Thick Hydride Vapor Phase Heteroepitaxy: A Novel Approach to Growth of Nonlinear Optical Materials
Abstract
:1. Introduction
2. Some Basics of Heteroepitaxy
2.1. The Three Modes of Heteroepitaxy
2.2. Major Transport Phenomena During Vapor Phase Growth
2.3. Nucleation and Growth under Equilibrium and Non-Equilibrium Conditions: Far and Close-to-Equilibrium Growth Techniques
2.4. When to Prefer Heteroepitaxy to Homoepitaxy and What are the Criteria to Make the Right Choice
- The lattice mismatch between substrate and growing layer: This is an important parameter, there are many examples when materials with larger lattice mismatch can grow more successfully than materials with smaller lattice mismatch.
- The thermal mismatch: The growth of especially thicker layers may lead to cracking in the case of a large difference in the thermal conductivity and the thermal expansion coefficients of the substrate and growing layer.
- The choice of the matching orientations: In some cases one material can be grown on a foreign substrate with different crystallographic orientation, or two phases of one of the same material can be grown on two completely different materials. For example, cubic GaN layers can be successfully grown on (100) GaAs substrates, whereas hexagonal GaN grows better on (111) oriented GaAs substrates [54]. Similarly, the cubic α-phase of gallium selenide Ga4Se6 has small lattice mismatch with GaP, while the hexagonal β-phase GaSe has the closest match with the hexagonal GaN.
- The quality of the substrate: In some cases a better substrate quality does not always mean a successful growth. For example, in spite of the negligible lattice mismatch between GaP and Si, GaP cannot be grown directly by HVPE on a Si-substrate, namely, due to its high quality (low EPD—compare EPD of Si < 100 cm−2 with the EPD of GaAs and GaP provided in Table 1). In such a case the substrate surface does not provide enough sites for the atoms approaching the surface to adhere. Growing on a near defect-free surface by a close-to equilibrium process such as HVPE is difficult—starting nucleation on a clean atomic surface is energetically unfavorable, plus the probabilities for nucleation and returning into the vapor phase are about the same. For this purpose, a far-from-equilibrium process such as MOCVD or MBE must be preferred for successful GaP/Si growth instead of HVPE.
- The maturity of the growth techniques for producing the bulk substrate material as well as the maturity of the vapor phase growth of the layer on the substrate.
- The maturity of the template preparation techniques: There are only a few materials with relatively well-developed techniques for OP template preparation—GaAs, GaP and GaN. This is one of the reasons to attempt a thick growth of OP-structures on foreign OP templates.
