Structural and Gas Retention Changes Induced by Ozonization of Cobalt(II) and Manganese(II) Hexacyanocobaltates(III)
Abstract
:1. Introduction
2. Results and Discussion
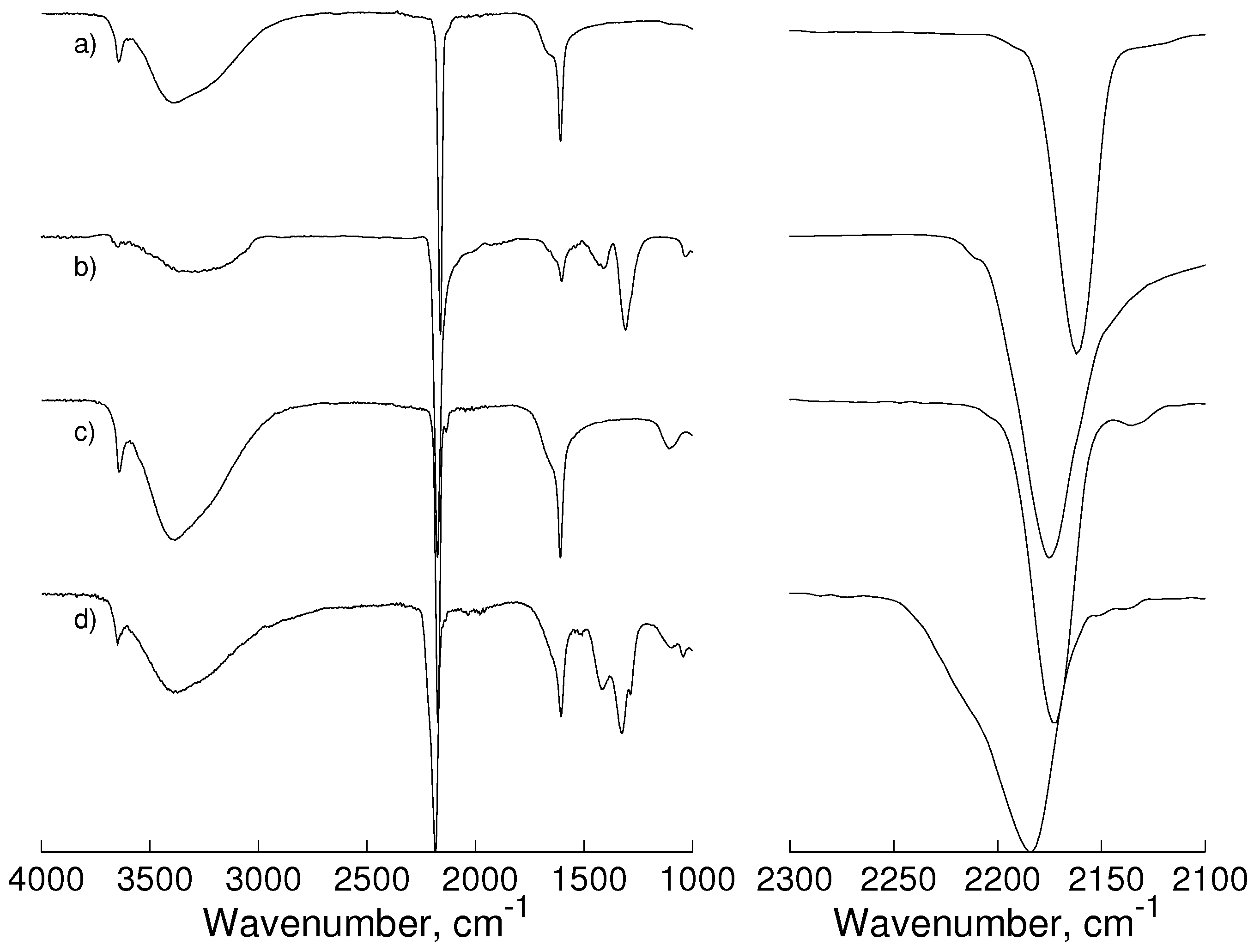
2.1. Infrared and Raman Spectroscopy
- stretching mode of C≡N.
- stretching mode of C≡N.
- stretching mode of Co−C≡N.
- stretching mode of C−Co−C.
