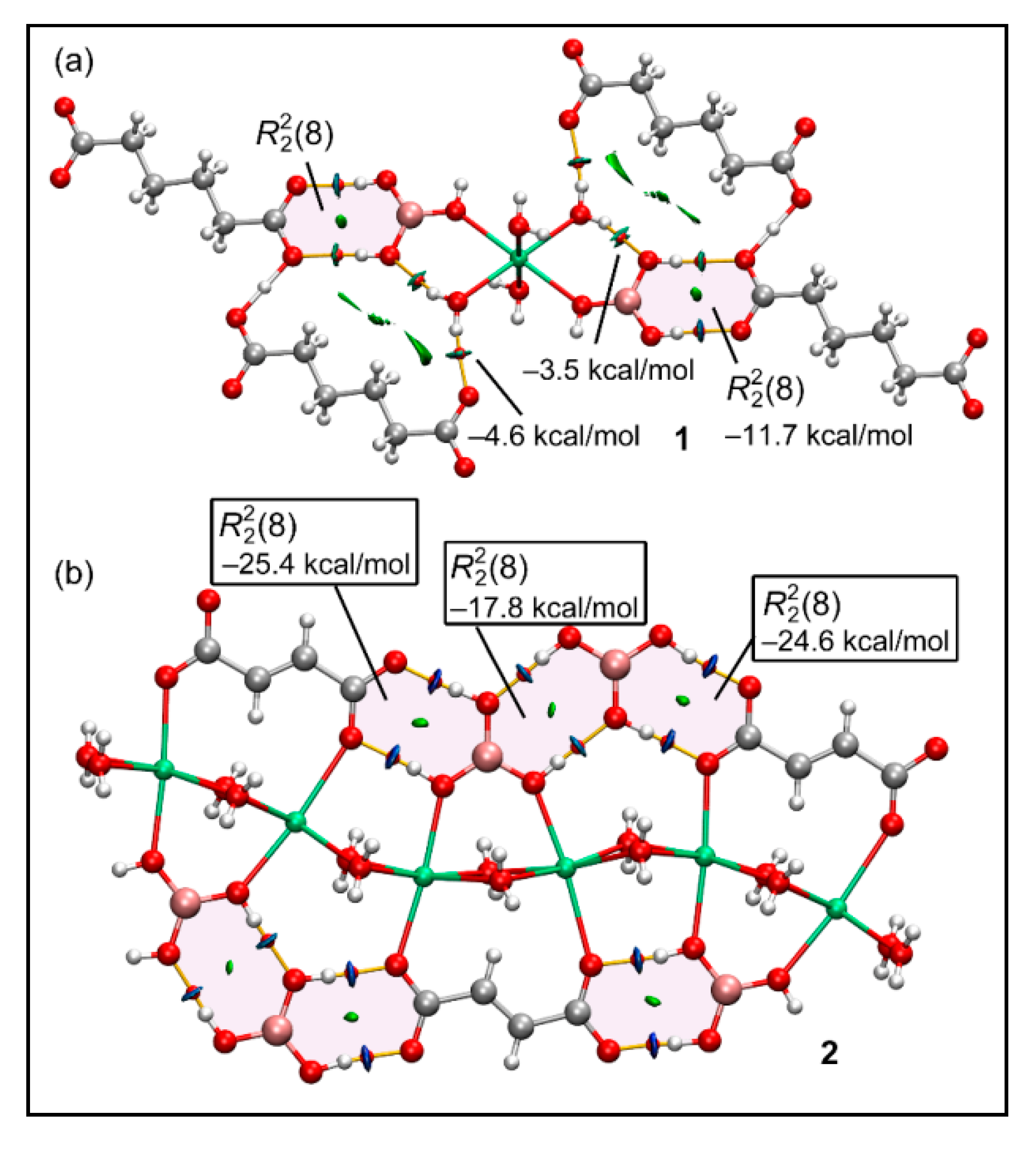
3.5. Theoretical Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Engel, E.R.; Scott, J.L. Advances in the Green Chemistry of Coordination Polymer Materials. Green Chem. 2020, 22, 3693–3715. [Google Scholar] [CrossRef]
- Biradha, K.; Ramanan, A.; Vittal, J.J. Coordination Polymers versus Metal–Organic Frameworks. Cryst. Growth Des. 2009, 9, 2969–2970. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hazra, A.; Mondal, A.; Banerjee, P. Weak Interactions: The Architect behind the Structural Diversity of Coordination Polymer. Inorg. Chim. Acta 2019, 488, 86–119. [Google Scholar] [CrossRef]
- Bureekaew, S.; Shimomura, S.; Kitagawa, S. Chemistry and Application of Flexible Porous Coordination Polymers. Sci. Technol. Adv. Mater. 2008, 9, 14108. [Google Scholar] [CrossRef] [PubMed]
- Scaeteanu, G.V.; Maxim, C.; Badea, M.; Olar, R. Zinc(II) Carboxylate Coordination Polymers with Versatile Applications. Molecules 2023, 28, 1132. [Google Scholar] [CrossRef]
- Ou, Y.C.; Zhong, R.M.; Wu, J.Z. Recent Advances in Structures and Applications of Coordination Polymers Based on Cyclohexanepolycarboxylate Ligands. Dalton Trans. 2022, 51, 2992–3003. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Solomon, M.B.; Leong, C.F.; D’Alessandro, D.M. Redox-Active Ligands: Recent Advances towards Their Incorporation into Coordination Polymers and Metal-Organic Frameworks. Coord. Chem. Rev. 2021, 439, 213891. [Google Scholar] [CrossRef]
- Fromm, K.M. Coordination Polymer Networks with S-Block Metal Ions. Coord. Chem. Rev. 2008, 252, 856–885. [Google Scholar] [CrossRef]
- Das, B.C.; Chokkalingam, P.; Masilamani, P.; Shukla, S.; Das, S. Stimuli-Responsive Boron-Based Materials in Drug Delivery. Int. J. Mol. Sci. 2023, 24, 2757. [Google Scholar] [CrossRef]
- Córdova-Chávez, R.I.; Carrasco-Ruiz, M.F.; Rodríguez-Vera, D.; Pérez-Capistran, T.; Tamay-Cach, F.; Scorei, I.R.; Abad-García, A.; Soriano-Ursúa, M.A. Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders. Biol. Trace Elem. Res. 2023, 201, 2222–2239. [Google Scholar]
- Ji, L.; Zhou, H. Recent developments in the synthesis of bioactive boron-containing compounds. Tetrahedron Lett. 2021, 82, 153411–153426. [Google Scholar] [CrossRef]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [PubMed]