2.5. Advantages of Growing on Patterned Substrates and on Buffer Layers
2.6. Major Techniques for Preparation of Orientation-Patterned Templates
3. Some Basics of Optics
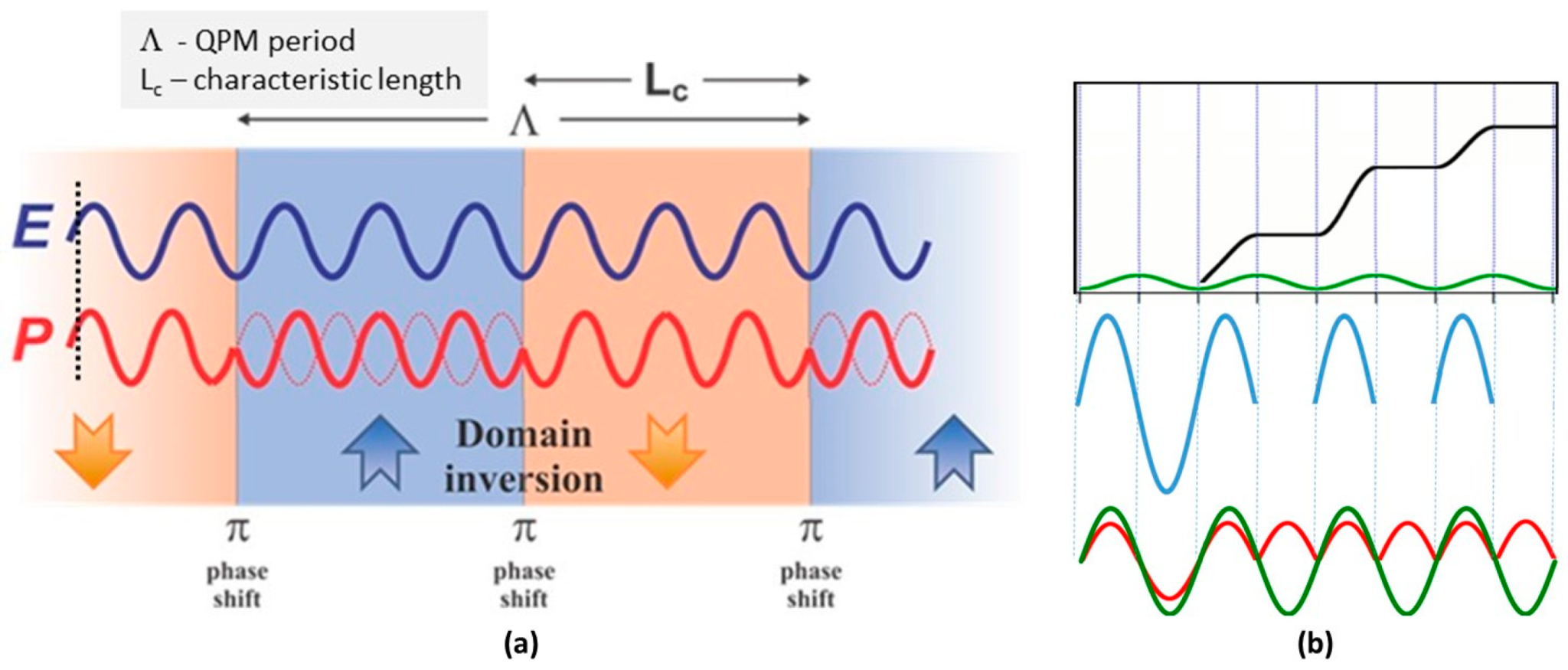
3.1. Wave Mixing Processes in Orientation-Patterned Optical Materials
3.2. Quasi-Phase Matching, Nonlinear Susceptibility and Two-Photon Absorption.
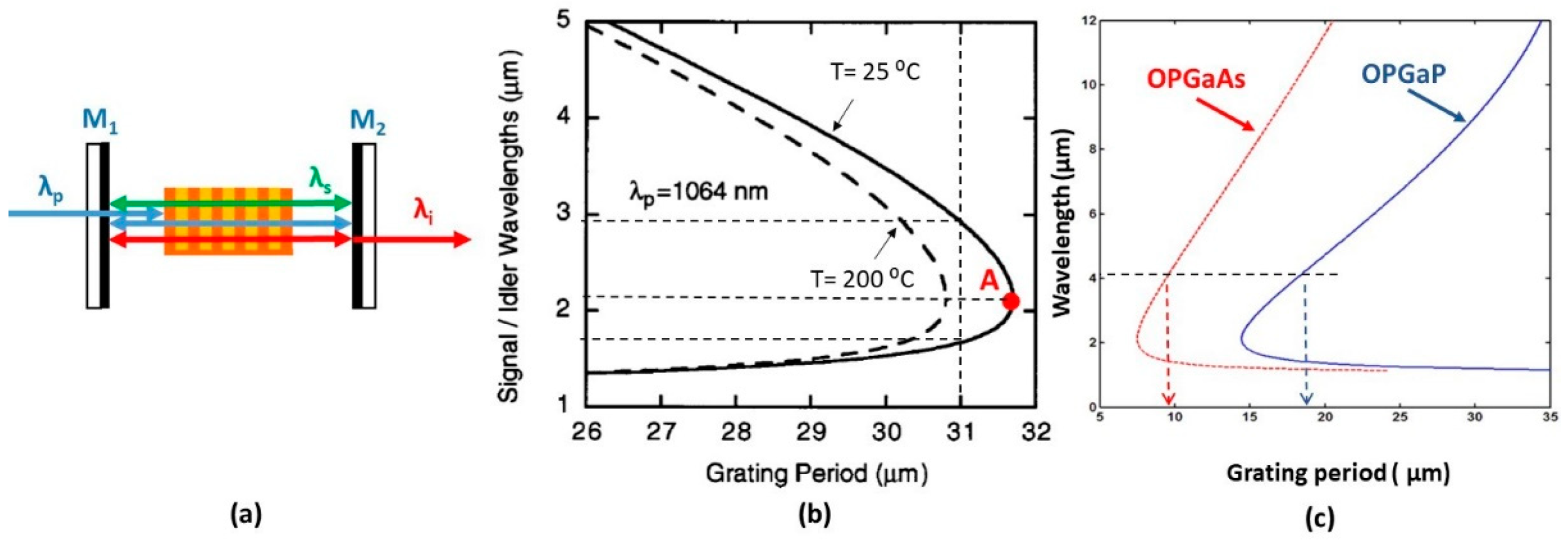
3.3. Optical Parametric Oscillator
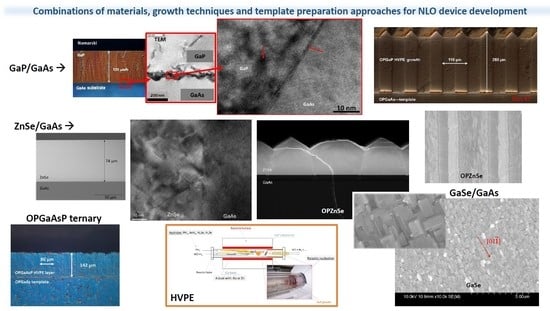
4. Heteroepitaxy of Nonlinear Optical Materials
4.1. Hydride/Halide Vapor Phase Epitaxy—Reactor, Process, Optimizing the Growth Conditions
4.2. Growth of GaP on Plain GaAs, Half-Patterned (HP) and OP-GaAs Templates
4.2.1. Growth of GaP on Plain GaAs
4.2.2. Growth of GaP on Half-Patterned (HP) GaAs Templates
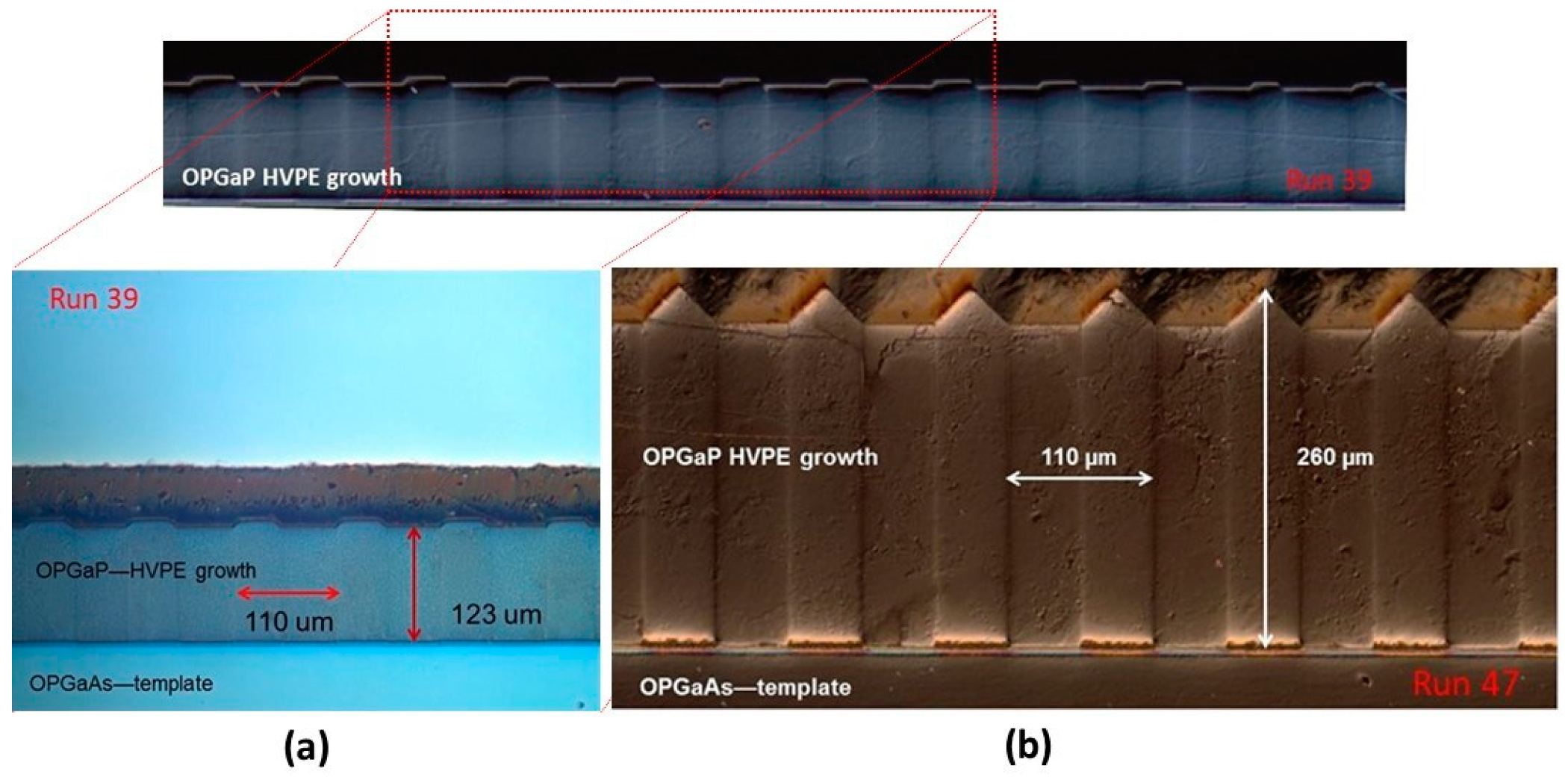
4.2.3. Growth of GaP on Orientation-Patterned (OP) GaAs Templates
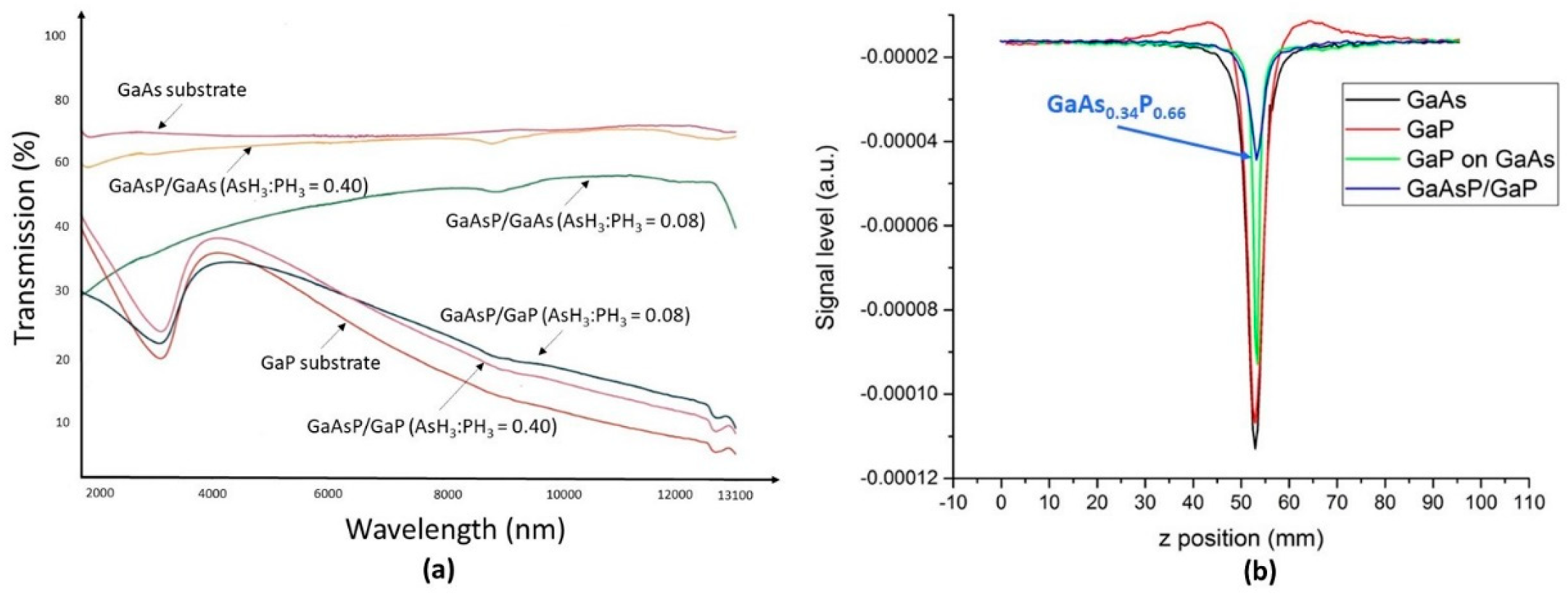
4.2.4. Growth of GaAsxP1−x Ternaries on Plain GaAs and GaP Substrates and on OP-GaAs Templates
4.3. Growth of Some Selenides: ZnSe on GaAs and GaSe on GaP, GaAs and GaN
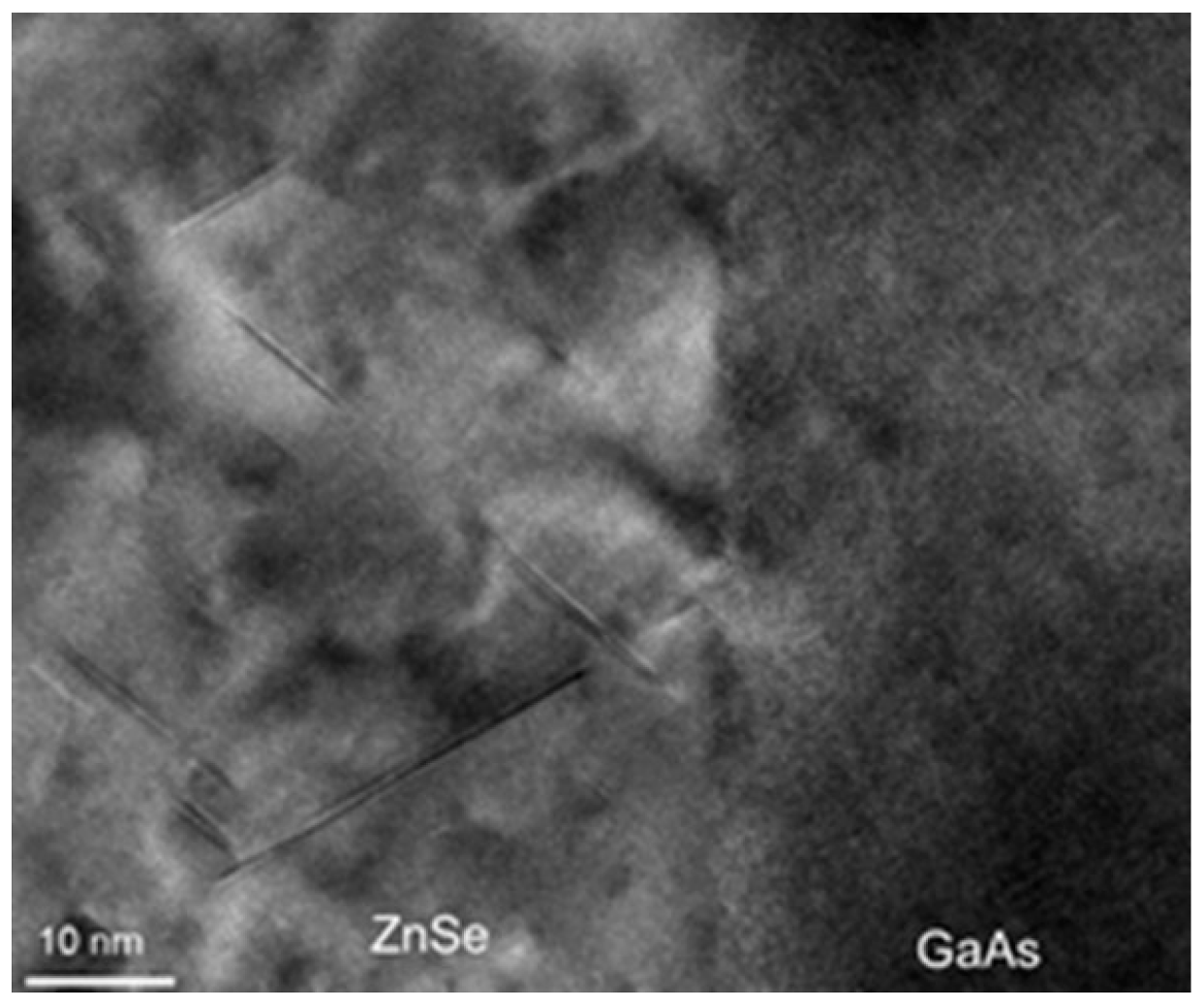
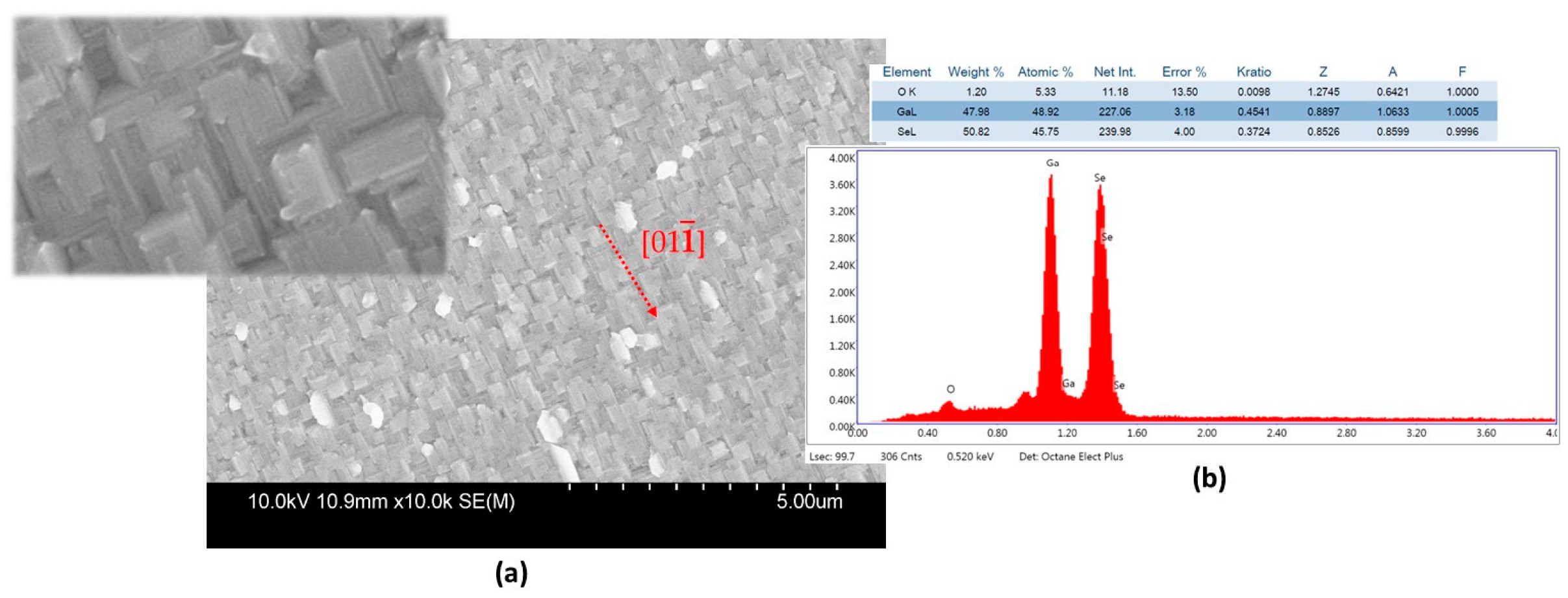
4.3.1. Growth of ZnSe on GaAs and on OP-GaAs Templates
4.3.2. Growth of GaSe on GaP, GaAs and GaN
4.4. More Prospective Heteroepitaxial Cases—ZnTe on GaSb and Many Others
4.5. Some Crystal Growth Considerations
5. Some Novel Techniques for OP Template Preparation
6. Some Novel Techniques for Growth/Fabrication of Hybrid QPM Structures
7. In Support of the HVPE Growth Technique
8. Conclusions
9. Patents
- Tassev, V.L.; Peterson, R.D. Heteroepitaxial growth of orientation-patterned materials on orientation-patterned substrates. U.S. Patent No 9,647,156, 9 May 2017 (on the material).
- Tassev, V.L.; Peterson, R.D. Heteroepitaxial growth of orientation-patterned materials on orientation-patterned substrates. U.S. Patent No 9,777,402, 3 October 2017 (on the method).
- Tassev, V.L. Growth/fabrication of organic-inorganic quasi phase-matching structures for frequency conversion devices, U.S. Patent No 9,960,568 B1, 1 May 2018.
- Tassev, V. Optimized thick heteroepitaxial growth of semiconductors with in-situ substrate pretreatment. Patent application No 16201446 submitted to USPTO on 27 November 2018.
- Tassev, V.L.; Vangala, S.R.; Tomich, D.H. Method for fabrications of orientation-patterned templates on common substrates, Patent application No 16447677 submitted to USPTO on 20 June 2019.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wavelengths of Commercially Available Lasers. Available online: https://en.wikipedia.org/wiki/List_of_laser_types#/media/File:Commercial_laser_lines.svg (accessed on 17 April 2019).
- Tacke, M. Lead laser sources. Philos. Trans. R. Soc. Lond. 2001, A359, 547–566. [Google Scholar] [CrossRef]
- Razeghi, M.; Zhou, W.; Slivken, S.; Lu, Q.Y.; Wu, D.; McClintock, R. Recent progress of quantum cascade laser research from 3 to 12 μm at the center for quantum devices. Appl. Opt. 2017, 56, H30–H44. [Google Scholar] [CrossRef] [PubMed]