2.2. X-ray Diffraction
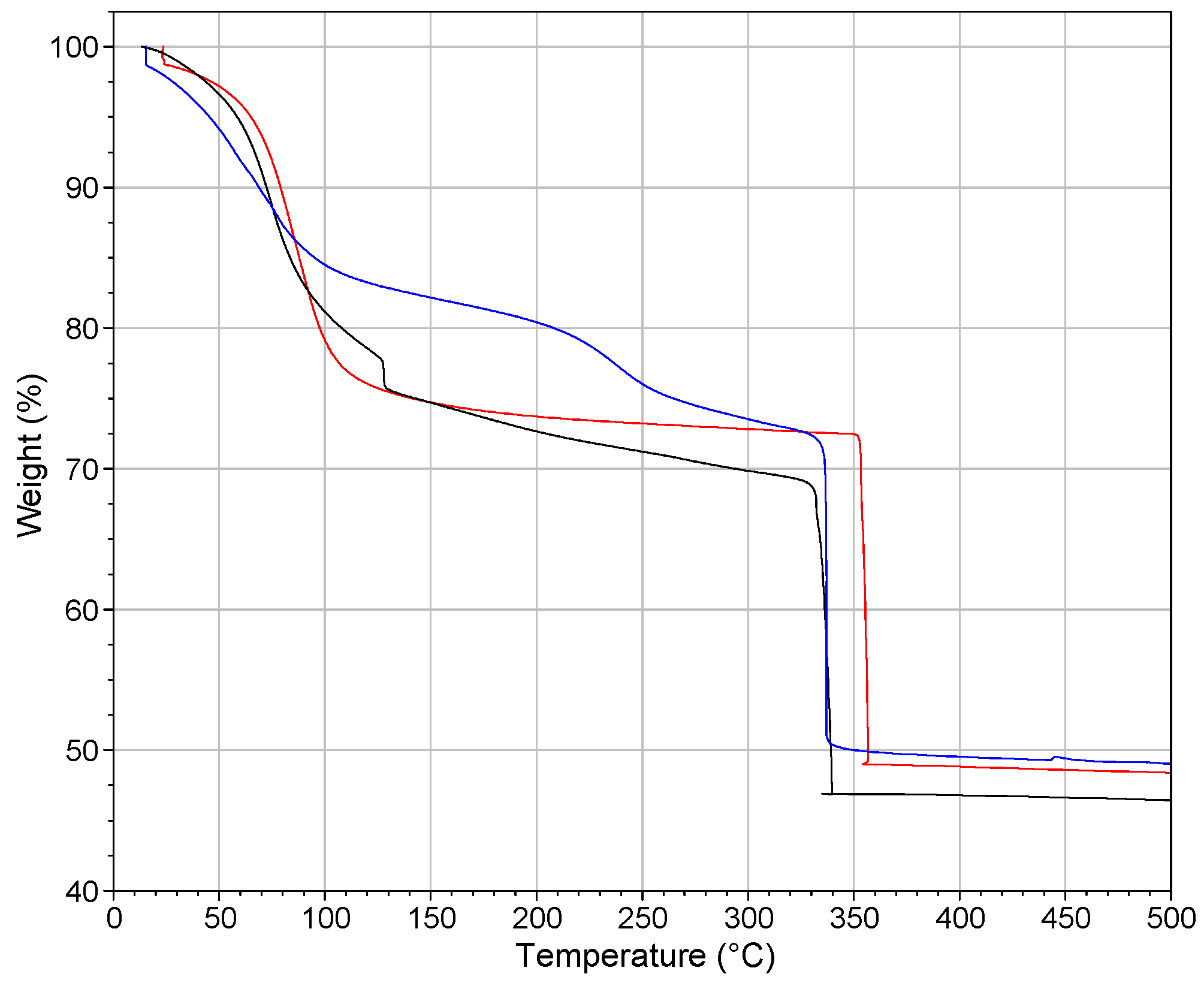
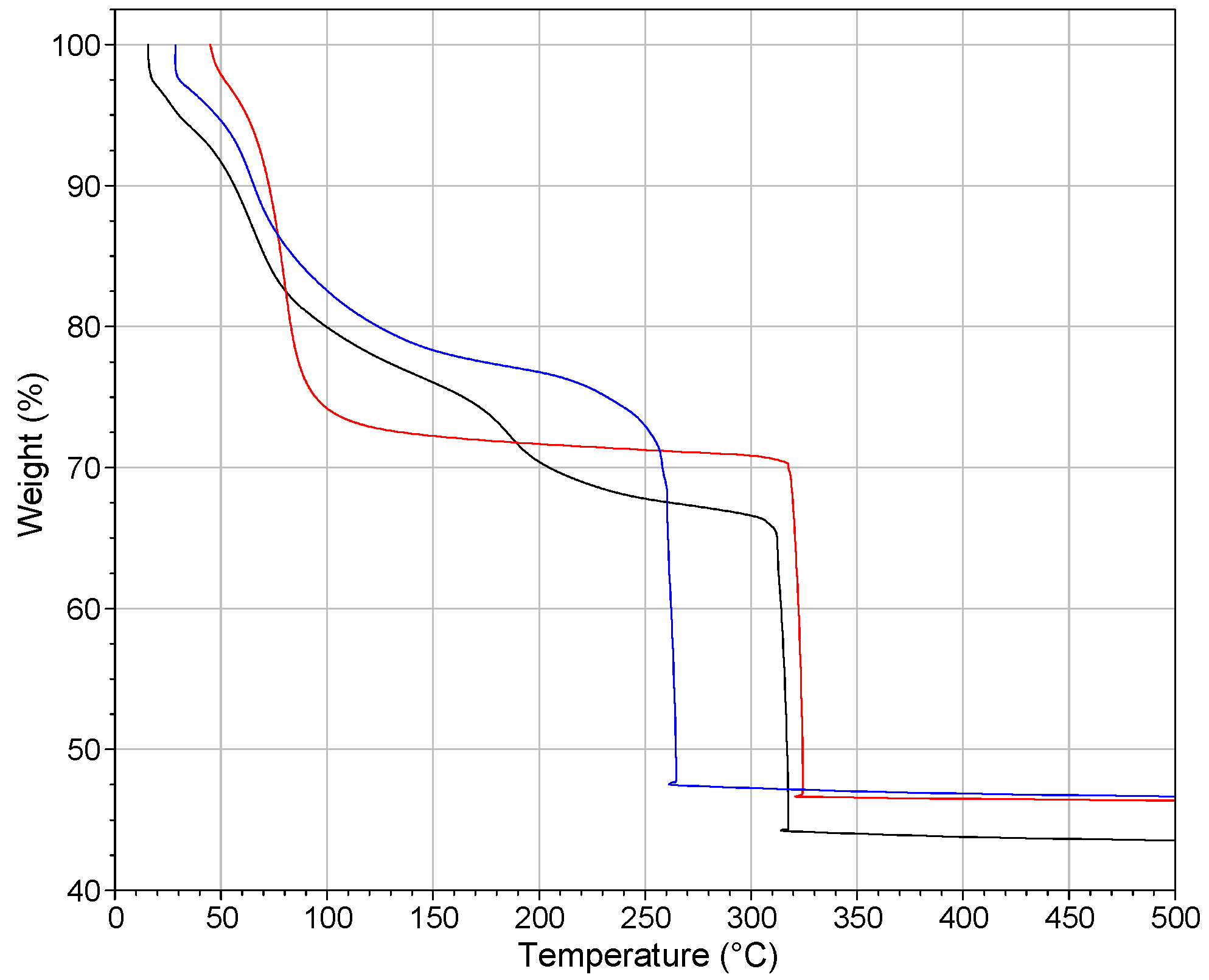
2.3. Thermogravimetric Analysis
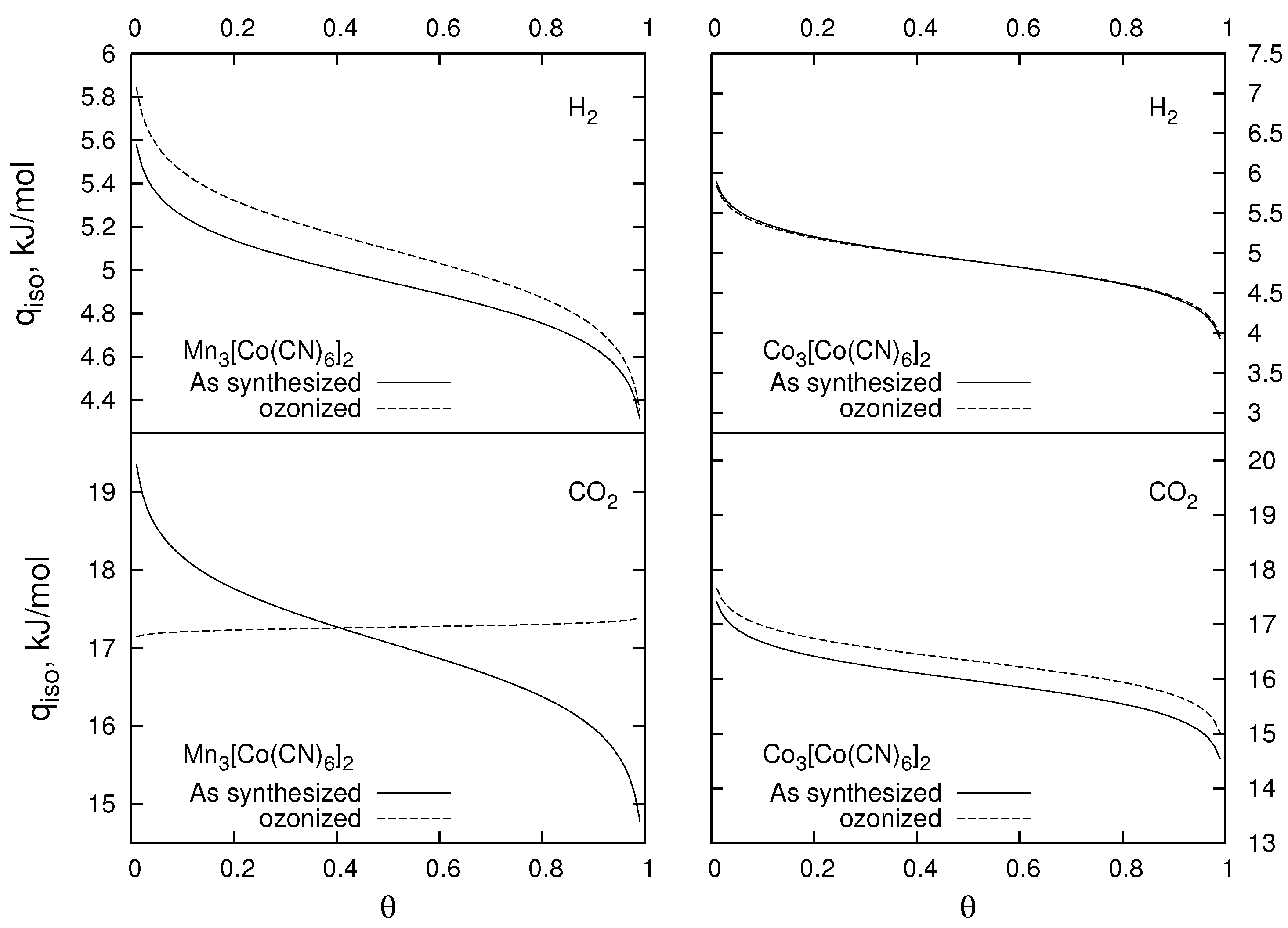
2.4. Gas Adsorption
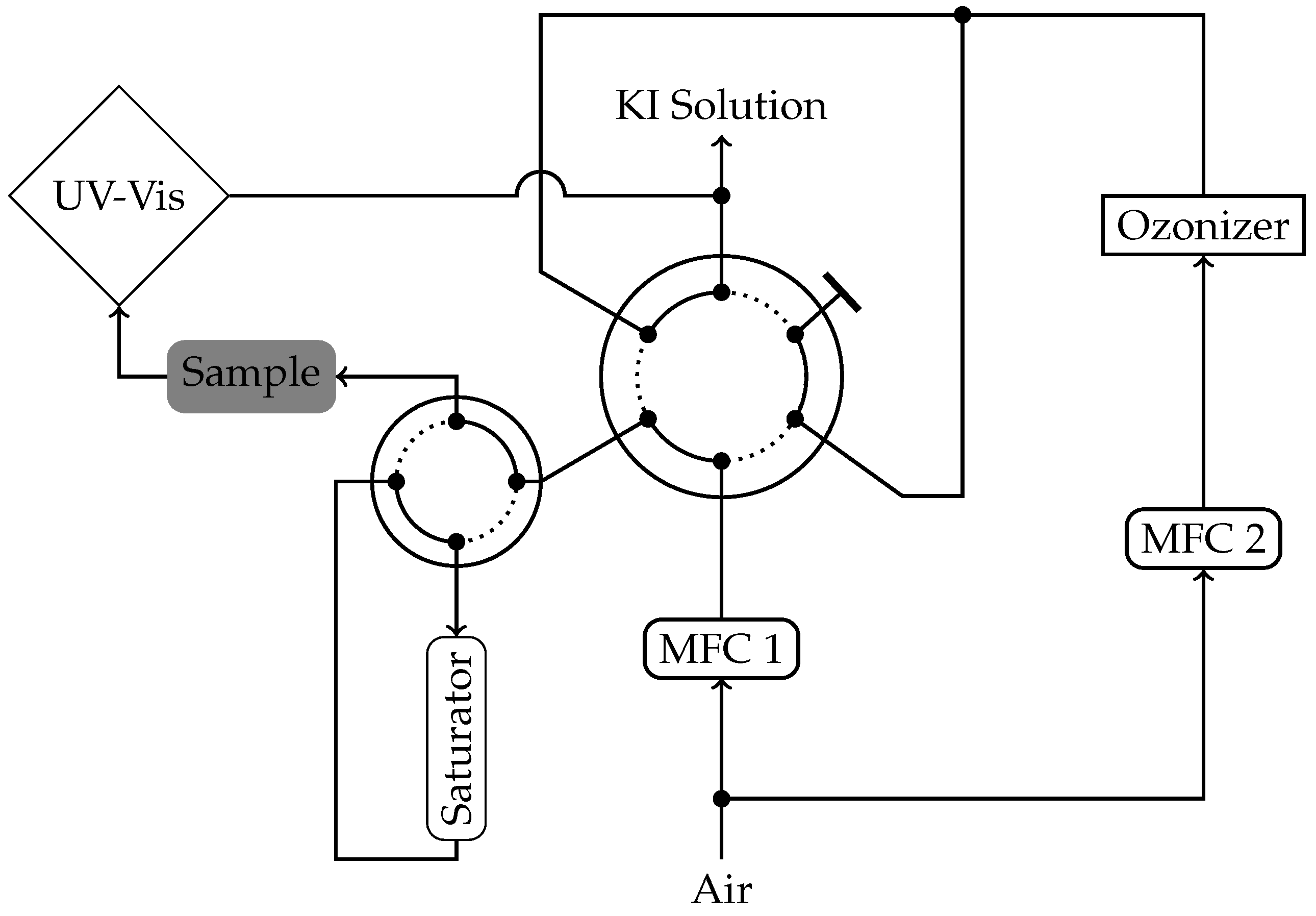
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MnHCC | Manganese(III) Hexacyanocobaltate(II) with molecular formula Mn3[Co(CN)6]2·nH2O |
| CoHCC | Cobalt(III) Hexacyanocobaltate(II) with molecular formula Co3[Co(CN)6]2·nH2O |
| CuHCC | Copper(III) Hexacyanocobaltate(II) with molecular formula Cu3[Co(CN)6]2·nH2O |
| MnHCCOz | Ozonized MnHCC |
| CoHCCOz | Ozonized CoHCC |
| FTIR | Fourier Transform Infrared Spectroscopy |
| PTFE | Polytetrafluoroethylene |
References
- Barton, T.J.; Bull, L.M.; Klemperer, W.G.; Loy, D.A.; McEnaney, B.; Misono, M.; Monson, P.A.; Pez, G.; Scherer, G.W.; Vartuli, J.C.; et al. Tailored Porous Materials. Chem. Mater. 1999, 11, 2633–2656. [Google Scholar] [CrossRef]
- Pentimalli, M.; Imperi, E.; Bellusci, M.; Alvani, C.; Santini, A.; Padella, F. Silica-Metal Composite for Hydrogen Storage Applications. Crystals 2012, 2, 690–703. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Yang, S.; Tsou, Y.J.; Lee, J.T.; Hsieh, C.J. Newly Designed Copolymers for Fabricating Particles with Highly Porous Architectures. Chem. Mater. 2016, 28, 6089–6095. [Google Scholar] [CrossRef]
- Breternitz, J.; Gregory, D.H. The Search for Hydrogen Stores on a Large Scale; A Straightforward and Automated Open Database Analysis as a First Sweep for Candidate Materials. Crystals 2015, 5, 617–633. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Chen, J.; Snurr, R.Q. High-Throughput Screening of Metal-Organic Frameworks for Hydrogen Storage at Cryogenic Temperature. J. Phys. Chem. C 2016, 120, 27328–27341. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen Storage in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Beall, G.W.; Milligan, W.O.; Petrich, J.A.; Swanson, B.I. Crystal Structure and Raman Spectral Study of Ligand Substitution in Mn3[Co(CN)6]2·xL. Inorg. Chem. 1978, 17, 2978–2981. [Google Scholar] [CrossRef]
- Roque, J.; Reguera, E.; Balmaseda, J.; Rodríguez-Hernández, J.; Reguera, L.; del Castillo, L. Porous Hexacyanocobaltates(III): Role of the Metal on the Framework Properties. Microporous Mesoporous Mater. 2007, 103, 57–71. [Google Scholar] [CrossRef]
- Avila, M.; Reguera, L.; Rodriguez-Hernandez, J.; Balmaseda, J.; Reguera, E. Porous Framework of T2[Fe(CN)6]·xH2O with T = Co, Ni, Cu, Zn, and H2 Storage. J. Solid State Chem. 2008, 181, 2899–2907. [Google Scholar] [CrossRef]
- Reguera, L.; Reguera, E.; Balmaseda, J.; Rodriguez-Hernandez, J.; Yee-Madeira, H. Mixed Valences System in Cobalt Iron Cyanide. Microporous Structure Stability. J. Porous Mater. 2008, 15, 719–729. [Google Scholar] [CrossRef]
- Reguera, L.; Balmaseda, J.; Krap, C.P.; Avila, M.; Reguera, E. Hydrogen Storage in Zeolite-Like Hexacyanometallates: Role of the Building Block. J. Phys. Chem. C 2008, 112, 17443–17449. [Google Scholar] [CrossRef]
- Reguera, L.; Krap, C.P.; Balmaseda, J.; Reguera, E. Hydrogen Storage in Copper Prussian Blue Analogues: Evidence of H2 Coordination to the Copper Atom. J. Phys. Chem. C 2008, 112, 15893–15899. [Google Scholar] [CrossRef]
