Study on Micro Morphology of Potassium Dihydrogen Phosphate Crystals Grown at Elevated Temperatures
Abstract
:1. Introduction
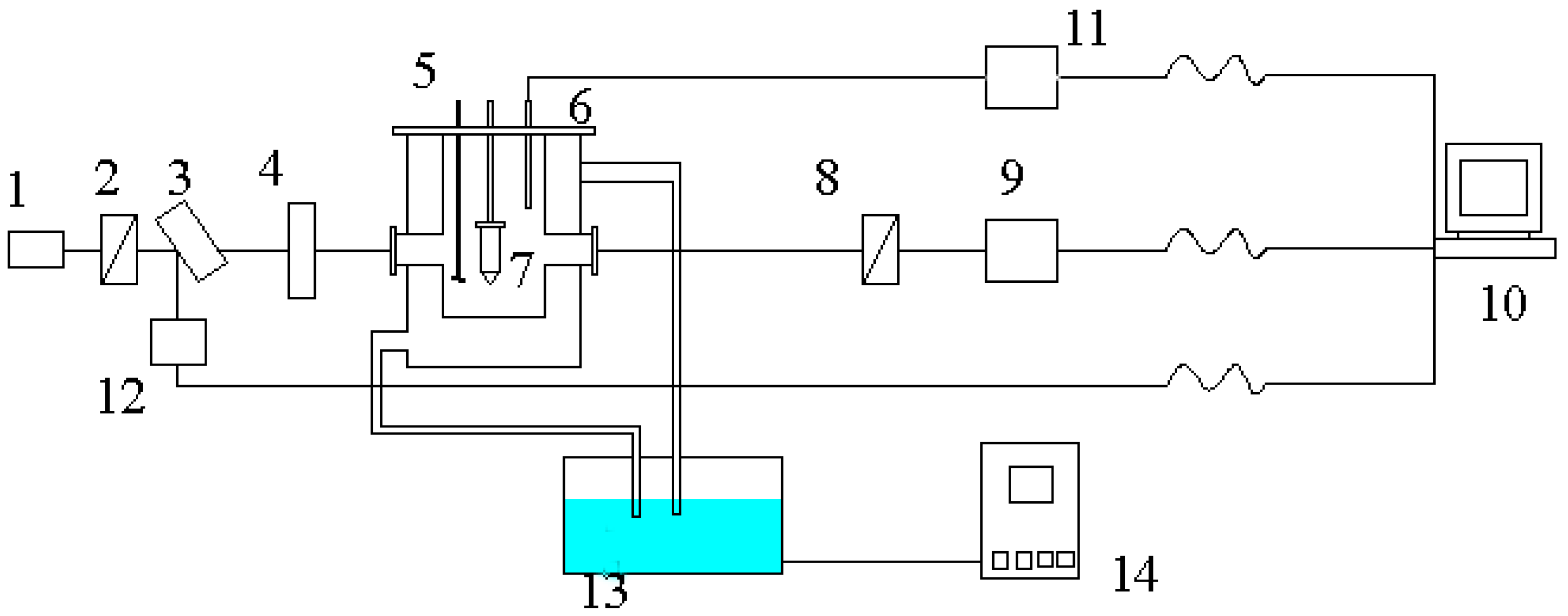
2. Experiments
2.1. Crystal Growth
2.2. AFM Characterization
2.3. Measurement of Growth Rate
3. Results
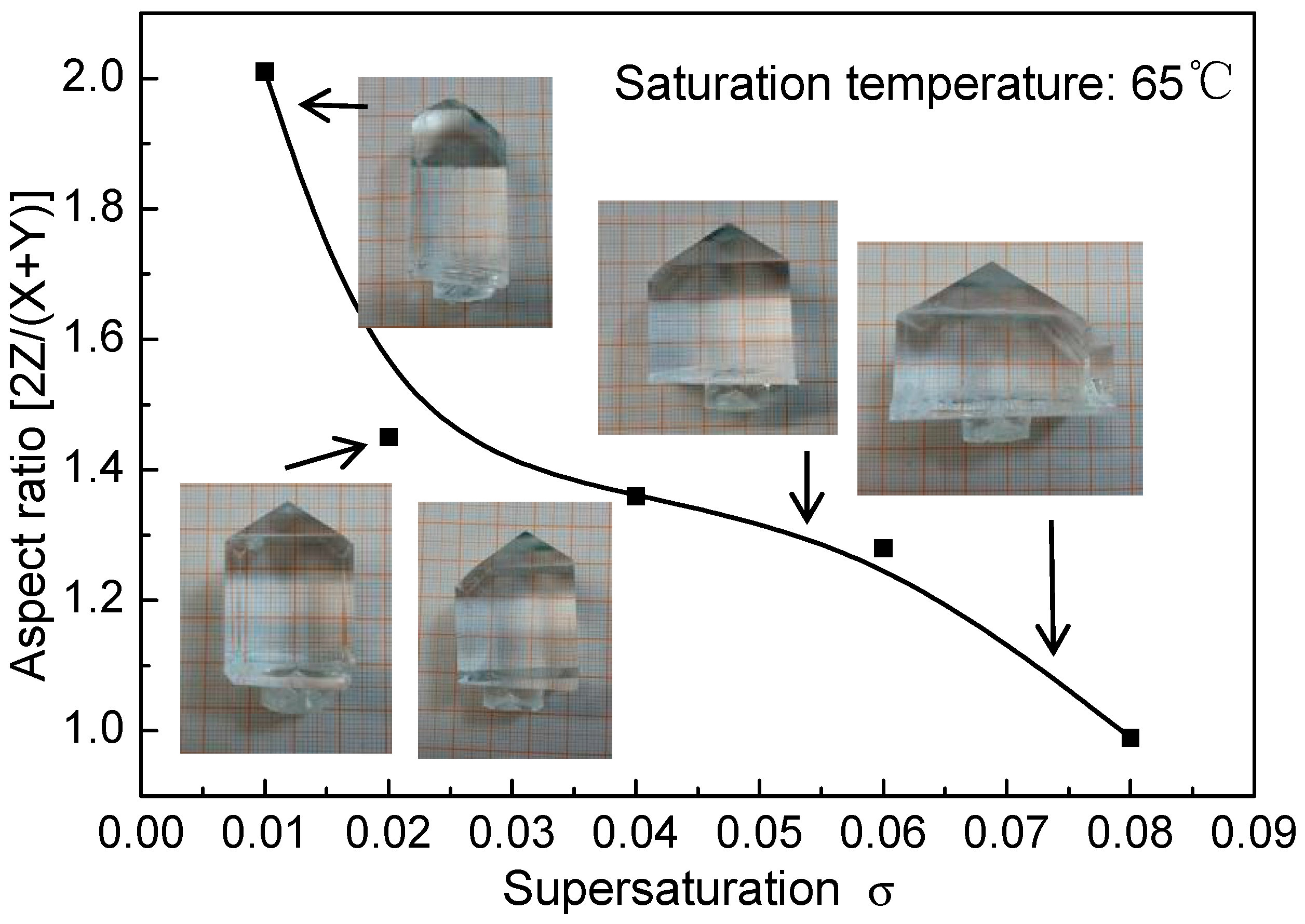
3.1. Crystals and Aspect Ratio
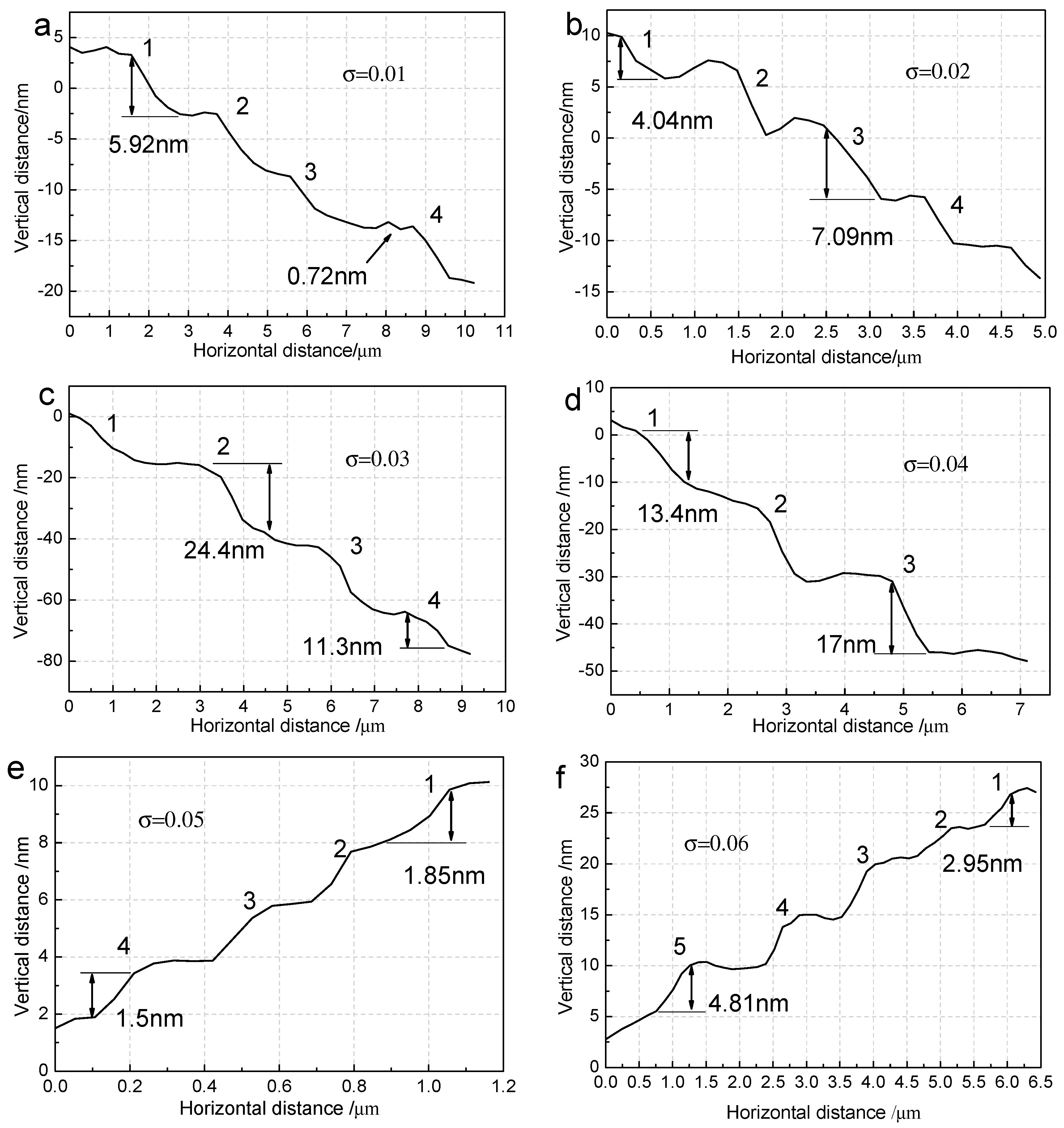
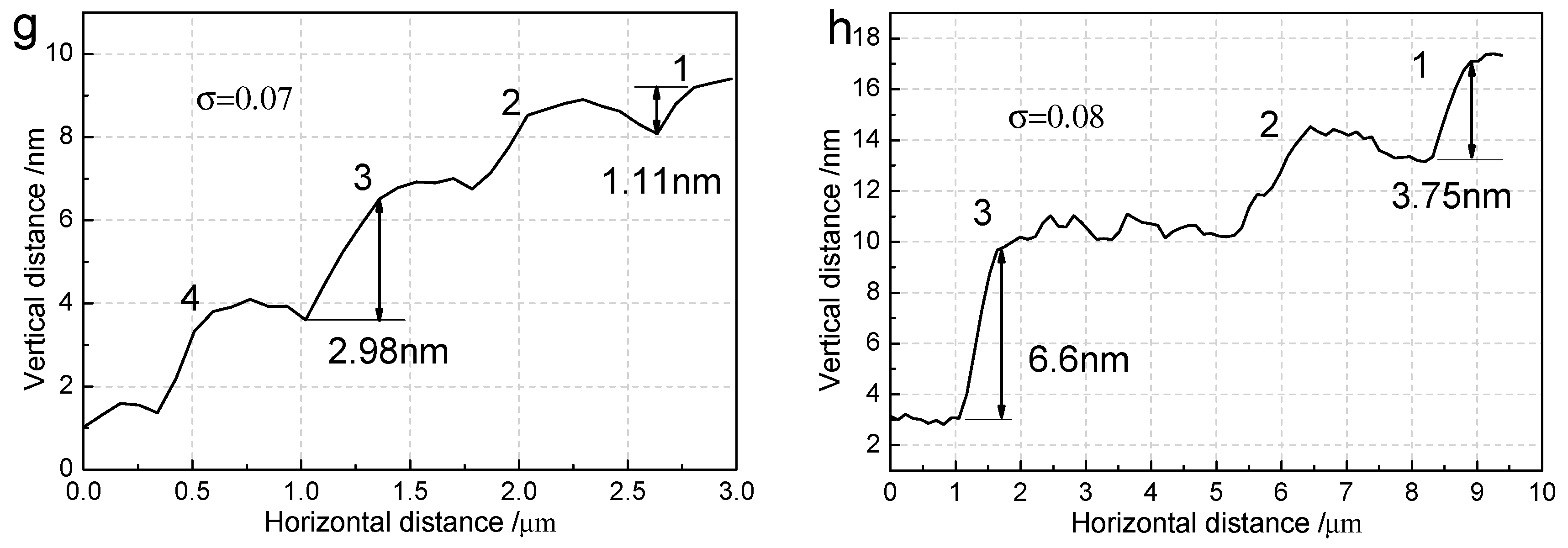
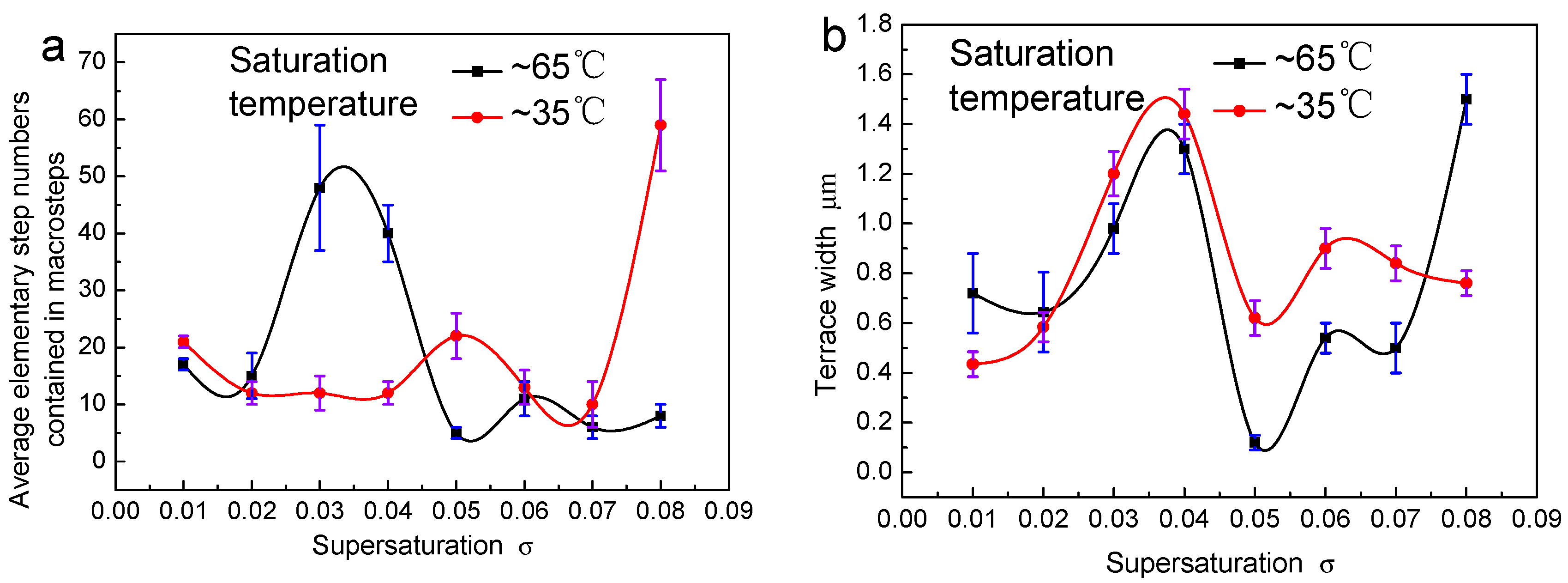
3.2. The Surface Step Morphology on (100) Faces
3.3. The Step Structure on (100) Faces
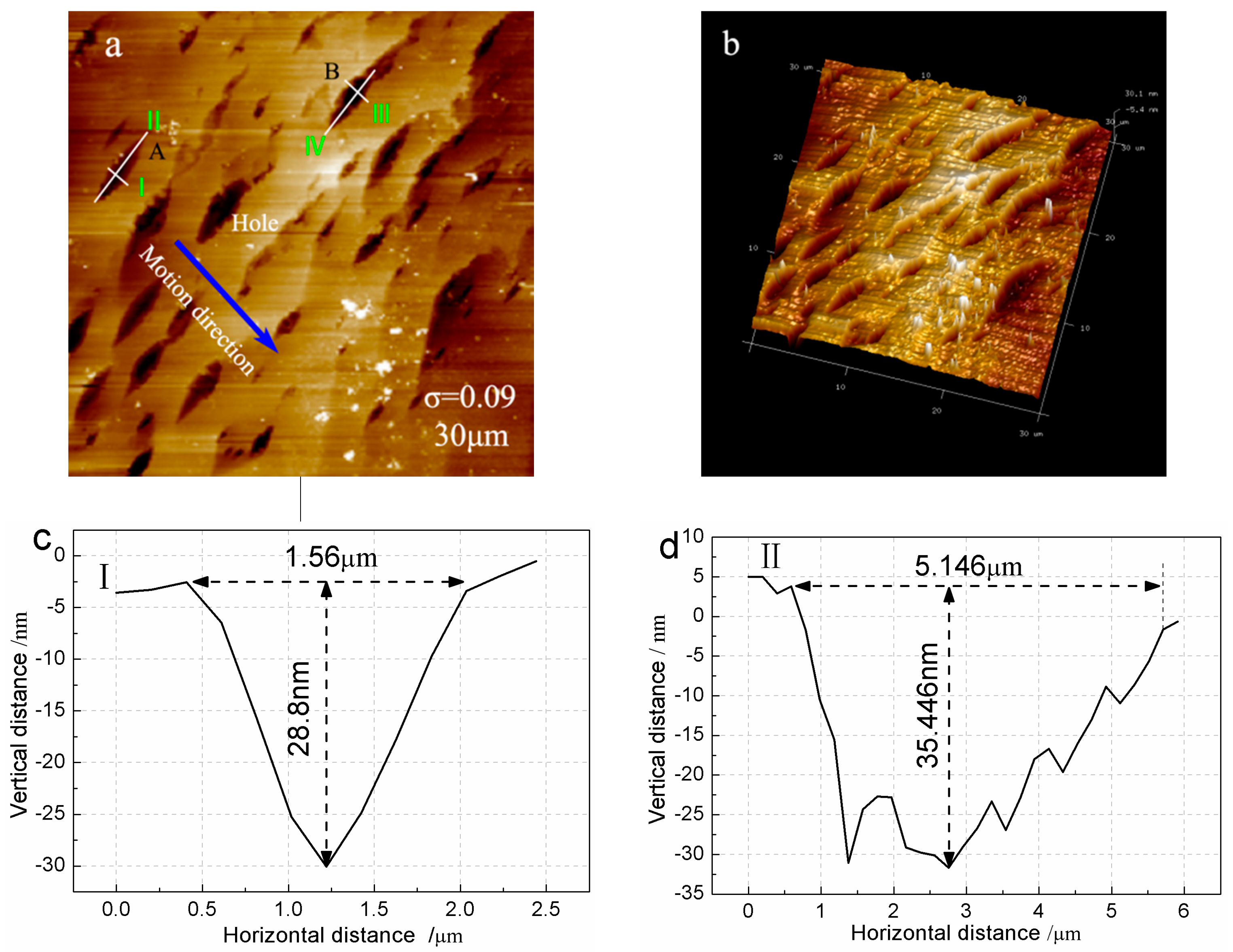
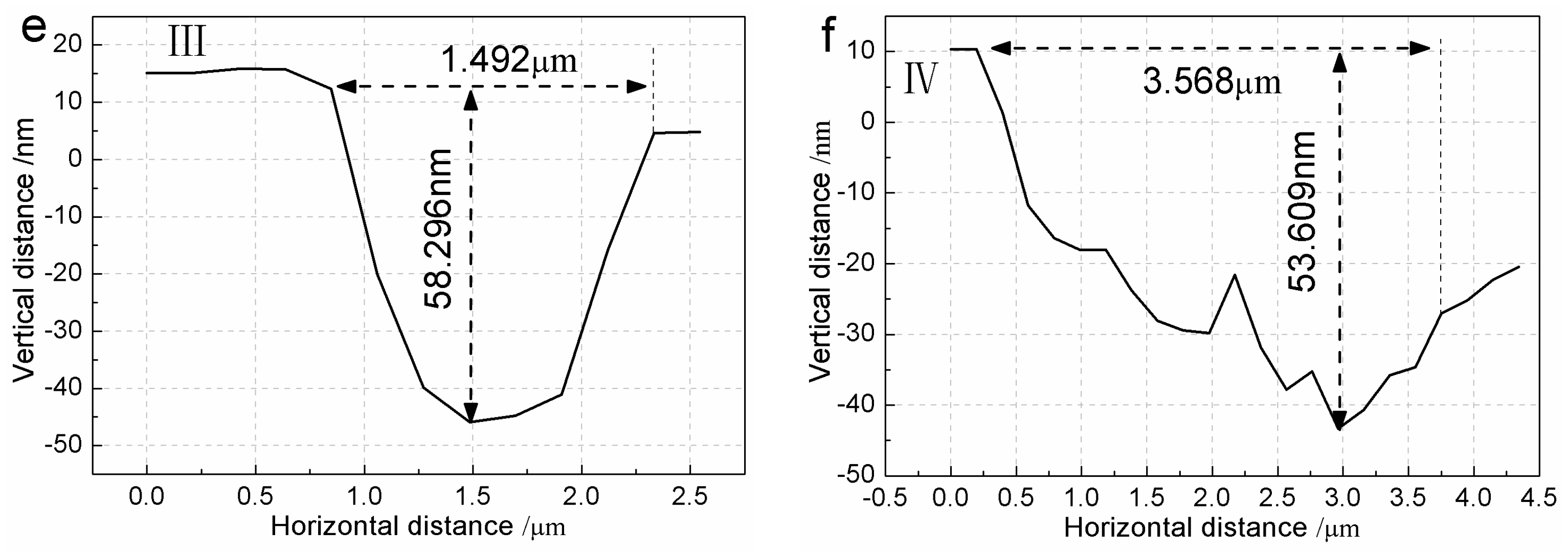
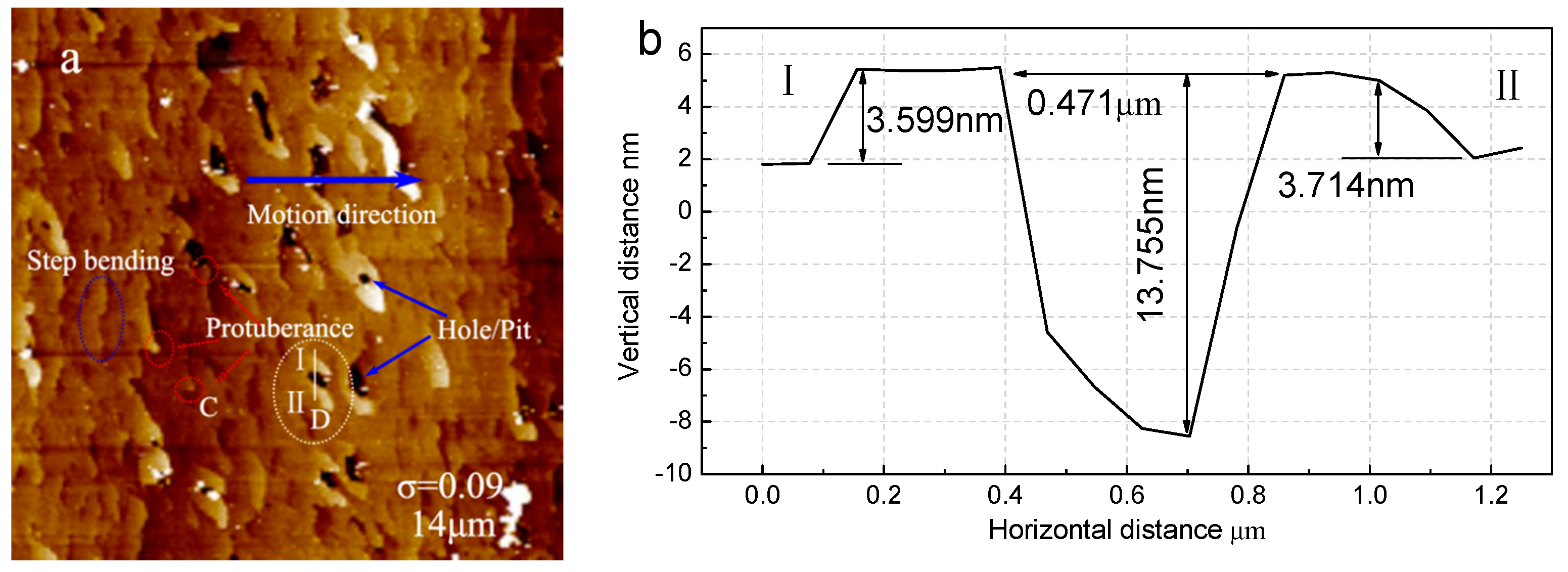
3.4. Step Structure on (100) Face for σ = 0.09
3.5. Growth Rate Versus the Supersaturation
4. Discussion
4.1. Effect of Temperature on Kinetic Coefficients
4.2. Effect of Temperature on Step Bunching