- Lopalco, A.; Lopedota, A.A.; Laquintana, V.; Denora, N.; Stella, V.J. Boric Acid, a Lewis Acid with Unique and Unusual Properties: Formulation Implications. J. Pharm. Sci. 2020, 109, 2375–2386. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Chen, T.; Liu, W.; Ye, F.; Zhao, S. Design and fabrication of boric acid functionalized hierarchical porous metal-organic frameworks for specific removal of cis-diol-containing compounds from aqueous solution. Appl. Surface Sci. 2021, 535, 147714. [Google Scholar] [CrossRef]
- Cebula, J.; Fink, K.; Boratyński, J.; Goszczyński, T.M. Supramolecular chemistry of anionic boron clusters and its applications in biology. Coord. Chem. Rev. 2023, 477, 214940. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not only hydrogen bonds: Other noncovalent interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Saha, U.; Dutta, D.; Frontera, A.; Verma, A.K.; Sharma, P.; Das, A. Unconventional DNA-Relevant π-Stacked Hydrogen Bonded Arrays Involving Supramolecular Guest Benzoate Dimers and Cooperative Anion–π/π–π/π–Anion Contacts in Coordination Compounds of Co(II) and Zn(II) Phenanthroline: Experimental and Theoretical Studies. New J. Chem. 2020, 44, 4504–4518. [Google Scholar] [CrossRef]
- Desiraju, G.R. Chemistry beyond the Molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hypervalency, Secondary Bonding and Hydrogen Bonding: Siblings under the Skin. Chem. Soc. Rev. 2017, 46, 1720–1729. [Google Scholar] [CrossRef]
- Karas, L.J.; Wu, C.-H.; Das, R.; Wu, J.I.-C. Hydrogen Bond Design Principles. WIREs Comput. Mol. Sci. 2020, 10, e1477. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Non-Covalent Interactions in the Synthesis of Coordination Compounds: Recent Advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Gutiérrez-Flores, J.; Ramos, E.; Mendoza, C.I.; Hernández-Lemus, E. Electronic Properties of DNA: Description of Weak Interactions in TATA-Box-like Chains. Biophys. Chem. 2018, 233, 26–35. [Google Scholar] [CrossRef]
- Allen, F.H.; Baalham, C.A.; Lommerse, J.P.M.; Raithby, P.R. Carbonyl–Carbonyl Interactions Can Be Competitive with Hydrogen Bonds. Acta Crystallogr. 1998, 54, 320–329. [Google Scholar] [CrossRef]
- Contejean, Z.I.; LaDuca, R.L. Nitroaromatic-Detecting Zinc and Cadmium Coordination Polymers with Methyl-Substituted Aliphatic Dicarboxylate and 4,4′-Dipyridylamine Ligands and Diverse Topologies. J. Solid State Chem. 2018, 266, 44–53. [Google Scholar] [CrossRef]