- Reguera, L.; Balmaseda, J.; del Castillo, L.F.; Reguera, E. Hydrogen Storage in Porous Cyanometalates: Role of the Exchangeable Alkali Metal. J. Phys. Chem. C 2008, 112, 5589–5597. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, J.; Reguera, E.; Lima, E.; Balmaseda, J.; Martinez-Garcia, R.; Yee-Madeira, H. An Atypical Coordination in Hexacyanometallates: Structure and Properties of Hexagonal Zinc Phases. J. Phys. Chem. Solids 2007, 68, 1630–1642. [Google Scholar] [CrossRef]
- Martinez-Garcia, R.; Reguera, E.; Balmaseda, J.; Ramos, G.; Yee-Madeira, H. On the Crystal Structures of Some Nickel Hexacyanoferrates(II,III). Powder Diffr. 2004, 19, 284–291. [Google Scholar] [CrossRef]
- Martinez-Garcia, R.; Reguera, E.; Rodriguez, J.; Balmaseda, J.; Roque, J. Crystal Structures of Some Manganese(II) and Cadmium Hexacyanoferrates(II,III) and Structural Transformations Related to the Sorption of Cesium. Powder Diffr. 2004, 19, 255–264. [Google Scholar] [CrossRef]
- Yuan, A.H.; Chu, C.X.; Zhou, H.; Yuan, P.; Liu, K.K.; Li, L.; Zhang, Q.F.; Chen, X.; Li, Y.Z. Syntheses, Crystal Structures and Gas Sorption Properties of Prussian Blue Analogues Constructed from [Cr(CN)6]3− Building Blocks. Eur. J. Inorg. Chem. 2010, 2010, 866–871. [Google Scholar] [CrossRef]
- Zamora, B.; Autie, M.; Contreras, J.L.; Centeno, M.; Reguera, E. Separation of Oxygen and Nitrogen by Porous Cyanometallates. Sep. Sci. Technol. 2010, 45, 692–699. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Lu, Y.; Liu, J.; Guo, B.; Xiao, P.; Lee, J.J.; Yang, X.Q.; Henkelman, G.; Goodenough, J.B. Removal of Interstitial H2O in Hexacyanometallates for a Superior Cathode of a Sodium-Ion Battery. J. Am. Chem. Soc. 2015, 137, 2658–2664. [Google Scholar] [CrossRef] [PubMed]