4.3. Appearance of Pits at High Supersaturation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoshida, H.; Jitsuno, T.; Fujita, H.; Nakatsuka, M.; Yoshimura, M.; Sasaki, T.; Yoshida, K. Investigation of bulk laser damage in KDP crystal as a function of laser irradiation direction, polarization, and wavelength. Appl. Phys. B 2000, 70, 195–201. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Burnham, A.K.; Whitman, P.K. Developing KH2PO4 and KD2PO4 crystal for world’s most powerful laser. Int. Mater. Rev. 2002, 47, 113–152. [Google Scholar] [CrossRef]
- Sangwal, K. Effects of impurities on crystal growth process. Cryst. Growth Charact. 1996, 32, 3–43. [Google Scholar] [CrossRef]
- Leroudier, J.; Zaccaro, J.; Ildefonso, M.; Veesler, S.; Baruchel, J.; Ibanez, A. Nucleation control and rapid growth of KDP crystals in stationaryconditions. Cryst. Growth Des. 2011, 11, 2592–2598. [Google Scholar] [CrossRef]
- Zaitseva, N.; Carman, L.; Smolsky, I.; Torres, R.; Yan, M. The effect of impurities and supersaturation on the rapid growth of KDP crystals. J. Cryst. Growth 1999, 204, 512–524. [Google Scholar] [CrossRef]
- Liu, G.X.; Wang, S.L.; Gu, Q.T.; Ding, J.X.; Sun, Y.; Liu, W.J.; Zhu, S.J.; Liu, L. Numerical simulation of temperature field of KDP crystal grown by traditional method. J. Synth. Cryst. 2013, 42, 1261–1266. [Google Scholar]
- Rashkovich, L.N.; Kronsky, N.V. Influence of Fe3+ and Al3+ ions on the kinetics of steps on the {100} faces of KDP. J. Cryst. Growth 1997, 182, 434–441. [Google Scholar] [CrossRef]
- Land, T.A.; Martin, T.L.; Potapenko, S. Recovery of surfaces from impurity poisoning during crystal growth. Nature 1999, 399, 442–445. [Google Scholar] [CrossRef]
- Sangwal, K.; Torrent-Burgues, J.; Gorostiza, P.; Sanz, F. AFM study of the behavior of growth steps on the (100) faces of KDP crystals and the tapering phenomenon. Cryst. Res. Technol. 1999, 34, 667–675. [Google Scholar] [CrossRef]