- White, C.L.; LaDuca, R.L. Nickel Adipate Coordination Polymers with Isomeric Dipyridylamide Ligands: Topological Disorder and Divergent Magnetic Properties. CrystEngComm. 2016, 18, 6789–6797. [Google Scholar] [CrossRef]
- Ignatyev, I.; Kondratenko, Y.; Fundamensky, V.; Kochina, T. Synthesis and Characterization of Cobalt(II) Complexes with Triethanolamine and Succinate and/or Nitrate Anions. Transit. Met. Chem. 2018, 43, 127–136. [Google Scholar] [CrossRef]
- Forlin, E.; Lanza, A.; Nicola, C.D.; Monari, M.; Gazzano, M.; Nestola, F.; Pettinari, C.; Pandolfo, L. 1D and 3D Coordination Polymers Based on the Cu3(Μ3-OH)(μ-Pz)3 and Cu(Hpz)3 SBUs Connected by the Flexible Glutarate Dianion. Inorg. Chim. Acta 2018, 470, 385–392. [Google Scholar] [CrossRef]
- Biswas, C.; Jana, A.D.; Drew, M.G.B.; Mostafa, G.; Ghosh, A. Segregated Self-Assembly and Pillaring Action of Aliphatic Dicarboxylic Acids in the Super Structure of Cu–Picolinate Complexes. Polyhedron 2009, 28, 653–660. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Damodaran, S.; Bisht, K.K.; Suresh, E. Hydrogen Bonded Binary Molecular Adducts Derived from Exobidentate N-Donor Ligand with Dicarboxylic Acids: Acid⋯imidazole Hydrogen-Bonding Interactions in Neutral and Ionic Heterosynthons. J. Mol. Struct. 2011, 985, 361–370. [Google Scholar] [CrossRef]
- Dutta, B.; Maity, S.; Ghosh, S.; Sinha, C.; Mir, M.H. An Acetylenedicarboxylato-Bridged Mn(Ii)-Based 1D Coordination Polymer: Electrochemical CO2 Reduction and Magnetic Properties. New J. Chem. 2019, 43, 5167–5172. [Google Scholar] [CrossRef]
- Arici, M.; Erer, H.; Karaçam, S.; Yeşilel, O.Z.J. Coordination polymers based on 3,3-dimethylglutarate and 1,4-bis ((1H-imidazol-1-yl) methyl) benzene: Hydrothermal synthesis and characterizations. J. Solid State Chem. 2019, 277, 811–818. [Google Scholar] [CrossRef]
- Chandra, A.; Dutta, B.; Pal, K.; Jana, K.; Sinha, C. Designing of an Adipic Acid Bridged Zn(II) Coordination Polymer: Synthesis and Biological Study. J. Mol. Struct. 2021, 1243, 130923. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xu, S.; Liu, S.N.; Hu, J.; Qiao, Y.X.; Liu, B.Q. Two Series of Substituent-Group-Directed Adipate-Based Lanthanide Coordination Polymers: Syntheses, Structures, Photoluminescence, and Magnetism. Eur. J. Inorg. Chem. 2020, 2020, 1233–1241. [Google Scholar] [CrossRef]