- Kildaland, H.; Mikkelsen, J.C. The nonlinear coefficient phase matching and optical damage in the Chalcopyrite AgGaSe2. Opt. Commun. 1973, 9, 315–318. [Google Scholar] [CrossRef]
- Myers, L.E.; Bosenberg, W.R. Periodically poled lithium niobate and quasi-phase-matched optical parametric oscillators. IEEE J. Quantum Electron. 1997, 33, 1663–1672. [Google Scholar] [CrossRef]
- Grisard, A.; Lallier, E.; Gérard, B. Quasi-phase-matched gallium arsenide for versatile mid-infrared frequency conversion. Opt. Mater. Express 2012, 2, 1021. [Google Scholar] [CrossRef]
- Lynch, C.; Bliss, D.F.; Zens, T.; Lin, A.; Harris, J.S.; Kou, P.H.; Fejer, M.M. Growth of mm-thick orientation-patterned GaAs. J. Cryst. Growth 2008, 310, 5241–5247. [Google Scholar] [CrossRef]
- Vodopyanov, K.L.; Levi, O.; Kuo, P.S.; Pinguet, T.J.; Harris, J.S.; Fejer, M.M.; Gerard, B.; Becouarn, L.; Lallier, E.B. Optical parametric oscillation in quasi-phase-matched GaAs. Opt. Lett. 2004, 29, 1912–1914. [Google Scholar] [CrossRef]
- Hurlbut, W.; Lee, Y.; Vodopyanov, K.; Kuo, P.; Fejer, M. Multi-photon absorption and nonlinear refraction of GaAs in the mid-infrared. Opt. Lett. 2007, 32, 668–670. [Google Scholar] [CrossRef]
- Schunemann, P.G.; Zetzler, S.D. Future directions in quasi-phase matched semiconductors for mid-infrared lasers. Proc. SPIE 2011, 7917, 79171F-1. [Google Scholar]
- Tassev, V.L.; Vangala, S.R.; Peterson, R.D.; Kimani, M.M.; Snure, M.; Stites, R.W.; Guha, S.; Slagle, J.E.; Ensley, T.R.; Syed, A.A.; et al. Heteroepitaxial growth of OPGaP on OPGaAs for frequency conversion in the IR and THz. Opt. Mater. Express 2016, 6, 1724–1737. [Google Scholar] [CrossRef]
- Yasakov, D.A.; Pikhtin, A.N.; Ulyanov, V.I. Optical properties of gallium phosphide grown by floating zone. Mater. Res. Bull. 1969, 4, 839–848. [Google Scholar] [CrossRef]
- Tassev, V.; Bliss, D.; Snure, M.; Bryant, G.; Peterson, R.; Bedford, R.; Yapp, C.; Goodhue, W.; Termkoa, K. HVPE growth and characterization of GaP on different substrates and patterned templates for frequency conversion devices. J. Eur. Opt. Soc. 2011, 6, 110171–110177. [Google Scholar] [CrossRef]
- Tassev, V.; Snure, M.; Peterson, R.; Bedford, R.; Bliss, D.; Bryant, G.; Mann, M.; Goodhue, W.; Vangala, S.; Termkoa, K.; et al. Epitaxial growth of quasi-phase matched GaP for nonlinear applications: Systematic process improvements. J. Cryst. Growth 2012, 352, 72–77. [Google Scholar] [CrossRef]
- Tassev, V.; Snure, M.; Peterson, R.; Schepler, K.L.; Bedford, R.; Mann, M.; Vangala, S.; Goodhue, W.; Lin, A.; Harris, J.; et al. Progress in orientation-patterned GaP for next-generation nonlinear optical devices. Proc. SPIE LASE 2013, 8604, 86040V. [Google Scholar]
- Schunemann, P.G.; Mohnkern, L.; Vera, A.; Yang, X.S.; Lin, A.C.; Harris, J.S.; Tassev, V.L.; Snure, M. All-epitaxial growth of orientation-patterned gallium phosphide (OPGaP). OSA Tech. Dig. 2012, ITh5B.5. [Google Scholar]
- Schunemann, P.G.; Pomeranz, L.A.; Magarrell, D.J. First OPO based on orientation-patterned gallium phosphide (OP-GaP). OSA Tech. Dig. 2015, SW3O.1. [Google Scholar]