- Min, C.; Li, M.W.; Guo, J.L.; Cao, Y.C. Ex situ and in situ AFM investigations on the growth of the (100) face of KDP with different pH values. Sci. China Technol. Sci. 2010, 53, 1554–1561. [Google Scholar]
- Ding, J.X.; Wang, S.L.; Xu, X.G.; Gu, Q.T.; Sun, X.; Li, Y.P.; Wang, B.; Liu, G.X.; Liu, W.J.; Sun, Y. Investigation on (100) face of KDP crystal poisoned by MoO42− impurity. J. Mater. Sci. 2012, 47, 5232–5240. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhang, L.S.; Yu, G.W.; Sun, X. Effect of pH value on the growth morphology of KH2PO4 crystal grown in defined crystallographic direction. Cryst. Res. Technol. 2015, 50, 164–170. [Google Scholar] [CrossRef]
- Zhu, S.J.; Wang, S.L.; Ding, J.X.; Liu, G.X.; Liu, W.J.; Liu, L.; Wang, D.L.; Li, W.D.; Gu, Q.T.; Xu, X.G. Imorovement of growth rate and optical performances of rapidly gown KDP crystal by adding cyclohexane tetraacetic acid in growth solution. J. Cryst. Growth 2014, 388, 98–102. [Google Scholar] [CrossRef]