- Dincǎ, M.; Long, J. Hydrogen Storage in Microporous Metal-Organic Frameworks with Exposed Metal Sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef] [PubMed]
- Zamora, B.; Al-Hajjaj, A.; Shah, A.; Bavykin, D.; Reguera, E. Kinetic and Thermodynamic Studies of Hydrogen Adsorption on Titanate Nanotubes Decorated with a Prussian Blue Analogue. Int. J. Hydrogen Energy 2013, 38, 6406–6416. [Google Scholar] [CrossRef]
- Poloni, R.; Lee, K.; Berger, R.F.; Smit, B.; Neaton, J.B. Understanding Trends in CO2 Adsorption in Metal-Organic Frameworks with Open-Metal Sites. J. Phys. Chem. Lett. 2014, 5, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Thallapally, P.K.; Motkuri, R.K.; Fernandez, C.A.; McGrail, B.P.; Behrooz, G.S. Prussian Blue Analogues for CO2 and SO2 Capture and Separation Applications. Inorg. Chem. 2010, 49, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Autie-Castro, G.; Autie, M.; Reguera, E.; Santamaría-González, J.; Moreno-Tost, R.; Rodríguez-Castellón, E.; Jiménez-López, A. Adsorption and Separation of Light Alkane Hydrocarbons by Porous Hexacyanocobaltates(III). Surf. Interface Anal. 2009, 41, 730–734. [Google Scholar] [CrossRef]
- Kim, H.K.; Yun, W.S.; Kim, M.B.; Kim, J.Y.; Bae, Y.S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal-Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Tanaka, H.; Parajuli, D.; Nakamura, T.; Minami, K.; Sugiyama, Y.; Hakuta, Y.; Ohkoshi, S.I.; Kawamoto, T. Historical Pigment Exhibiting Ammonia Gas Capture beyond Standard Adsorbents with Adsorption Sites of Two Kinds. J. Am. Chem. Soc. 2016, 138, 6376–6379. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Thallapally, P.K.; McGrail, B.P.; Annapureddy, H.V.R.; Dang, L.X.; Mei, D.; Karri, N.; Alvine, K.J.; Olszta, M.J.; Arey, B.W.; et al. Adsorption Kinetics in Nanoscale Porous Coordination Polymers. ACS Appl. Mater. Interfaces 2015, 7, 21712–21716. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.I. Aspects of the Structure and Bonding in Prussian Blues. Single-Crystal Raman Study of Trimanganese Hexacyanocobaltate Hydrate (Mn3[Co(CN)6]·xH2O) and Tricadmium Bis(hexacyanocobaltate) Hydrate (Cd3[Co(CN)6]2·xH2O). Inorg. Chem. 1976, 15, 253–259. [Google Scholar] [CrossRef]
- Bertrán, J.F.; Pascual, J.B.; Reguera Ruiz, E. The CN Stretch of Hexacyanometallates as a Sensor of Ligand-Outer Cation Interactions-I. Ferricyanides and Cobalticyanides. Spectrochim. Acta A Mol. Spectrosc. 1990, 46, 685–689. [Google Scholar] [CrossRef]
- Reguera, E.; Bertrán, J.; Diaz, C.; Blanco, J.; Rondón, S. Mössbauer and Infrared Spectroscopic Studies of Novel Mixed Valence States in Cobaltous Ferrocyanides and Ferricyanides. Hyperfine Interact. 1990, 53, 391–395. [Google Scholar] [CrossRef]
- Reguera, E.; Fernández-Bertrán, J.; Díaz, C.; Molerio, J. Behaviour of Prussian Blue during Its Interaction with Ozone. Hyperfine Interact. 1992, 73, 285–294. [Google Scholar] [CrossRef]
- Reguera, E.; Fernández-Bertrán, J. Study of the Interaction of Ozone with Solid Ferrocyanides. Eur. J. Solid State Inorg. Chem. 1994, 31, 1021–1027. [Google Scholar]
- Reguera, E.; Fernández-Bertrán, J.; Duque, J. On the Interactions of Ozone with Manganous Hexacyanoferrates. Polyhedron 1994, 13, 479–484. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume B. [Google Scholar]
- Lide, D.R. (Ed.) Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2003.
