Abstract
The one-pot synthesis of a Cu(II) complex with partially oxidized tetrathiafulvalene (TTF) moieties in its capping MT-Hsae-TTF ligands, [CuII(MT-sae-TTF)2] [CuICl2] was realized by the simultaneous occurrence of Cu(II) complexation and CuIICl2 mediated oxidation of TTF moieties. The crystal structure was composed of one-dimensional columns formed by partially oxidized TTF moieties and thus the cation radical salt showed relatively high electrical conductivity. Tight binding band structure calculations indicated the existence of a Peierls gap due to the tetramerization of the TTF moieties in the one-dimensional stacking column at room temperature, which is consistent with the semiconducting behavior of this salt.
1. Introduction
The interplay between electrical conductivity and magnetism provides a number of interesting physical phenomena such as colossal magneto-resistance and the Kondo effect, which have been widely investigated in inorganic compounds. In molecular based materials, although the interaction between the conduction electrons and local magnetic moments is rather weak, attractive properties, such as paramagnetic superconductivity or magnetic field induced superconductivity, were reported in so-called π-d systems, where organic π-electron donors like TTF (tetrathiafulvalene) derivatives are responsible for the conduction and counter anions with magnetic moments for magnetism [1,2,3,4]. Compared with conventional TTF-based radical cation molecular conductors with paramagnetic counter ions, metal complexes usually show a relatively strong interaction due to the direct coordination of π-electron ligands with the central paramagnetic metal ions. For example, [Cu(DCNQI)] has a band structure based on the hybridization of the d-orbitals of Cu and the π-orbitals of DCNQI [5,6], the paramagnetic Fe(III) complex of Phthalocyanine (Pc), TPP[Fe(Pc)(CN)2]2 (TPP = tetraphenylphosphonium) exhibits a giant negative magnetoresistance [7,8], and the single-component molecular metal based on TTF-dithiolato ligand, [Au(tmdt)2], undergoes an antiferromagnetic transition [9,10,11,12]. In all cases, the π-d interaction through direct coordination plays an important role in the unique physical properties.
In order to enhance the π-d interaction in molecular based materials, paramagnetic metal complexes with TTF moiety-incorporating ligands have been widely prepared [13]. In most cases, the TTF moieties in metal complexes are in their neutral state, resulting in electrical insulators with the exception of a few examples in which the TTF moieties are in their complete or partial oxidation states; a Mo(0) carbonyl complex with a TTF-phosphine ligand [14] and a Ni(II) dinuclear complex with TTF-pyridine ligands [15] were reported to afford cation radical salts with completely oxidized TTF moieties resulting in insulating behavior. On the other hand, a Cu(II) complex with TTF-pyridine ligands was isolated with partially oxidized TTF moieties, but the complex behaved as an insulator because of the charge disproportionation on the TTF sites [16]. A diamagnetic Cu(I) complex coordinated by pyrazine-annulated diselenadithiafulvalene was shown to have partially oxidized ligands and displayed relatively high electrical conduction [17]. The key issue in the generation of highly conducting TTF-based metal complex is therefore the preparation of stable complexes with partially oxidized TTF moieties.
In order to obtain TTF-based metal complexes with partially oxidized TTF moieties, we have synthesized several TTF-ligands incorporating Schiff base-type coordination sites and metal complexes based on such TTF-ligands [18,19]. The TTF-based metal complexes are expected to be stable upon oxidation of the TTF moieties in the ligands because of the chelating coordination site of the Schiff base moieties. Recently, TTF-based Cu(II) and Ni(II) complexes with two Schiff base coordination sites were reported, but the partially oxidized species were not prepared [20]. In contrast, the Cu(II) complex of ET-Hsae-TTF [4-(N-salicyli-deneiminoethylthio)-5-methyl-4',5'-ethylene-dithio-TTF] (Figure 1) led to charge transfer salts and a cation radical salt with partially oxidized TTF moieties, which exhibited fairly high conductivity [18]. In this paper, we describe the thiomethyl analogue of Hsae-TTF, MT-Hsae-TTF [4-(N-salicylidene-iminoethylthio)-5-methyl-4',5'-bis(methyl-thio)-TTF] (Figure 1), and the one-pot synthesis of a partially oxidized TTF-based Cu(II) complex. The crystal structure and conducting behavior are discussed.
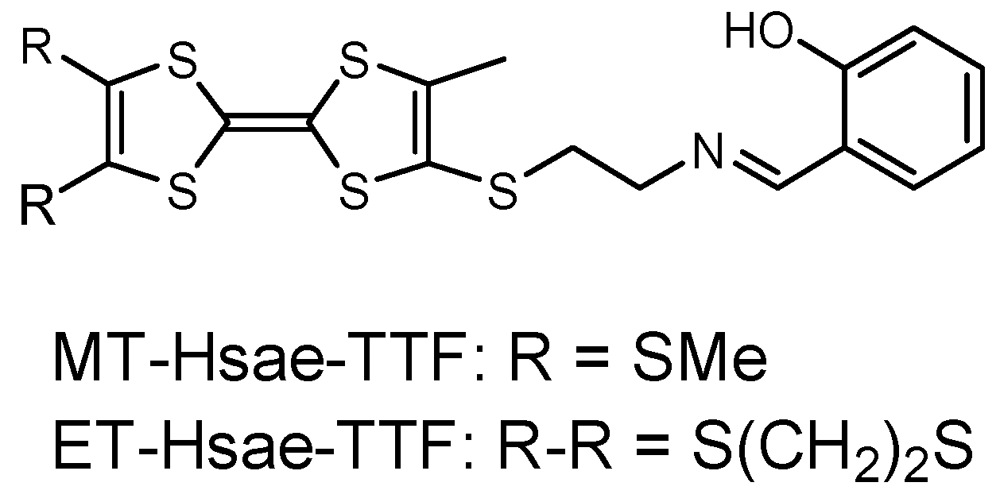
Figure 1.