- Tassev, V.L.; Vangala, S.R.; Peterson, R.D.; Snure, M. Heteroepitaxy on orientation-patterned nonlinear optical materials. J. Cryst. Growth 2018, 486, 155–161. [Google Scholar] [CrossRef]
- Tassev, V.L.; Peterson, R.D. Heteroepitaxial Growth of Orientation-Patterned Materials on Orientation-Patterned Substrates. U.S. Patent 9,647,156, 9 May 2017. [Google Scholar]
- Tassev, V.L. Heteroepitaxy, an amazing contribution to the world of optics and electronics. Crystals 2017, 7, 178. [Google Scholar] [CrossRef]
- MTI Corporation Website. Available online: https://www.mtixtl.com/crystalssubstratesa-z.aspx (accessed on 1 May 2019).
- Collazo, R.; Mita, S.; Aleksov, A.; Schlesser, R.; Sitar, Z. Growth of Ga- and N- polar gallium nitride layers by metalorganic vapor phase epitaxy on sapphire wafers. J. Cryst. Growth 2006, 287, 586–590. [Google Scholar] [CrossRef]
- Sumiya, M.; Fuke, S. Review on polarity determination and control in GaN. MRS Internet J. Nitride Semicond. Res. 2004, 9, e1. [Google Scholar] [CrossRef]
- Mita, S.; Collazo, R.; Sitar, Z. Fabrication of a GaN lateral polarity junction by metalorganic chemical vapor deposition. J. Cryst. Growth 2009, 311, 3044–3048. [Google Scholar] [CrossRef]
- Matsumura, H.; Kanematsu, Y.; Shimura, T.; Tamaki, T.; Ozeki, Y.; Itoh, K.; Sumiya, M.; Nakano, T.; Fuke, S. Lateral polarity control in GaN based selective growth procedure using carbon mask layers. Appl. Phys. Express 2009, 2, 101001. [Google Scholar] [CrossRef]
- Ebert, C.B.; Eyres, L.A.; Fejer, M.M.; Harris, J.S. MBE growth of antiphase GaAs films using GaAs/Ge/GaAs heteroepitaxy. J. Cryst. Growth 1999, 201, 187. [Google Scholar] [CrossRef]
- Faye, D.; Grisard, A.; Lallier, E.; Gérard, B.; Kieleck, C.; Hirth, A. Orientation-patterned gallium arsenide: Engineered materials for infrared sources. SPIE Newsroom 2008. Available online: http://spie.org/news/1164-orientation-patterned-gallium-arsenide-engineered-materials-for-infrared-sources?SSO=1 (accessed on 26 July 2019). [CrossRef]
- Tassev, V.L.; Vangala, S.R.; Tomich, D.H. Method for Fabrications of Orientation-Patterned Templates on Common Substrates. U.S. Patent 1,644,767,7, 20 June 2019. [Google Scholar]
- Tassev, V.L. Growth/fabrication of organic/inorganic quasi phase-matching structures for frequently conversion devices. U.S. Patent 9,960,568, 1 May 2018. [Google Scholar]
- Hong, Y.I.; Lee, C.H. Van der Waals heteroepitaxy of semiconductor nanowires. In Semiconductors and Semimetals; Elsevier: Cambridge, MA, USA, 2015; Volume 93, pp. 125–172. [Google Scholar]
- Bauer, E. Phänomenologische Theorie der Kristallabscheidung an Oberflächen. Kristallografiya 1958, 110, 372. [Google Scholar] [CrossRef]
- Wang, L.G.; Kratzer, P.; Schefller, M.; Moll, N. Formation and stability of self-assembled coherent islands in highly mismatched heteroepitaxy. Phys. Rev. Lett. 1999, 82, 4042–4045. [Google Scholar] [CrossRef]