- Kaye, S.S.; Long, J.R. Hydrogen Storage in the Dehydrated Prussian Blue Analogues M3[Co(CN)6]2 (M = Mn, Fe, Co, Ni, Cu, Zn). J. Am. Chem. Soc. 2005, 127, 6506–6507. [Google Scholar] [CrossRef] [PubMed]
- Ludi, A.; Guedel, H.U.; Ruegg, M. Structural Chemistry of Prussian Blue Analogs. Single-Crystal Study of Manganese (II) Hexacyanocobaltate (III), Mn3[Co(DcN)6]2·xH2O. Inorg. Chem. 1970, 9, 2224–2227. [Google Scholar] [CrossRef]
- Beall, G.W.; Milligan, W.O.; Korp, J.; Bernal, I. Crystal Structure of Mn3[Co(CN)6]2·12H2O and Cd3[Co(CN)6]2·12H2O by Neutron and X-ray Diffraction. Inorg. Chem. 1977, 16, 2715–2718. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic Hydration Enthalpies. J. Chem. Educ. 1977, 54, 540. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Culp, J.T.; Matranga, C.; Bockrath, B. Adsorption Properties of Hydrogen and Carbon Dioxide in Prussian Blue Analogues M3[Co(CN)6]2, M = Co, Zn. J. Phys. Chem. C 2007, 111, 1055–1060. [Google Scholar] [CrossRef]
- Flambard, A.; Köhler, F.H.; Lescouëzec, R.; Revel, B. Probing Spin Density and Local Structure in the Prussian Blue Analogues CsCd[FeCo(CN)6]·0.5H2O and Cd3[FeCo(CN)6]2·15H2O with Solid-State MAS NMR Spectroscopy. Chem. Eur. J. 2011, 17, 11567–11575. [Google Scholar] [CrossRef] [PubMed]
- Olvera, L.I.; Ruiz-Treviño, F.A.; Balmaseda, J.; Ronova, I.A.; Zolotukhin, M.G.; Carreón-Castro, M.P.; Lima, E.; Cárdenas, J.; Gaviño, R. Microporous Polymers from Superacid Catalyzed Polymerizations of Fluoroketones with p-Quaterphenyl: Synthesis, Characterization, and Gas Sorption Properties. Polymer 2016, 102, 221–230. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry, 7th ed.; W. H. Freeman and Company: New York, NY, USA, 2002. [Google Scholar]
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, C.M.; Somiya, I. Guideline for Measurement of Ozone Concentration in the Process Gas From an Ozone Generator. Ozone Sci. Eng. 1996, 18, 209–229. [Google Scholar] [CrossRef]