Tetrathiafulvalene (TTF)-ligand Hsae-TTF.
2. Results and Discussion
2.1. Synthesis of MT-Hsae-TTF, and One-Pot Preparation of the Partially Oxidized TTF-based Metal Complex, [CuII(MT-sae-TTF)2][CuICl2]
The synthesis of MT-Hsae-TTF was performed following the same method for that of ET-Hsae-TTF [18]. The aminoethyl-TTF, prepared from the cyanoethyl-protected TTF derivative [21] via N-BOC-protected TTF (BOC = t-butoxycarbonyl), was reacted with salicylaldehyde in tetrahydrofuran (THF) to give MT-Hsae-TTF. The cyclic voltammogram of MT-Hsae-TTF was composed of two oxidation waves corresponding to the redox of the TTF moieties at E1 = 0.44 and E2 = 0.84 V (vs. SCE). The Cu(II) complex of the MT-Hsae-TTF ligand was synthesized by the addition of a methanolic solution of CuIICl2·2H2O to a CH2Cl2 solution of MT-Hsae-TTF in the presence of triethylamine (Scheme 1). The complex obtained was revealed to be a cation radical salt, [CuII(MT-sae-TTF)2][CuICl2], in which the TTF moiety was partially oxidized. A number of studies on the chemical oxidation of TTF derivatives with copper(II) halides have been reported [17,22,23]. In our reaction, the complexation and oxidation processes occured simultaneously, which is in contrast with the Cu(II) complex of ET-Hsae-TTF, [CuII(ET-sae-TTF)2], which could be isolated as a neutral complex due to the lower solubility of [CuII(ET-sae-TTF)2] compared to the present complex.
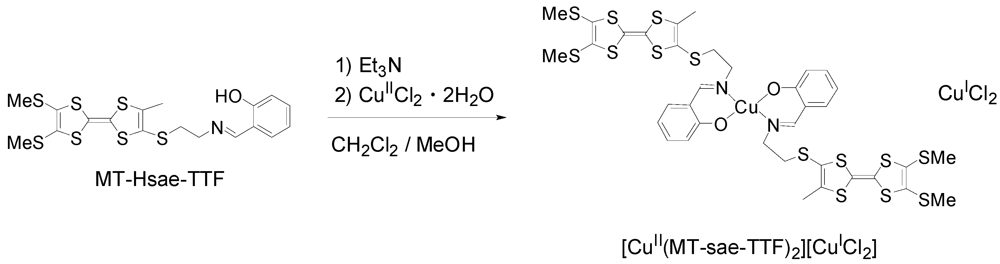
Scheme 1.
Synthesis of the cation radical salt [CuII(MT-sae-TTF)2][CuICl2].
2.2. X-ray Structural Analysis of [CuII(MT-sae-TTF)2][CuICl2]
X-ray data collection of [CuII(MT-sae-TTF)2][CuICl2] was performed at 100 K; crystallographic data are summarized in Table 1. [CuII(MT-sae-TTF)2][CuICl2] crystallizes in the triclinic space group P 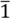 . The asymmetric unit is composed of one cation complex [CuII(MT-sae-TTF)2]+ and two CuICl2− anions, each of them located on an inversion center. Figure 2 shows the molecular structure of [CuII(MT-sae-TTF)2]+. Disorder of one 4,5-bis(methylthio)-1,3-dithiole ring was observed in TTF A. The central Cu(II) ion adopts an N2O2 configuration through complexation by the two Schiff base moieties, which form a square planar coordination environment with trans conformation. The 1:1 ratio of the Cu(II) complexes and the CuICl2− anions indicates that the charge of the Cu(II) complex is +1. The two TTF moieties in one complex form a dimer with staggered face-to-face overlap; the torsion angle of the staggered configuration is 38.6° (Figure 2(a)). The molecular structure of [CuII(MT-sae-TTF)2]+ is completely different from that of the neutral [MII(ET-sae-TTF)2] complex (M = Ni(II) and Cu(II)) [18], in which the square planar Schiff base coordination site was sandwiched by two neutral TTF moieties, and similar to that for the cation radical salt, [CuII(ET-sae-TTF)2]PF6.
. The asymmetric unit is composed of one cation complex [CuII(MT-sae-TTF)2]+ and two CuICl2− anions, each of them located on an inversion center. Figure 2 shows the molecular structure of [CuII(MT-sae-TTF)2]+. Disorder of one 4,5-bis(methylthio)-1,3-dithiole ring was observed in TTF A. The central Cu(II) ion adopts an N2O2 configuration through complexation by the two Schiff base moieties, which form a square planar coordination environment with trans conformation. The 1:1 ratio of the Cu(II) complexes and the CuICl2− anions indicates that the charge of the Cu(II) complex is +1. The two TTF moieties in one complex form a dimer with staggered face-to-face overlap; the torsion angle of the staggered configuration is 38.6° (Figure 2(a)). The molecular structure of [CuII(MT-sae-TTF)2]+ is completely different from that of the neutral [MII(ET-sae-TTF)2] complex (M = Ni(II) and Cu(II)) [18], in which the square planar Schiff base coordination site was sandwiched by two neutral TTF moieties, and similar to that for the cation radical salt, [CuII(ET-sae-TTF)2]PF6.