- Brehm, M.; Montalenti, F.; Grydlik, M.; Vastola, G.; Lichtenberger, H.; Hrauda, N.; Beck, M.J.; Fromherz, T.; Schäffler, F.; Miglio, L.; et al. Key role of the wetting layer in revealing the hidden path of Ge/Si(001) Stranski-Krastanow growth onset. Phys. Rev. B 2009, 80, 205321. [Google Scholar] [CrossRef] [Green Version]
- Stranski, I.; Krastanov, L. Über die kristallisation von alkalihalogenidkristallen auf fluorit. Akad. Wiss. Lit. Mainz 1939, 146, 797. [Google Scholar]
- Zangwill, A. Physics at Surfaces; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Bhattacharyya, A.; Moustakas, T.D.; Zhou, L.; Smith, D.J.; Hug, W. Deep ultraviolet emitting AlGaN quantum wells with high internal quantum efficiency. Appl. Phys. Lett. 2009, 94, 181907. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.H.; Jia, H.Q.; Guo, L.W.; Xing, Z.G.; Wang, Y.; Pei, X.J.; Zhou, J.M.; Chen, H. White light-emitting diodes based on a single InGaN emission layer. Appl. Phys. Lett. 2007, 91, 161912. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Zhou, G.; Tang, G.; Wang, Z. Wetting layer evolution and its temperature dependence during self-assembly of InAs/GaAs quantum dots. Nanoscale Res. Lett. 2012, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, B.; Cai, D.; Zheng, L.; Bliss, D.F.; Tassev, V.L. Group III nitride vapor growth systems: Modeling and experiments. In Focus on Crystal Growth Research, 1st ed.; Karas, G.V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2006; Volume 1, pp. 64–68. [Google Scholar]
- Markov, I.V. Crystal growth for beginners. In Fundamentals of Nucleation, Crystal Growth and Epitaxy, 2nd ed.; World Scientific: Hackensack, NY, USA, 2003; p. 43. [Google Scholar]
- Lourdudoss, S. Potential use of Hydride Vapor Phase Epitaxy for discrete and integrated photonic devices. In Proceedings of the AFRL, WPAFB, Montgomery, OH, USA, 1 June 2018. [Google Scholar]
- Badikov, V.V.; Badikov, D.V.; Laptev, V.B.; Mitin, K.V.; Shevyrdyaeva, G.S.; Shchebetova, N.I.; Petrov, V. Crystal growth and characterization of new quaternary chalcogenide nonlinear crystals for the mid-IR: BaGa2GeS6 and BaGa2GeSe6. Opt. Mater. Express 2016, 6, 2933–2938. [Google Scholar] [CrossRef]
- Galazka, Z.; Uecker, R.; Klimm, D.; Naumann, M.; Kwasniewski, A.; Bertram, R.; Ganschow, S.; Bickermann, M. Scaling-up of bulk β-Ga2O3 single crystals by the Czochralski method. ECS J. Solid State Sci. Technol. 2017, 6, Q3007–Q3011. [Google Scholar] [CrossRef]
- Leach, J.H.; Udwary, K.; Rumsey, J.; Dodson, G.; Splawn, H.; Evans, K.R. Halide vapor phase epitaxial growth of β-Ga2O3 and α-Ga2O3 films. APL Mater. 2019, 7, 022504. [Google Scholar] [CrossRef]
- Rafique, S.; Han, L.; Neal, A.T.; Mou, S.; Tadjer, M.J.; French, R.H.; Zhao, H. Heteroepitaxy of N-type β-Ga2O3 thin films on sapphire substrate by low pressure chemical vapor deposition. App. Phys. Lett. 2016, 109, 132103. [Google Scholar] [CrossRef]
- Fornari, R. Single Crystals of Electronic Materials: Growth and Properties; Woodhead Publishing, Elsevier: Duxford, UK; Cambridge, MA, USA, 2019. [Google Scholar]