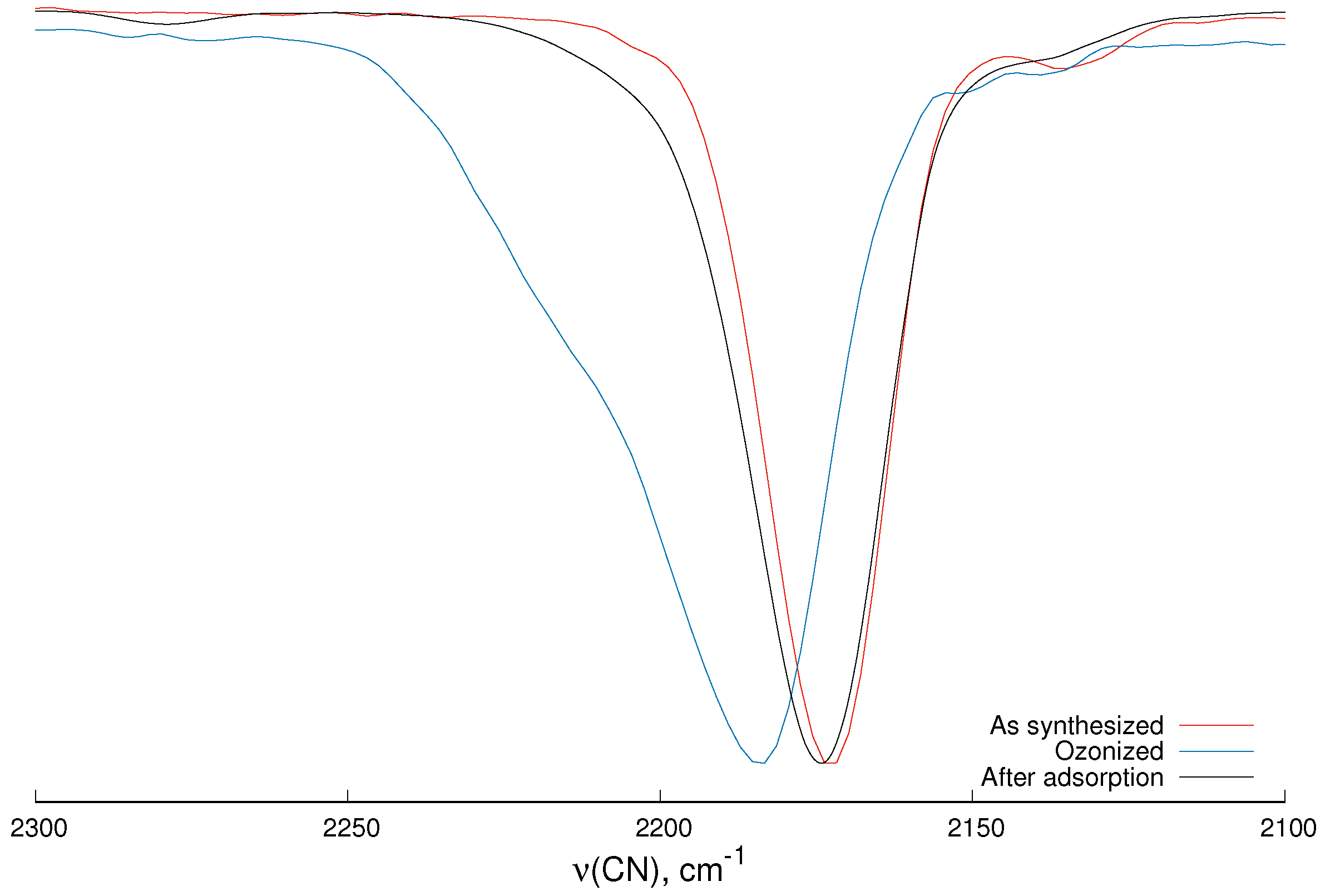
| Mode | MnHCC | MnCoO3 | Reference MnHCC [31] |
|---|---|---|---|
| (, CN) | 2192 | 2197 | 2191 |
| (, CoC) | 484 | 486 | 485 |
| (, CN) | 2173 | 2177 | 2172 |
| (, CoC) | 469 | 470 | 470 |
| (, CoCN) | 480 | 484 | 480 |
| (, CCoC) | 202 | 202 a | 202 |
| M | Hydrated | Dehydrated | , % | Hydrated Ozonized | , % |
|---|---|---|---|---|---|
| Mn | 10.4228(1) | 10.3272(4) | 2.7 | 10.3511(6),10.2734(8) a | 2.0, 4.2 |
| Co | 10.2191(5) | 10.143(7) | 2.2 | 10.1092(8) | 3.2 |
| Adsorbate | Sample | mmol/g | mmHg | g |
|---|---|---|---|---|
| H2 | MnHCC | 11.1 ± 0.1 | 202 ± 6 | 1.22 ± 0.01 |
| MnHCCOz | 9.3 ± 0.1 | 160 ± 5 | 1.25 ± 0.02 | |
| CoHCC | 10.8 ± 0.2 | 214 ± 10 | 1.33 ± 0.02 | |
| CoHCCOz | 10.4 ± 0.1 | 215 ± 8 | 1.32 ± 0.04 | |
| CO2 | MnHCC | 9.2 ± 0.1 | 589 ± 20 | 1.220 ± 0.009 |
| MnHCCOz | 6.74 ± 0.03 | 539 ± 5 | 0.988 ± 0.003 | |
| CoHCC | 7.40 ± 0.06 | 948 ± 16 | 1.139 ± 0.003 | |
| CoHCCOz | 7.0 ± 0.1 | 809 ± 31 | 1.127 ± 0.008 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos, M.L.; Rodríguez-Hernández, J.; Del Castillo, L.F.; Balmaseda, J. Structural and Gas Retention Changes Induced by Ozonization of Cobalt(II) and Manganese(II) Hexacyanocobaltates(III). Crystals 2017, 7, 16. https://doi.org/10.3390/cryst7010016
Ríos ML, Rodríguez-Hernández J, Del Castillo LF, Balmaseda J. Structural and Gas Retention Changes Induced by Ozonization of Cobalt(II) and Manganese(II) Hexacyanocobaltates(III). Crystals. 2017; 7(1):16. https://doi.org/10.3390/cryst7010016
Chicago/Turabian StyleRíos, María Laura, Joelis Rodríguez-Hernández, Luis Felipe Del Castillo, and Jorge Balmaseda. 2017. "Structural and Gas Retention Changes Induced by Ozonization of Cobalt(II) and Manganese(II) Hexacyanocobaltates(III)" Crystals 7, no. 1: 16. https://doi.org/10.3390/cryst7010016