- Song, Y.; Yan, Y.; Zhang, H.; Wang, X. Synthesis and Structural Characterization of a Novel 2D Supramolecular Lead Coordination Polymer with Phenanthroline Derivate and Adipic Acid. Main Group Met. Chem. 2021, 44, 239–242. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Ma, J.S. Hydrothermal Syntheses and Characterization of Novel 3D Open-Framework and 2D Grid Lanthanide Fumarates: Ln2(Fum)3(H2fum)(H2O)2 (Ln = Ce or Nd), [Sm2(Fum)3(H2O)4](H2O)3, and [Yb2(Fum)3(H2O)4](H2O)2. Cryst. Growth Des. 2006, 6, 933–939. [Google Scholar] [CrossRef]
- Gogoi, A.; Das, A.; Frontera, A.; Verma, A.K.; Bhattacharyya, M.K. Energetically Significant Unconventional π-π Contacts Involving Fumarate in a Novel Coordination Polymer of Zn(II): In-Vitro Anticancer Evaluation and Theoretical Studies. Inorg. Chim. Acta 2019, 493, 1–13. [Google Scholar] [CrossRef]
- Ozer, D.; Köse, D.A.; Şahin, O.; Oztas, N.A. Synthesis and Characterization of Boric Acid Mediated Metal-Organic Frameworks Based on Trimesic Acid and Terephthalic Acid. J. Mol. Struct. 2017, 1141, 261–267. [Google Scholar] [CrossRef]
- APEX3, SAINT, SADABS and XP; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond 3.1f; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Ozer, D.; Köse, D.A.; Sahin, O.; Oztas, N.A. Study of Structural, Surface and Hydrogen Storage Properties of Boric Acid Mediated Metal (Sodium)-Organic Frameworks. J. Mol. Struct. 2018, 1157, 159–164. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Nashre-ul-Islam, S.M.; Dutta, D.; Guha, A.K.; Bhattacharyya, M.K. An Unusual Werner Type Clathrate of Mn(II) Benzoate Involving Energetically Significant Weak CH⋯C Contacts: A Combined Experimental and Theoretical Study. J. Mol. Struct. 2019, 1175, 130–138. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistri, S.; Jana, A.D. A Rare Supramolecular Assembly Involving Ion Pairs of Coordination Complexes with a Host–Guest Relationship: Synthesis, Crystal Structure, Photoluminescence and Thermal Study. CrystEngComm 2012, 14, 7415–7422. [Google Scholar] [CrossRef]
- Nath, H.; Dutta, D.; Sharma, P.; Frontera, A.; Verma, A.K.; Barceló-Oliver, M.; Devi, M.; Bhattacharyya, M.K. Adipato Bridged Novel Hexanuclear Cu(II) and Polymeric Co(II) Coordination Compounds Involving Cooperative Supramolecular Assemblies and Encapsulated Guest Water Clusters in a Square Grid Host: Antiproliferative Evaluation and Theoretical Studies. Dalton Trans. 2020, 49, 9863–9881. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-F.; Zhou, J.; Zou, H.-H.; Xiao, H.-P.; Tang, Q.; Jiang, T.; Zhang, X. Synthesis, Crystal Structures and Properties of a Series of Lanthanide Adipates [Ln2(Ad)3(H2O)4] (Ln = Y3+, Ho3+, Er3+, Tm3+). J. Clust. Sci. 2016, 27, 2025–2033. [Google Scholar] [CrossRef]