- Burton, W.K.; Cabrera, N.; Frank, F.C. The growth of crystals and the equilibrium structure of their surfaces. Philos. Trans. R. Soc. Lond. 1951, 243, 299–358. [Google Scholar] [CrossRef]
- Liu, B.; Fang, C.S.; Wang, S.L. In situ measurement of growth kinetics of (100) KDP crystal faces in the presence of polyphosphate impurities. Cryst. Res. Technol. 2008, 43, 700–708. [Google Scholar] [CrossRef]
- Li, W.D.; Wang, S.L.; Yu, G.W. Influence of supersaturation on the growth and surface morphology of KDP crystals. J. Chin. Ceram. Soc. 2016, in press. [Google Scholar]
- Thomas, T.N.; Land, T.A.; Martin, T.; Casey, W.H.; de Yoreo, J.J. AFM investigation of step kinetics and hillock morphology of the {100} face of KDP. J. Cryst. Growth 2004, 260, 566–579. [Google Scholar] [CrossRef]
- Belustin, A.V.; Kolina, A.V. Growth kinetics of potassium dihydrophosphate crystals. Kristallografyia 1975, 20, 206–207. [Google Scholar]
- Rashkovich, L.N. KDP Family of Crystals; Hilger: Bristol, UK, 1991. [Google Scholar]
- Frank, F.C. Crystal Growth; Butterworths: London, UK, 1959. [Google Scholar]
- Arrhenius, S.A. Uber die Dissociationswarme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef]
- Ding, J.X.; Wang, S.L.; Mu, X.M.; Yu, G.W.; Xu, X.G.; Sun, X.; Liu, W.J. AFM investigation on step bunching on (100) face of KDP crystal. J. Inorg. Mater. 2010, 25, 1191–1194. [Google Scholar] [CrossRef]
- Zaitseva, N.; Carman, L. Rapid growth of KDP-type crystals. Prog. Cryst. Growth Charact. Mater. 2001, 43, 1–118. [Google Scholar] [CrossRef]
- Rashkovich, L.N.; Mkrtchyan, A.A.; Chernov, A.A. Optical interference investigation of growth morphology and kinetics of (100) face of ADP from aqueous solution. Sov. Phys. Crystallogr. 1985, 30, 380–387. [Google Scholar]