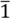 . The asymmetric unit is composed of one cation complex [CuII(MT-sae-TTF)2]+ and two CuICl2− anions, each of them located on an inversion center. Figure 2 shows the molecular structure of [CuII(MT-sae-TTF)2]+. Disorder of one 4,5-bis(methylthio)-1,3-dithiole ring was observed in TTF A. The central Cu(II) ion adopts an N2O2 configuration through complexation by the two Schiff base moieties, which form a square planar coordination environment with trans conformation. The 1:1 ratio of the Cu(II) complexes and the CuICl2− anions indicates that the charge of the Cu(II) complex is +1. The two TTF moieties in one complex form a dimer with staggered face-to-face overlap; the torsion angle of the staggered configuration is 38.6° (Figure 2(a)). The molecular structure of [CuII(MT-sae-TTF)2]+ is completely different from that of the neutral [MII(ET-sae-TTF)2] complex (M = Ni(II) and Cu(II)) [18], in which the square planar Schiff base coordination site was sandwiched by two neutral TTF moieties, and similar to that for the cation radical salt, [CuII(ET-sae-TTF)2]PF6.
. The asymmetric unit is composed of one cation complex [CuII(MT-sae-TTF)2]+ and two CuICl2− anions, each of them located on an inversion center. Figure 2 shows the molecular structure of [CuII(MT-sae-TTF)2]+. Disorder of one 4,5-bis(methylthio)-1,3-dithiole ring was observed in TTF A. The central Cu(II) ion adopts an N2O2 configuration through complexation by the two Schiff base moieties, which form a square planar coordination environment with trans conformation. The 1:1 ratio of the Cu(II) complexes and the CuICl2− anions indicates that the charge of the Cu(II) complex is +1. The two TTF moieties in one complex form a dimer with staggered face-to-face overlap; the torsion angle of the staggered configuration is 38.6° (Figure 2(a)). The molecular structure of [CuII(MT-sae-TTF)2]+ is completely different from that of the neutral [MII(ET-sae-TTF)2] complex (M = Ni(II) and Cu(II)) [18], in which the square planar Schiff base coordination site was sandwiched by two neutral TTF moieties, and similar to that for the cation radical salt, [CuII(ET-sae-TTF)2]PF6. 
Table 1.
Crystallographic data for [CuII(MT-sae-TTF)2][CuICl2] at 100 K.
| [CuII(MT-sae-TTF)2][CuICl2] | |
|---|---|
| Empirical formula | C36H36Cl2Cu2N2O2S14 |
| Formula weight | 1175.49 |
| Temperature/K | 100(2) |
| Wavelength/Å | 0.71073 |
| Crystal system | Triclinic |
| Space group | P 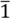 |
| a/Å | 12.903(2) |
| b/Å | 13.390(2) |
| c/Å | 15.561(3) |
| α/° | 67.636(2) |
| β/° | 71.690(2) |
| γ/° | 73.487(2) |
| V/Å3 | 2318.4(7) |
| Z | 2 |
| Density (calculated)/g cm−3 | 1.684 |
| Absorption coeffient/mm−1 | 1.700 |
| F(000) | 1196 |
| Crystal size/mm | 0.20 × 0.10 × 0.10 |
| Theta range for data collection/° | 1.45 to 25.92 |
| Reflections collected | 11860 |
| Independent reflections | 8764 [R(int) = 0.0220] |
| Completeness | 96.9 |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restrains/parameter | 8764 116/698 |
| Goodness-of-fit on F2 | 1.014 |
| Final R indices [I > 2σ(I)] | R1 = 0.0718, wR2 = 0.1760 |
| R indices (all data) | R1 = 0.0947, wR2 = 0.1959 |
| Largest diff. peak and hole/e.Å−3 | 1.609 and −1.416 |
Consideration of the charge balance in the Cu(II) cation complex and the similarity of the molecular structure to that seen for [CuII(ET-sae-TTF)2]+, allows us to suggest that the TTF moieties are in their partially oxidized states. The two TTF moieties in the dimer are crystallographically independent (TTF A and TTF B), and no S···S contacts shorter than the sum of van der Waals radii (3.70 Å) were observed in the dimer. As shown in Figure 2(b), the TTF skeletons are almost planar, as expected for partially oxidized groups, and the average C=C bond distances in TTF A and TTF B are 1.373 and 1.351 Å, respectively, further supporting their oxidation state assignment [16]. Although different charges on TTF A and TTF B, namely charge disproportionation, cannot be ruled out as the two TTF moieties are crystallographically non-equivalent, the difference between the central C=C bond lengths is very slight and we can consider the valence states of the TTF moieties to be approximately +0.5 per group. The interatomic distance between nearest neighboring Cu(II) ions in the lattice is 11.421 Å, and the shortest distance between a Cu(II) ion and a TTF sulfur atom is relatively short (3.649 Å).
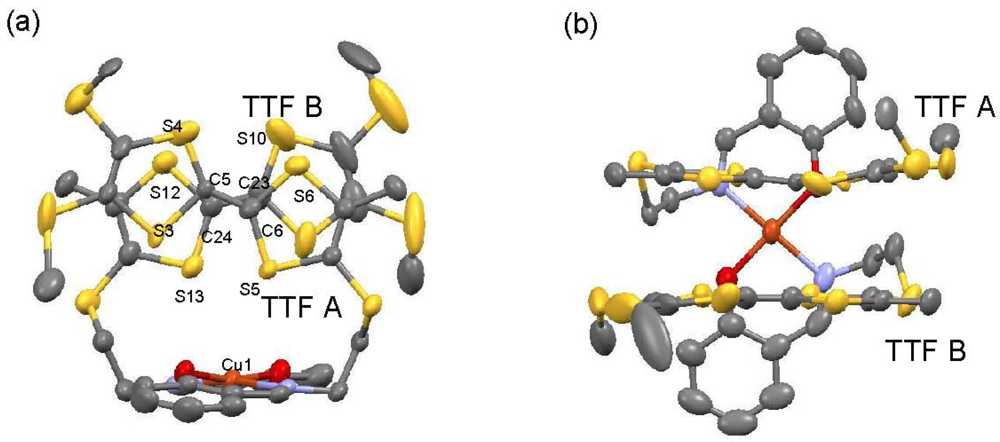
Figure 2.