- Jumanath, E.C.; Pradyumnan, P.P. Thermal Degradation and Spectroscopic Studies of Single-Crystalline Organometallic Calcium Adipate Monohydrate. J. Therm. Anal. Calorim. 2020, 140, 567–575. [Google Scholar] [CrossRef]
- Jumanath, E.C.; Pradyumnan, P.P. Growth, Characterization and Dielectric Proprty Studies of Zinc Adipate Dihydrate Crystals. AIP Conf. Proc. 2019, 2082, 1–5. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bora, S.J.; Das, B.K. Synthesis, structure and properties of a fumarate bridged Ni (II) coordination polymer. J. Mol. Struct. 2011, 999, 83–88. [Google Scholar] [CrossRef]
- Hernández, M.F.; Suárez, G.; Cipollone, M.; Conconi, M.S.; Aglietti, E.F.; Rendtorff, N.M. Formation, Microstructure and Properties of Aluminum Borate Ceramics Obtained from Alumina and Boric Acid. Ceram. Int. 2017, 43, 2188–2195. [Google Scholar] [CrossRef]
- Wang, C.G.; Xing, Y.H.; Li, Z.P.; Li, J.; Zeng, X.Q.; Ge, M.F.; Niu, S.Y. A Series of Three-Dimensional Lanthanide-Rigid-Flexible Frameworks: Synthesis, Structure, and Luminescent Properties of Coordination Polymers with 2,5-Pyridine Dicarboxylic Acid and Adipic Acid. Cryst. Growth Des. 2009, 9, 1525–1530. [Google Scholar] [CrossRef]
- Téllez-López, A.; Sánchez-Mendieta, V.; Jaramillo-García, J.; Rosales-Vázquez, L.D.; García-Orozco, I.; Morales-Luckie, R.A.; Escudero, R.; Morales-Leal, F. Modification of the Structure and Magnetic Properties of Fumarato-Bridged Mn Coordination Polymers through Different Dimethyl-2,2′-Bipyridine Co-Ligands. Transit. Met. Chem. 2016, 41, 879–887. [Google Scholar] [CrossRef]




| Crystal Parameters | 1 | 2 |
|---|---|---|
| Empirical formula | C6H19B2NaO12 | C4H16B2Na2O14 |
| Formula weight | 327.82 | 355.77 |
| Temperature (K) | 100.0 | 294.0 |
| Wavelength (Å) | 1.54178 | 1.54178 |
| Crystal system | Triclinic | Orthorhombic |
| Space group | P | Pnma |
| a (Å) | 3.7723 (4) | 14.0708 (12) |
| b (Å) | 8.6376 (9) | 6.7995 (6) |
| c (Å) | 10.9248 (12) | 14.9868 (12) |
| α (°) | 87.123 (4) | 90 |
| β (°) | 84.622 (4) | 90 |
| γ (°) | 82.919 (4) | 90 |
| Volume (Å3) | 351.43 (7) | 1433.9 (2) |
| Z | 1 | 4 |
| Calculated density (g/cm3) | 1.549 | 1.648 |
| Absorption coefficient (mm−1) | 1.543 | 1.950 |
| F(000) | 172.0 | 736.0 |
| Crystal size (mm3) | 0.26 × 0.24 × 0.15 | 0.39 × 0.26 × 0.21 |