- De Yoreo, J.J.; Land, T.A.; Dair, B. Growth morphology of vicinal hillocks on the {101} face of KH2PO4: From step-flow to layer-by-layer growth. Phys. Rev. Lett. 1994, 73, 838. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.L.; U, D.X.; Sun, D.L. Atomic force microscopy studies on growth steps and 2D nuclei on the {100} faces of deuterated L-arginine phosphate crystals. Surface Rev. Lett. 2004, 11, 379–383. [Google Scholar] [CrossRef]
- Zhu, S.J.; Wang, S.L.; Ding, J.X.; Liu, G.X.; Wang, D.L.; Liu, L.; Gu, Q.T.; Xu, X.G. Effect of supersaturation on growth and optical properties of KDP crystal. J. Synth. Cryst. 2013, 42, 1973. [Google Scholar]


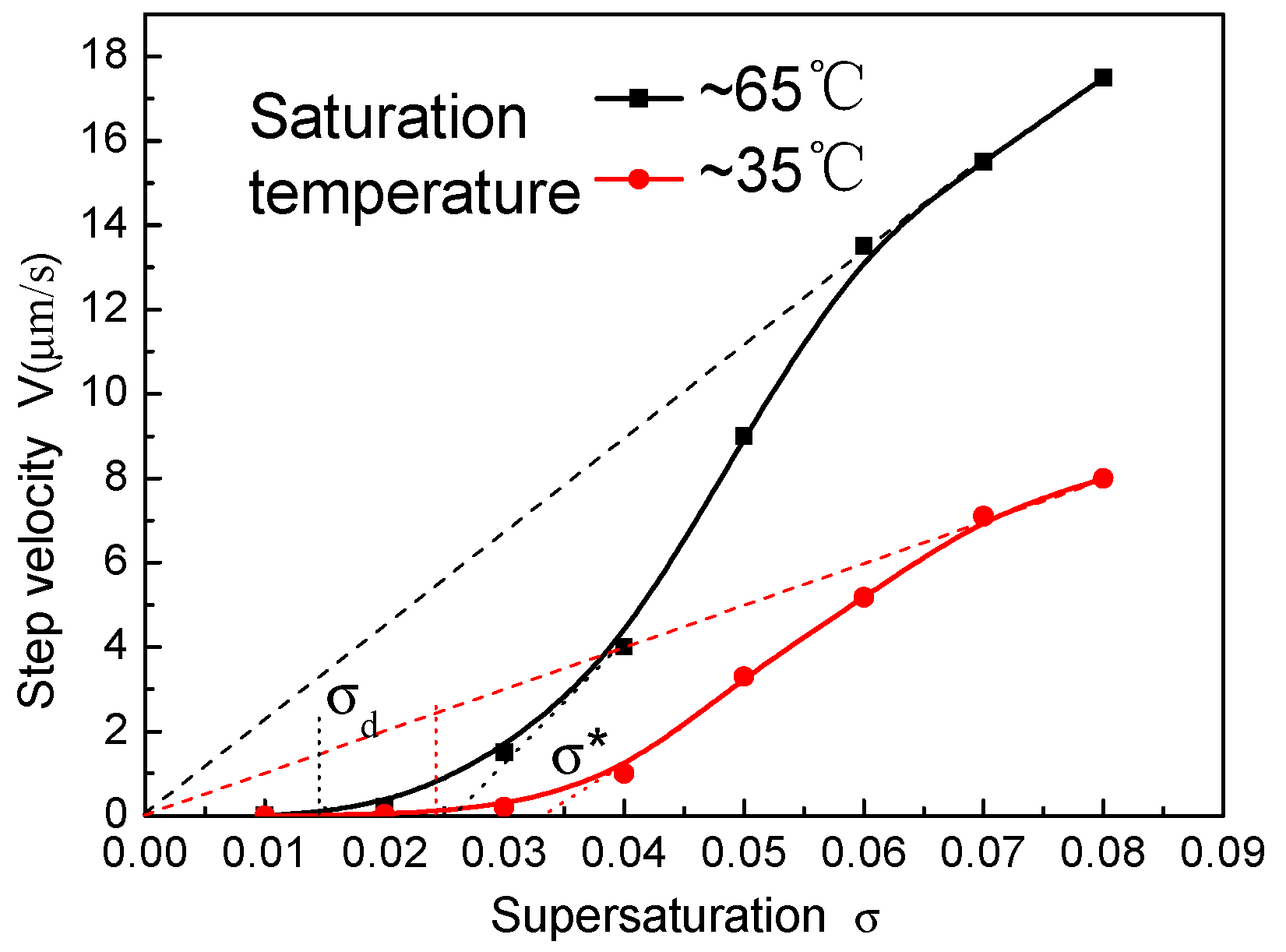
| Saturation Temperature | σd (%) | σ* (%) | A (10−6 m/s) | (10−6 m/s) | (10−6 m/s) [18] |
|---|---|---|---|---|---|
| ~35 °C | 2.4 | 3.3 | 5.075 | 1.261 | 1.394 (30 °C) |
| ~65 °C | 1.51 | 2.6 | 42.58 | 5.096 | — |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, S.; Ding, J.; Yu, G.; Wang, D.; Huang, P.; Liu, H.; Gu, Q.; Xu, X. Study on Micro Morphology of Potassium Dihydrogen Phosphate Crystals Grown at Elevated Temperatures. Crystals 2017, 7, 118. https://doi.org/10.3390/cryst7040118
Li W, Wang S, Ding J, Yu G, Wang D, Huang P, Liu H, Gu Q, Xu X. Study on Micro Morphology of Potassium Dihydrogen Phosphate Crystals Grown at Elevated Temperatures. Crystals. 2017; 7(4):118. https://doi.org/10.3390/cryst7040118
Chicago/Turabian StyleLi, Weidong, Shenglai Wang, Jianxu Ding, Guangwei Yu, Duanliang Wang, Pingping Huang, Hui Liu, Qingtian Gu, and Xinguang Xu. 2017. "Study on Micro Morphology of Potassium Dihydrogen Phosphate Crystals Grown at Elevated Temperatures" Crystals 7, no. 4: 118. https://doi.org/10.3390/cryst7040118
APA StyleLi, W., Wang, S., Ding, J., Yu, G., Wang, D., Huang, P., Liu, H., Gu, Q., & Xu, X. (2017). Study on Micro Morphology of Potassium Dihydrogen Phosphate Crystals Grown at Elevated Temperatures. Crystals, 7(4), 118. https://doi.org/10.3390/cryst7040118