- Galazka, Z.; Fornari, R. A novel crystal growth technique from the melt: Levitation-assisted self-seeding crystal growth method. J. Cryst. Growth 2014, 388, 61–69. [Google Scholar] [CrossRef]
- Stringfellow, G.B. Organometallic vapor-phase epitaxy. Theory and Practice, 2nd ed.; Academic Press: San Diego, CA, USA, 1999; p. 37. [Google Scholar]
- Bauer, E.G.; Dodson, B.W.; Ehrlich, D.J.; Feldman, L.C.; Flynn, C.P.; Geis, M.W.; Harbison, J.P.; Matyi, R.J.; Peercy, P.S.; Petroff, P.M.; et al. Fundamental issues in heteroepitaxy—A department of energy, council on materials science panel report. J. Mat. Res. 1990, 5, 852–894. [Google Scholar] [CrossRef]
- Ayers, J.E. Heteroepitaxy of Semiconductors: Theory, Growth and Characterization, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 63. [Google Scholar]
- Lourdudoss, S.; Kjebon, O. Hydride vapor phase epitaxy revisited. IEEE J. Sel. Top. Quantum Electron. 1997, 3, 749–767. [Google Scholar] [CrossRef]
- Shaw, D.W. Kinetic aspects in the vapor phase epitaxy of III-V compounds. J. Cryst. Growth 1975, 31, 130–141. [Google Scholar] [CrossRef]
- Moustakas, T.D. Growth of wide-bandgap nitride semiconductors by MBE. Proc. SPIE. 2002, 10303, 1030302. [Google Scholar]
- Tsuchiy, H.; Hasegawa, F.; Okukura, H.; Yoshida, S. Comparison of hydride vapor phase epitaxy of GaN layers on cubic GaN/(100) GaAs and hexagonal Ga/(111) GaAs substrates. Jpn. J. Appl. Phys. 1994, 33, 6448–6453. [Google Scholar] [CrossRef]
- Paskova, T.; Tungasmita, S.; Valcheva, E.; Svedberg, E.B.; Arnaudov, B.; Evtimova, S.; Persson, P.A.; Henry, A.; Beccard, R.; Heuken, M.; et al. Hydride vapour phase homoepitaxial growth of GaN on MOCVD-grown ‘Templates’. Mater. Res. Soc. Int. J. Nitride Semicond. Res. 2000, 5, 131–137. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Yang, W.; Zhu, Y.; Li, G. A new approach to heteroepitaxially growth of high-quality GaN films on Si substrates: The combination of MBE and PLD. Nat. Sci. Rep. 2016, 6, 24448. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.C.; Rowland, W.H.; Johnson, M.A.L.; Fujita, S.; Cook, J.W.; Schetzina, J.F.; Ren, J.; Edmund, J.A. Molecular beam epitaxy growth and properties of GaN films on GaN/SiC substrates. J. Vac. Sci. Technol. B 1995, 13, 1571–1577. [Google Scholar] [CrossRef]
- Park, H.; Chan, H.M. A novel process for the generation of pristine sapphire surfaces. Thin Solid Films 2002, 422, 135–140. [Google Scholar] [CrossRef]
- Park, H.; Chan, H.M.; Vinci, R.P. Patterning of sapphire substrates via a solid state conversion process. Mater. Res. 2005, 20, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Cho, S.J.; Seo, J.H.; Kim, K.; Kim, M.; Shi, J.; Yin, X.; Choi, W.; Zhang, C.; Kim, J.; et al. Lattice Mismatched Semiconductor Heterostructures. Appl. Phys. 2019, arXiv:1812.10225. Available online: https://arxiv.org/ftp/arxiv/papers/1812/1812.10225.pdf (accessed on 9 June 2018).
- Kuech Group: Heteroepitaxy of Large Lattice Mismatch Compound Semiconductors. Available online: https://amps.che.wisc.edu/research_latticemismatch.php (accessed on 14 May 2019).