Molecular structure of the cation complex [CuII(MT-sae-TTF)2]+ (a) view along a perpendicular direction to TTF planes (b) side view of the complex. Hydrogen atoms, disordered 4,5-bis(methylthio)-1,3-dithiol rings and CuCl2− anions are omitted for clarity.
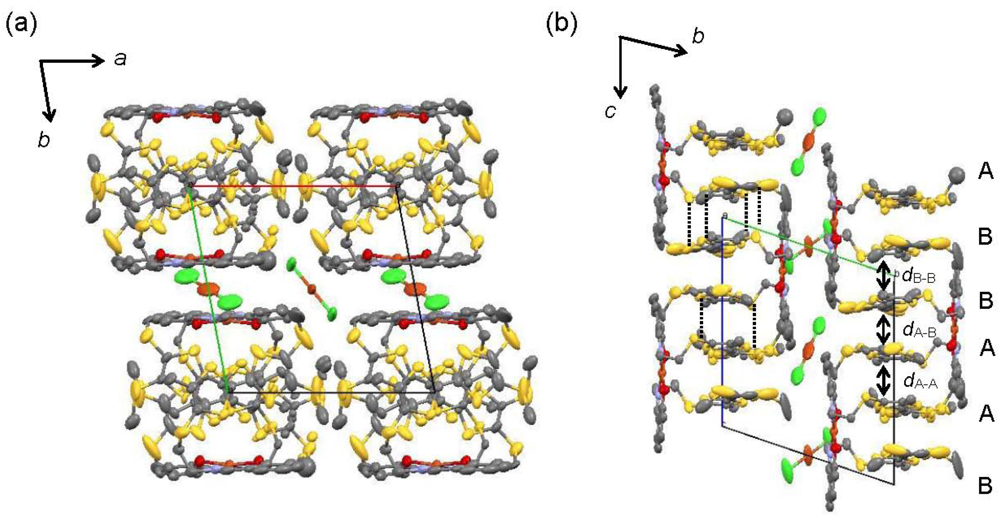
Figure 3.
Crystal structure of [CuII(MT-sae-TTF)2][CuICl2] (a) Projection view along the c-axis, the stacking direction of the TTF moieties (b) Projection view along the a-axis, with interplanar distanced within the column; dA-A = 3.531, dB-B = 3.534, dA-B = 3.373–3.413 Å. Dotted lines represent S···S contacts shorter than the sum of van der Waals radii (3.70 Å).
The crystal structure of [CuII(MT-sae-TTF)2][CuICl2] is shown in Figure 3. The TTF dimer in the complex forms stacks along the c-axis (Figure 3(a)). There are S···S contacts shorter than the sum of van der Waals radii between TTF dimers of neighboring complexes (Figure 3(b)). As the Cu(II) complex moieties and anion layers are located between TTF columns running along the b-axis, no side-by-side intermolecular contact was observed between TTF moieties.
Within the column, neighboring [CuII(ET-sae-TTF)2]+ complexes are related by an inversion center, and thus four TTF units (A–B···B–A) constitute the periodic unit of the column structure. The interplanar distances between the average molecular planes of the TTF moieties of adjacent complexes, i.e. the distances between TTF A···TTF A and between TTF B···TTF B, were calculated to be very close, 3.531 and 3.534 Å, respectively, where the best planes were calculated for C5, C6, S3-S6 for TTF A and for C23, C24, S10-S13 for TTF B. In terms of the interplanar distances, the gap between the TTF moieties in the dimer, namely the distance between TTF A and TTF B, was estimated to be 3.373–3.413 Å. As a result, the TTF dimers corresponding to the unit of TTF A–TTF B from the Cu(II) complex stack along the c-axis to form an almost uniform one-dimensional column.
2.3. Electrical Conductivity of [CuII(MT-sae-TTF)2][CuICl2]
The TTF moieties in [CuII(MT-sae-TTF)2][CuICl2] are partially oxidized and form a one-dimensional columnar structure through intermolecular interactions. Sizeable electrical conductivity may be expected. Temperature dependence of resistivity of [CuII(MT-sae-TTF)2][CuICl2] was measured by the four-probe method along the one-dimensional stacking column direction, the c-axis. Figure 4 shows the temperature dependence of the resistivity in the temperature range of 298–130 K. The electrical transport behavior of the Cu(II) complex salt is semiconductive; electrical conductivity at 298 K was 3.48 × 10−3 Scm−1 with an activation energy of Ea = 0.13 eV. Based on the interplanar distance within the one-dimensional column, the dimer units comprising two TTF moieties in the Cu(II) complex (TTF A···TTF B) stack uniformly leading to columns of TTF dimers. Therefore, the Cu(II) complex salt should have a Fermi surface due to the quarter-filled band structure.