| θ range for data collection (°) | 8.134 to 137.124 | 8.62 to 136.55 |
| Index ranges | −4 ≤ h ≤ 4, −10 ≤ k ≤ 10, −13 ≤ l ≤ 13 | −16 ≤ h ≤ 16, −8 ≤ k ≤ 7, −18 ≤ l ≤ 18 |
| Reflections collected | 7484 | 15,582 |
| Unique data (Rint) | 1276 | 1421 |
| Refinement method | Full-matrix least squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 1276/0/118 | 1421/0/161 |
| Goodness-of-fit on F2 | 1.074 | 1.065 |
| Final Rindices [I > 2σ (I)] R1/wR2 | 0.0422/0.1152 | 0.0228/0.0616 |
| Rindices (all data) R1/wR2 | 0.0427/0.1161 | 0.0229/0.0617 |
| Largest diff. peak and hole (e·Å−3) | 0.31 and −0.24 | 0.25 and −0.19 |
| D–H∙∙∙A | d(D–H) | d(D∙∙∙A) | d(H∙∙∙A) | <(DHA) |
|---|---|---|---|---|
| Compound 1 | ||||
| O1W-H1WB∙∙∙O4B#1 | 0.86 | 2.91 | 2.09 | 159.4 |
| O2B-H2B∙∙∙O3B#3 | 0.82 | 2.769 | 1.95 | 175 |
| C4A-H4AB∙∙∙O3A | 0.99 | 3.02 | 2.64 | 103.1 |
| C4A-H4AA∙∙∙O4B | 0.99 | 3.726 | 2.74 | 175.1 |
| O3B-H3B∙∙∙O1A#2 | 0.77 | 2.658 | 1.89 | 175 |
| O4B-H4B∙∙∙O3A#2 | 0.83 | 2.891 | 2.06 | 176.5 |
| Compound 2 | ||||
| O4A-H4A∙∙∙O7#6 | 0.89 | 2.6 | 1.71 | 178 |
| O2A-H2A∙∙∙O8#6 | 0.87 | 2.665 | 1.8 | 174 |
| O2W-H2WB∙∙∙O7#4 | 0.83 | 2.902 | 2.08 | 168.9 |
| O2B-H2B∙∙∙O1 | 0.88 | 2.646 | 1.77 | 178 |
| O3B-H3B∙∙∙O3 | 0.9 | 2.638 | 1.75 | 172 |
| O3A-H3A∙∙∙O2B#7 | 0.81 | 2.677 | 1.87 | 177 |
| O4B-H4B∙∙∙O4A#6 | 0.87 | 2.682 | 1.82 | 176 |
| O2W-H2WA∙∙∙O3B#5 | 0.86 | 2.777 | 1.92 | 175.3 |
| O1W-H1WB∙∙∙O4B#5 | 0.8 | 3.016 | 2.22 | 169.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baishya, T.; Dutta, K.K.; Frontera, A.; Gomila, R.M.; Barceló-Oliver, M.; Bhattacharyya, M.K. On the Importance of H-Bonding Interactions in the Enclathration of Boric Acids in Na(I) Polymers: Experimental and Theoretical Studies. Crystals 2023, 13, 895. https://doi.org/10.3390/cryst13060895
Baishya T, Dutta KK, Frontera A, Gomila RM, Barceló-Oliver M, Bhattacharyya MK. On the Importance of H-Bonding Interactions in the Enclathration of Boric Acids in Na(I) Polymers: Experimental and Theoretical Studies. Crystals. 2023; 13(6):895. https://doi.org/10.3390/cryst13060895
Chicago/Turabian StyleBaishya, Trishnajyoti, Kamal K. Dutta, Antonio Frontera, Rosa M. Gomila, Miquel Barceló-Oliver, and Manjit K. Bhattacharyya. 2023. "On the Importance of H-Bonding Interactions in the Enclathration of Boric Acids in Na(I) Polymers: Experimental and Theoretical Studies" Crystals 13, no. 6: 895. https://doi.org/10.3390/cryst13060895