- Zheleva, T.S.; Ashmawy, W.M.; Jones, K.A. Pendeo-Epitaxy versus lateral epitaxial overgrowth of GaN: A comparative study via finite element analysis. Phys. Status Solidi 1999, 176, 545–551. [Google Scholar] [CrossRef]
- Davis, R.F.; Gehrke, T.; Linthicum, K.J.; Zheleva, T.S.; Preble, E.A.; Rajagopal, P.; Zorman, C.A.; Mehregany, M. Pendeo-epitaxial growth of thin films of gallium nitride and related materials and their characterization. J. Cryst. Growth 2001, 225, 134–140. [Google Scholar] [CrossRef]
- Montalenti, F.; Rovaris, F.; Bergamaschini, R.; Miglio, L.; Salvalaglio, M.; Isella, G.; Isa, F.; Känel, H. Dislocation-Free SiGe/Si Heterostructures. Crystals 2018, 8, 257. [Google Scholar] [CrossRef]
- Wong, W.S.; Krüger, J.; Cho, Y.; Linder, B.P.; Weber, E.R.; Cheung, N.W.; Sands, T. Selective UV-laser processing for lift-off of GaN thin films from sapphire substrates, In Proceedings of the Symposium on LED for Optoelectronic Applications and the 28th State of the Art Programs on Compound Semiconductors; The Electrochemical Society: Pennington, NJ, USA, 1998; Volume 98, p. 377. [Google Scholar]
- Delmdah, R.; Pätzel, R.; Brune, J. Large-area laser-lift-off processing in microelectronics. Phys. Proc. 2013, 41, 241–248. [Google Scholar] [CrossRef]
- Berkeley University: Laser Lift-off & Film Transfer. Available online: https://www.mse.berkeley.edu/groups/Sands/HEMI/Liftoff.html (accessed on 15 June 2019).
- Disco Co: DISCO has developed a unique Laser Lift-off optimal for high brightness vertical structured LED manufacturing. Available online: https://www.disco.co.jp/eg/news/press/20151207.html (accessed on 15 June 2019).
- OPTEK laser systems: Laser Lift Off. Available online: https://optec-laser-systems.com/applications/laser-lift-off-llo/ (accessed on 15 June 2019).
- Chang, T.M.; Fang, H.K.; Liao, C.; Hsu, W.Y.; Wu, Y.C.S. Laser Lift-Off Mechanisms of GaN Epi-Layer Grown on Pattern Sapphire Substrate. ECS J. Solid State Sci. Technol. 2015, 4, R20–R22. [Google Scholar] [CrossRef]
- Lallier, E.; Brevignon, M.; Leho, J. Efficient second-harmonic generation of a CO2 laser with a quasi-phase-matched GaAs crystal. Opt. Lett. 1998, 23, 1511–1513. [Google Scholar] [CrossRef]
- Lin, A.; Fenner, D.B.; Termkoa, K.; Allen, M.; Moulton, P.; Lynch, C.; Bliss, D.F.; Goodhue, W.D. Wafer-bonding of GaP, Wafer-fused orientation-patterned GaAs. Proc. SPIE 2008, 6875. [Google Scholar]
- Vangala, S.R.; Tassev, V.L.; Kimani, M.; Snure, M.; Peterson, R. Development of thick orientation patterned GaP for frequency conversion in the Mid IR and THz region. In Proceedings of the 2014 39th International Conference on Infrared, Millimeter, and Terahertz waves (IRMMW-THz), Tucson, AZ, USA, 14–19 September 2014. [Google Scholar]
- Lin, A.C. All-Epitaxial Orientation-Patterned III-V Semiconductors for Nonlinear Optics. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2012. [Google Scholar]
- Hum, D.S.; Fejer, M.M. Quasi-phase-matching. Comptes Rendus Phys. 2007, 8, 180–198. [Google Scholar] [CrossRef]
- Boyd, R.W. Nonlinear Optics, 3rd ed.; Elsevier, Academic press: San Diego, CA, USA, 2008. [Google Scholar]
- Shen, Y.R. The Principles of Nonlinear Optics; Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Christodoulides, D.N.; Khoo, I.C.; Salamo, J.W.; Stegeman, J.I.; Van Stryland, E.W. Nonlinear refraction and absorption: mechanisms and magnitudes. Adv. Optics Photonics 2010, 2, 60–200. [Google Scholar] [CrossRef]
- Myers, L.E.; Eckardt, C.R.; Fejer, M.M.; Bayer, R.L.; Bosenberg, W.R.; Pierce, J.W. Quasi-phase-matched optical parametric oscillators in bulk periodically poled LiNb03. J. Opt. Soc. Am. 1995, 12, 2102–2116. [Google Scholar] [CrossRef]
- Madarasz, F.L.; Dimmock, J.O.; Dietz, N.; Bachmann, K.J. Sellmeier parameters for ZnGaP2 and Ga. J. Appl. Phys. 2000, 87, 1564–1565. [Google Scholar] [CrossRef]
- Skauli, T.; Kuo, P.S.; Vodopyanov, K.L.; Pinguet, T.J.; Levi, O.; Eyres, L.A.; Harris, J.S.; Fejer, M.M.; Gerard, B.; Becouarn, L.; et al. Improved dispersion relations for GaAs and applications to nonlinear optics. J. Appl. Phys. 2003, 94, 6447–6454. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Bloembergen, N.; Ducuing, J.; Pershan, P.S. Interactions between light waves in a nonlinear dielectric. Phys. Rev. 1962, 127, 1918–1939. [Google Scholar] [CrossRef]
- Simon, J.; Schulte, K.L.; Horowitz, K.A.; Remo, T.; Young, D.L.; Ptak, A.J. III-V-based optoelectronics with low-cost dynamic hydride vapor phase epitaxy. Crystals 2019, 9, 3. [Google Scholar] [CrossRef]
- Tassev, V.; Snure, M.; Vangala, S.; Kimani, M.; Peterson, R.; Schunemann, P. Growth and study of nonlinear optical materials for frequency conversion devices with applications in defense and security. Proc. SPIE 2014, 9253. [Google Scholar]
- Gil, E.; Andre, Y.; Cadoret, R.; Trassoudaine, A. Hydride vapor phase epitaxy for current III-V and nitride semiconductor compound issues. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–93. [Google Scholar]
- Gruter, K.; Deschler, M.; Jurgensen, H.; Beccard, R.; Balk, P. Deposition of high quality GaAs films at fast rates in the LP-CVD system. J. Cryst. Growth 1989, 94, 607. [Google Scholar] [CrossRef]
- Putz, E.; Veuhoff, E.; Bachem, K.H.; Balk, P.; Luth, H. Epitaxial nucleation and growth kinetics of gallium arsenide in a chloride transport system. J. Electrochem. Soc. 1981, 128, 2202. [Google Scholar]
- Madelung, O. Semiconductors: Data Handbook, 3rd ed.; Springer: Berlin, Germany; New York, USA; Tokyo, Japan, 2003. [Google Scholar]