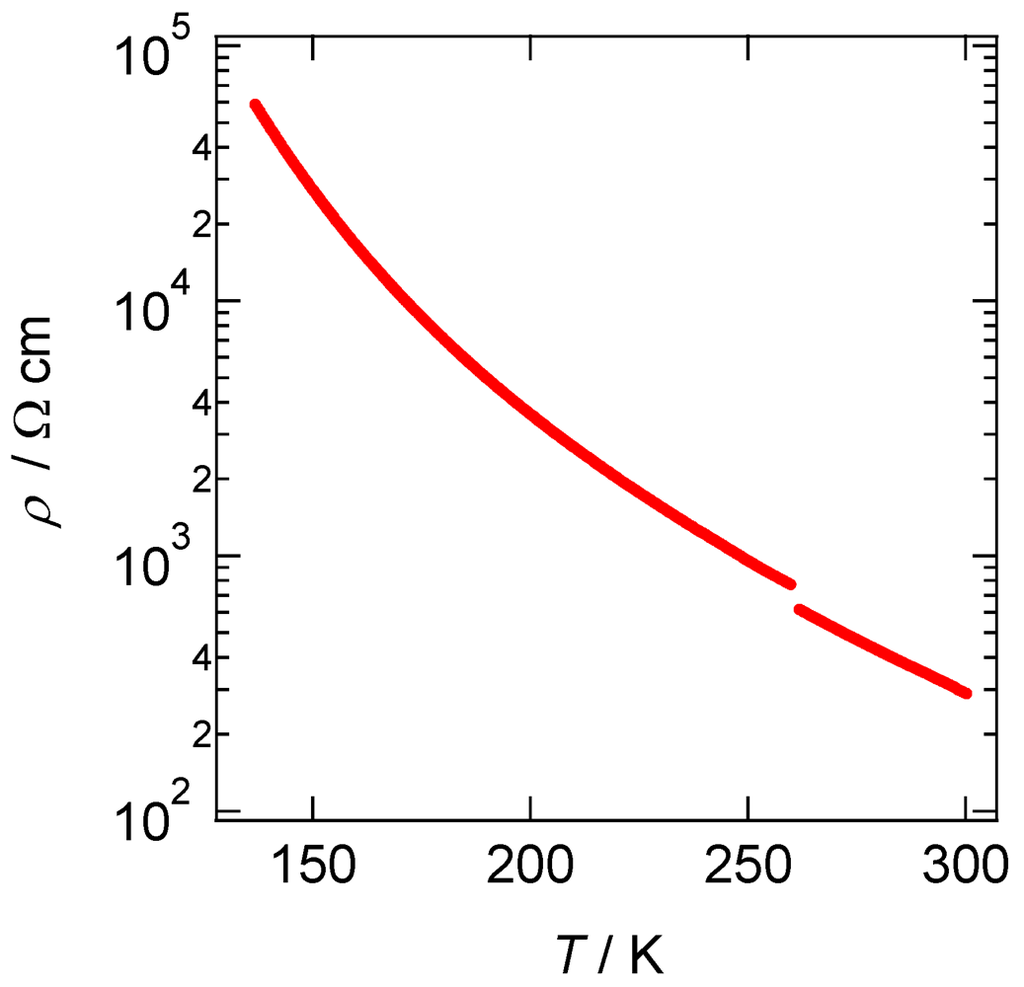
Figure 4.
Temperature dependence of resistivity of [CuII(MT-sae-TTF)2][CuICl2] measured along the c-axis.
2.4. Band Calculation of [CuII(MT-sae-TTF)2][CuICl2]
In order to understand the semiconducting behavior of [CuII(MT-sae-TTF)2][CuICl2], the band structure was calculated based on the overlap integrals (S) between the highest occupied molecular orbitals (HOMOs) of the TTF moieties. The HOMOs were calculated for the TTF fragments where the Cu(II) Schiff-base coordination site was substituted by a methyl group as shown in Figure 5, at extended Hückel level. The intra-dimer interaction between TTF moieties gives the largest overlap integral (c1), consistent with it having the shortest interplanar distance. From the interplanar distances between TTF moieties within the stacking column, the TTF dimers are stacked almost uniformly, but the calculated overlap integrals do not suggest a perfectly uniform stack of dimers; the inter-dimer overlap between TTF moieties was found to be larger for TTF B···TTF B, (c2) than for TTF A···TTF A, (c3), leading to a non-uniform stack of the TTF dimers and slight tetramerization of the TTF moieties. X-ray crystallography revealed that the Cu(II) Schiff-base coordination moieties and CuICl2– anions exist between the TTF groups and suppress intermolecular interaction between columns, resulting in no orthogonal overlap integral being obtained, suggesting relatively strong one-dimensionality. The band structure obtained by tight-binding method is shown in Figure 6. According to the one-dimensional character along the c-axis, there is no dispersion along the Γ→Y direction. The one-dimensional electronic structure with tetramerization of the TTF moieties opens a Peierls gap at Fermi level even at room temperature as shown in Figure 6, resulting in the band insulator for [CuII(MT-sae-TTF)2][CuICl2], consistent with the semiconducting behavior observed in the transport measurements.
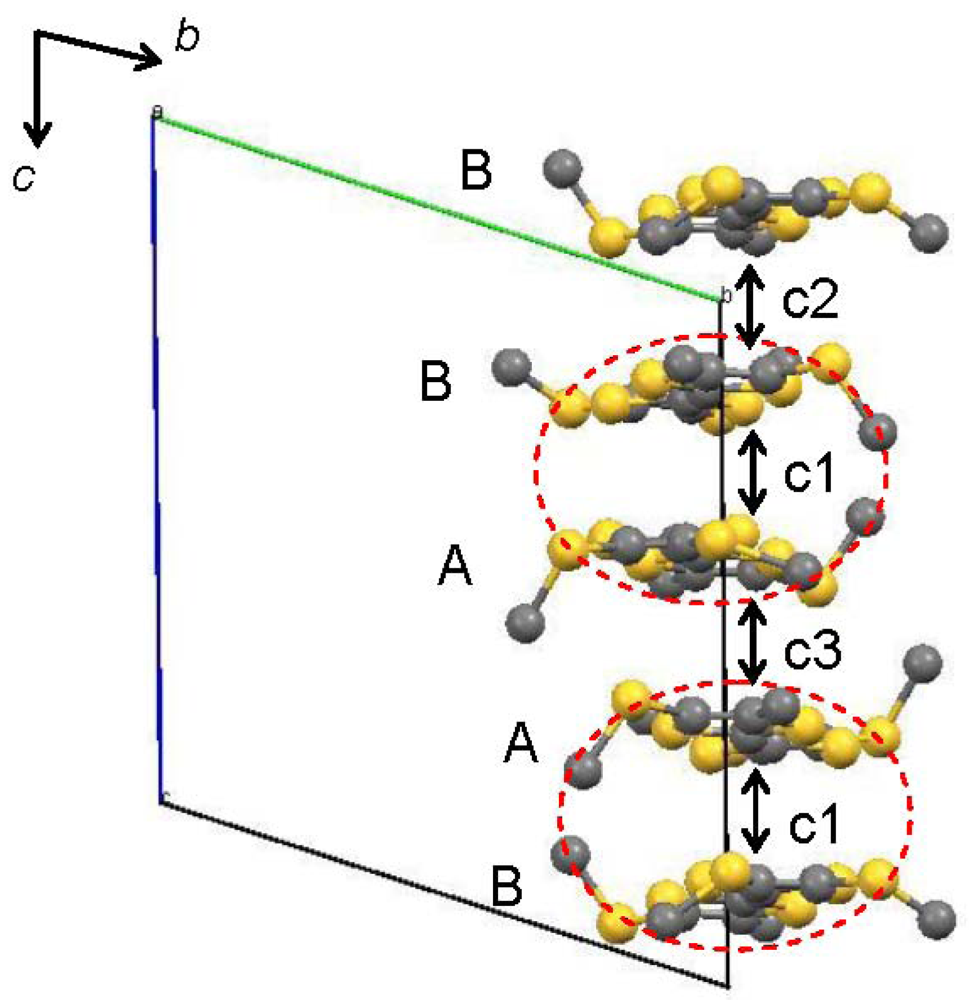
Figure 5.
Overlap integrals (×103) between the TTF moieties in [CuII(MT-sae-TTF)2] [CuICl2]: c1 = −22.05, c2 = 19.90, c3 = 15.64. The HOMOs were calculated for the TTF fragments. The red dotted circle represents the TTF moieties from the same [CuII(MT-sae-TTF)2]+ complex.
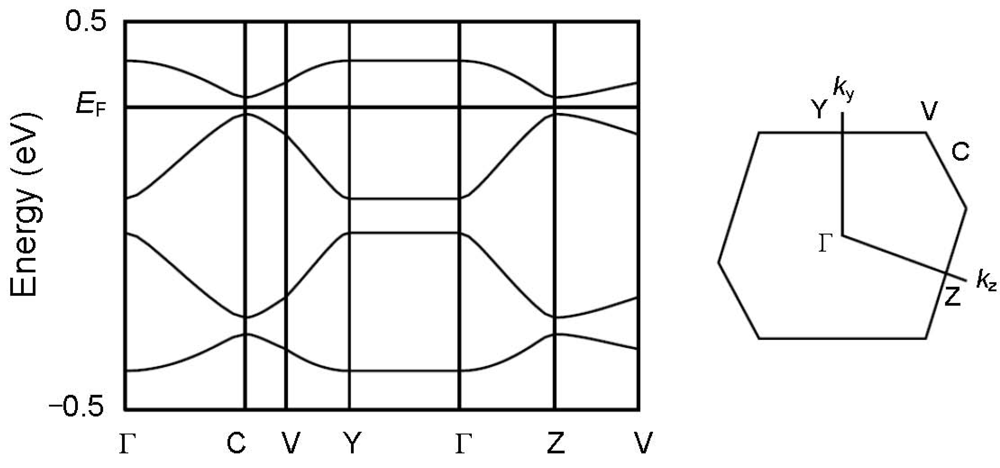
Figure 6.
Band structure and the first Brillouin zone of [CuII(MT-sae-TTF)2] [CuICl2].
3. Experimental Section
3.1. Syntheses of [CuII(MT-sae-TTF)2][CuICl2]
All solvents and chemicals were reagent-grade, purchased commercially, and used without further purification unless otherwise noted. Tetrahydrofuran (THF) was distilled from sodium benzophenone ketyl under a nitrogen atmosphere. Dichloromethane was distilled from calcium hydride. Tetra-n-butylammonium hexafluorophosphate, used as electrolyte of electrochemical measurements, was recrystallized from ethanol before use.
[CuII(MT-sae-TTF)2][CuICl2]: To a CH2Cl2 solution (3 mL) of MT-Hsae-TTF (10 mg, 0.02 mmol) was added triethyl amine (2.0 μL, 0.014 mmol) and then a methanolic solution (1 mL) of CuIICl2∙2H2O (4.0 mg, 0.02 mmol). The reaction mixture was stirred for 30 min. allowing the color to change from orange to dark red. Slow evaporation of the solvent afforded [CuII(MT-sae-TTF)2][CuICl2] (5.4 mg, 4.5 μmol) black plate crystals in 45% yield: IR(KBr): 1614, 1537, 1421, 1199, 758 cm−1; Anal. Calcd for C36H36N2O2S14Cu2Cl2∙H2O: C, 36.23; H, 3.21; N, 2.35. Found: C, 36.08; H, 3.07; N, 2.47.
3.2. X-ray Crystallography
Diffraction data were collected on a Bruker SMART APEX diffractometer fitted with a CCD type area detector, and a full sphere of data were collected using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). The data frames were integrated using SAINT and merged to give a unique data set for structure determination. The structures were solved by direct methods and refined by the full-matrix least-squares method on all F2 data using the SHELEX program (Bruker Analytical X-ray System). Empirical absorption corrections by SADABS were carried out. Non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were included in calculated positions and refined with isotropic thermal parameters riding on those of the parent atoms. The disordered 4,5-bis(methylthio)-1,3-dithiole ring in TTF A converged in three sites with respective occupancies of 0.4, 0.3 and 0.3. CCDC-873100 containing the supplementary crystallographic data for [CuII(MT-sae-TTF)2][CuICl2] can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif or from Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ UK; fax +44-1223-336-033; or deposite@ccdc.cam.ac.uk.
3.3. Physical Measurements
Cyclic voltammetry measurements were carried out in a standard one-compartment cell under a nitrogen atmosphere at 25 °C equipped with a platinum-wire counter electrode, and an SCE reference electrode, and a glassy carbon working electrode using a BAS 620A electrochemical analyzer. The measurements were performed in MeCN with 0.1 M tetra-n-butylammonium hexafluorophosphate as the supporting electrolyte. Infrared absorption spectra on KBr pellet samples were recorded using a SHIMADZU FT-IR 8400 spectrometer. The temperature dependence of resistivity was measured for a single crystal by the four-probe dc method using gold wire connected to the crystal by carbon paste.
3.4. Band Structure Calculation
The band structure calculation was performed by tight-binding method using transfer integrals tij from equation tij = ESij, where E is the energy level of the HOMO of the TTF moieties (−10 eV) and Sij are overlap integrals between the HOMOs of adjacent TTF moieties calculated on the basis of the extended Hückel MO method [24]. The molecular orbitals are calculated with the Slater-type atomic orbitals with single exponent zeta basis sets. Because the disorder of 4,5-bis(methylthio)-1,3-dithiole moiety exists in TTF A, overlap integrals between two TTF A moieties and between TTF A and TTF B are the average of the calculated values using three disordered conformations.
4. Conclusions
A TTF-based Cu(II) complex with partially oxidized TTF moieties, [CuII(MT-sae-TTF)2][CuICl2], was newly synthesized by one-pot reaction of a Schiff base-type TTF-ligand, MT-Hsae-TTF with CuIICl2, where the Cu(II) ion complexation and CuIICl2 mediated TTF moiety oxidation occurred simultaneously. The partially oxidized TTF moieties in the cation complex dimerized through face-to-face interactions, and stacked to form a one-dimensional columnar architecture. The Cu(II) complex salt exhibited semiconducting behavior with relatively high conductivity compared with other TTF-based metal complexes. Calculation of the overlap integrals showed the tetramerization of TTF moieties, through the non-uniform stacking of TTF dimers. Tight binding band calculations based on the extended Hückel level indicated the existence of a Peierls gap, resulting from the tetramerization of the TTF moieties in the one-dimensional stacking column, present even at room temperature. This result is consistent with the conducting behavior of this salt.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 20550118) and a Grant-in-Aid for challenging Exploratory Research (No. 23655114) and a Grant-in-Aid for Scientific Research on Innovative Areas of Molecular Degree of Freedom (No. 20110007) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Coronado, E.; Day, P. Magnetic molecular conductors. Chem. Rev. 2004, 104, 5419–5448, and references therein.. [Google Scholar] [CrossRef]
- Enoki, T.; Miyazaki, A. Magnetic TTF-based charge-transfer complexes. Chem. Rev. 2004, 104, 5449–5477, and references therein.. [Google Scholar] [CrossRef]
- Uji, S.; Shinagawa, C.; Terakura, T.; Terashima, T.; Yakabe, Y.; Terai, M.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef]
- Xiao, X.; Hayashi, T.; Fujiwara, H.; Sugimoto, T.; Noguchi, S.; Weng, Y.; Yoshino, H.; Murata, K.; Katori, H.A. An antiferromagnetic molecular metal based on a new bent-donor molecule. J. Am. Chem. Soc. 2007, 129, 12618–12619. [Google Scholar]
- Hünig, S.; Herberth, E. N,N'-Dicyanoquinone diimines (DCNQIs): Versatile acceptors for organic conductors. Chem. Rev. 2004, 104, 5535–5563, and references therein.. [Google Scholar] [CrossRef]
- Uji, S.; Terachima, T.; Aoki, H.; Brooks, J.S.; Kato, R.; Sawa, H.; Aonuma, S.; Tamura, M.; Kinoshita, M. Coexistence of one- and three-dimensional Fermi surfaces and heavy cyclotron mass in the molecular conductor (DMe-DCNQI)2Cu. Phys. Rev. B 1994, 50, 15597–15601. [Google Scholar]
- Inabe, T.; Tajima, H. Phthalocyanines—Versatile components of molecular conductors. Chem. Rev. 2004, 104, 5503–5534, and references therein.. [Google Scholar] [CrossRef]
- Hanasaki, N.; Tajima, H.; Matsuda, M.; Naito, T.; Inabe, T. Giant negative magnetoresistance in quasi-one-dimensional conductor TPP[Fe(Pc)(CN)2]2: Interplay between local moments and one-dimensional conduction electrons. Phys. Rev. B 2000, 62, 5839–5842. [Google Scholar]
- Kobayashi, A.; Fujiwara, E.; Kobayashi, H. Single-component molecular metals with extended-TTF dithiolate ligands. Chem. Rev. 2004, 104, 5243–5264, and references therein.. [Google Scholar] [CrossRef]
- Suzuki, W.; Fujiwara, E.; Kobayashi, A.; Fujishiro, Y.; Nishibori, E.; Tanaka, M.; Sakata, H.; Fujiwara, H.; Kobayashi, H. Highly conducting crystals based on single-component gold complexes with extended TTF-dithiolate ligands. J. Am. Chem. Soc. 2003, 125, 1486–1487. [Google Scholar]
- Zhou, B.; Shimamura, M.; Fujiwara, E.; Kobayashi, A.; Higashi, T.; Nishibori, E.; Sakata, M.; Cui, H.-B.; Takahashi, K.; Kobayashi, H. Magnetic transition of single-component molecular metal [Au(tmdt)2] and its alloy systems. J. Am. Chem. Soc. 2006, 128, 3872–3873. [Google Scholar]
- Hara, Y.; Miyagawa, K.; Kanoda, K.; Shimamura, M.; Zhou, B.; Kobayashi, A.; Kobayashi, H. NMR evidence for antiferromagnetic transition in the single-component molecular conductor, [Au(tmdt)2] at 110 K. J. Phys. Soc. Jpn. 2008, 77, 053706:1–053706:4. [Google Scholar]
- Lorcy, D.; Bellec, N.; Fourmigué, M.; Avarvari, N. Tetrathiafulvalene-based group XV ligands: Synthesis, coordination chemistry and radical cation salts. Coord. Chem. Rev. 2009, 253, 1398–1438. [Google Scholar] [CrossRef]
- Avarvari, N.; Fourmigué, M. First cation radical salt of a tetrathiafulvalene-based phosphine metal complex. Chem. Commun. 2004, 1300–1301. [Google Scholar] [CrossRef]
- Liu, S.-X.; Ambrus, C.; Dolder, S.; Neels, A.; Decurtins, S. A dinuclear Ni(II) complex with two types of intramolecular magnetic couplings: Ni(II)-Ni(II) and Ni(II)-TTF·+. Inorg. Chem. 2006, 45, 9622–9624. [Google Scholar]
- Setifi, F.; Ouahab, L.; Golhen, S.; Yoshida, Y.; Saito, G. First radical cation salt of paramagnetic transition metal complex containing TTF as ligand, [CuII(hfac)2(TTF-py)2](PF6)·2CH2Cl2 (hfac = hexafluoroacetylacetonate and TTF-py = 4-(2-tetrathiafulvalenyl-ethenyl)pyridine). Inorg. Chem. 2003, 42, 1791–1793. [Google Scholar] [CrossRef]
- Ichikawa, S.; Mori, H. High conductivity of the new supramolecular copper complex with oxidized pyrazinoselenathiafulvalene (= pyra-STF) as the ligand, [CuICl1.5(pyra-STF)0.5+]. Inorg. Chem. 2009, 48, 4643–4645. [Google Scholar] [CrossRef]
- Nishikawa, H.; Oshima, H.; Narita, K.; Oshio, H. Syntheses of new TTF-based metal complexes for conducting and magnetic systems: Schiff base-type metal complex with partially oxidized TTF moiety. Phys. B Condens. Matter 2010, 405, S55–S60. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kitabatake, R.; Mitsumoto, K.; Oshio, H. Syntheses and properties of new metal complexes based on TTF-ligands with multidentate coordination sites. Phys. Status Solidi 2012. [Google Scholar]
- Wu, J.-C.; Liu, S.-X.; Keene, T.D.; Neels, A.; Mereacre, V.; Powell, A.K.; Decurtins, S. Coordination Chemistry of a π-extended, rigid and redox-active tetrathiafulvelene-fused Schiff-base ligand. Inorg. Chem. 2008, 47, 3452–3459. [Google Scholar]
- Nishikawa, H.; Kojima, S.; Kodama, T.; Ikemoto, I.; Suzuki, S.; Kikuchi, K.; Fujitsuka, M.; Luo, H.; Araki, Y.; Ito, O. Photophysical study of new methanofullerene-TTF dyads: An obvious intramolecular charge transfer in the ground states. J. Phys. Chem. A 2004, 108, 1881–1890. [Google Scholar]
- Siedel, A.E.; Cnadela, G.A.; Finnegan, T.F.; van Duyne, R.P.; Cape, T.; Kokoszka, G.F.; Woyciejes, P.M.; Hashmall, J.A. Copper and gold metallotetrathiaethylenes. Inorg. Chem. 1981, 20, 2635–2640. [Google Scholar]
- Kanehama, R.; Umemiya, M.; Iwahori, F.; Miyasaka, H.; Sugiura, K.; Yamashita, M.; Yokochi, Y.; Ito, H.; Kuroda, S.; Kishida, H.; Okamoto, H. Novel ET-coordinated copper(I) complexes: Synthesis, structures, and physical properties (ET = BEDT-TTF = bis(ethylenedithio)tetrathiaful-valene). Inorg. Chem. 2003, 42, 7173–7181. [Google Scholar]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The intermolecular interaction of tetrathiafulvalene and bis(ethylenedithio)tetrathiafulvalene in organic metals. Calculation of orbital overlaps and models of energy-band structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).