- Weber, M.J. CRC Handbook of Laser Science and Technology, Volume III Optical Materials: Part. 1; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Vangala, S.; Peterson, R.; Snure, M.; Tassev, V. Development of orientation-patterned GaP grown on foreign substrates for QPM frequency conversion devices. Proc. SPIE 2017, 10088. [Google Scholar]
- Vangala, S.; Kimani, M.; Peterson, R.; Sites, R.; Snure, M.; Tassev, V. Thick orientation-patterned growth of GaP on wafer-fused GaAs templates by hydride vapor phase epitaxy for frequency conversion. Opt. Mater. 2016, 60, 62–66. [Google Scholar] [CrossRef]
- Tassev, V.L.; Peterson, R.D. Heteroepitaxial Growth of Orientation-Patterned Materials on Orientation-Patterned Substrates. U.S. Patent 9,777,402, 3 October 2017. [Google Scholar]
- Tassev, V.L.; Vangala, S.R.; Tomich, D.; Dass, C. Heteroepitaxy of nonlinear optical materials for frequency conversion in the mid and longwave IR. J. Cryst. Growth. J. Cryst. Growth 2019, 522, 210–213. [Google Scholar] [CrossRef]
- Ru, Q.; Lee, N.; Chen, X.; Zhong, K.; Tsoy, G.; Miro, M.; Vasilyev, S.; Mirov, S.B.; Vodopyanov, K.L. Optical parametric oscillation in a random polycrystalline medium. Optics 2017, 4, 617–618. [Google Scholar]
- Tassev, V. Optimized thick heteroepitaxial growth of semiconductors with in-situ substrate pretreatment. Patent 1,620,144,6, 27 November 2018. [Google Scholar]
- Vangala, S.R.; Brinegar, D.; Tassev, V.L.; Snure, M. Thick hydride vapor phase epitaxial growth of ZnSe on GaAs (100) vicinal and orientation patterned substrates. J. Cryst. Growth 2019, 522, 230–234. [Google Scholar] [CrossRef]
- Kleiman, J.; Park, R.M.; Qadri, S.B. Determination of the onset of plastic deformation in ZnSe layers grown on GaAs by molecular-beam epitaxy. J. Appl. Phys. 1986, 61, 2067. [Google Scholar] [CrossRef]
- Gaines, J.M.; Petruzzello, J.; Greenberg, B. Structural properties of ZnSe films grown by migration enhanced epitaxy. J. Appl. Phys. 1993, 73, 2835. [Google Scholar] [CrossRef]
- Singh, N.B.; Kanner, G.S.; Berghmans, A.; Kahler, D.; Lin, A.; Wagner, B.; Kelley, S.P.; Knuteson, D.J.; Holmstrom, R.; Schepler, K.L.; et al. Characteristics of thick ZnSe films on quasi-phase-matched (QPM) GaAs substrates. J. Cryst. Growth 2010, 312, 1142–1145. [Google Scholar] [CrossRef]
- Kanner, G.S.; Marable, M.L.; Singh, N.B.; Bergham, A.; Kahler, D.; Wagner, B.; Lin, A.; Fejer, M.M.; Harris, J.S.; Schepler, K.L. Optical probes of orientation-patterned ZnSe quasi-phase-matched devices. Opt. Eng. 2009, 48, 114201. [Google Scholar] [CrossRef]
- Schunemann, P.G.; Zawilski, K.T. Vapor transport growth of single crystal zinc selenide. Photonics West 2019. In Proceedings of the Nonlinear Frequency Generation-Conversion, San Francisco, CA, USA, 4 March 2019. [Google Scholar]
- Youbao, N.; Haixin, W.; Huang, C.; Mao, M.; Wang, Z.; Cheng, X. Growth and quality of gallium selenide (GaSe) crystals. J. Cryst. Growth 2013, 381, 10–14. [Google Scholar] [Green Version]
- Li, X.; Lin, M.; Puretzki, A.A.; Idrodo, J.C.; Ma’, C.; Chi, M.; Yoon, M.; Rouleau, C.; Kravchenko, I.I.; Geohegan, D.B.; et al. Controlled vapor phase growth of single crystalline two-dimensional GaSe crystals with High photo-response. Sci. Rep. 2014, 5497. [Google Scholar]
- Vinh, L.T.; Eddrief, M.; Sebenne, C.; Sacuto, A.; Balkanski, M. Heteroepitaxy of GaSe layered semiconductor compound on Si (111) 7x7 substrate: A Van der Waals epitaxy. J. Cryst. Growth 1994, 135. [Google Scholar] [CrossRef]
- Kojima, N.; Sato, K.; Yamada, A.; Konagai, M.; Takahashi, K. Epitaxial growth of GaSe films by molecular beam epitaxy on GaAs (111), (001) and (112) substrates. Jap. J. Appl. Phys. 1994, 33, L1482. [Google Scholar] [CrossRef]
- Edward, G.; Gillan, E.G.; Barron, A.R. Chemical vapor deposition of hexagonal gallium selenide and telluride films from cubane precursors: understanding the envelope of molecular control. Chem. Mater. 1997, 9, 3037–3048. [Google Scholar]
- Materials Project. Available online: https://materialsproject.org/materials/mp-1943/ (accessed on 18 June 2019).
- Ng, T.L.; Maung, N.; Fan, G.; Pole, I.B.; Williams, J.O.; Wright, A.C.; Foster, D.F.; Cole-Hamilton, D.J. Metal-organic vapor phase epitaxial growth of cubic Gallium selenide, Ga2Se3. Chem. Vapor Dep. 1996, 2, 185–189. [Google Scholar] [CrossRef]
- Dong, L.; Schnitker, J.; Smith, R.; Srolovitz, D. Stress relaxation and misfit dislocations nucleation in the growth of misfitting films: A molecular dynamics simulation Study. J. App. Phys. 1998, 83, 217–227. [Google Scholar] [CrossRef]
- Prieto, J.E.; Markov, I. Stranski-Krastanov mechanism of growth and the effect of misfit sign on quantum dots nucleation. Surf. Sci. 2017, 664, 172–184. [Google Scholar] [CrossRef]
- Simmonds, P.J.; Lee, M.L. Tensile-strained growth on low-index GaAs. Appl. Phys. Lett. 2011, 99, 123111. [Google Scholar] [CrossRef]
- Budevski, E.; Bostanov, V.; Titanov, T. Electro-crystallization and the electrolytic deposition mechanism for silver. In Growth of Crystals; Consultants Bureau: New York, NY, USA, 1976; p. 231. [Google Scholar]
- Natarajan, S.; Devi, N.R.; Martin, S.D.; Dhas, B.; Athimoolam, S. Crystal growth and structure of Lmethionine L-methioninium hydrogen maleate—A new NLO material. Sci. Technol. Adv. Mater. 2008, 9, 025012. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, V.; Manohar, S.; Nagalakshmi, R. Semiorganic nonlinear optical l-lysine sulphate growth and characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Soldera, A.; Monterrat, E. Mid-infrared optical properties of a polymer film: Comparison between classical molecular simulations, spectrometry, and ellipsometry techniques. Polymer 2002, 43, 6027–6035. [Google Scholar] [CrossRef]
- Kula, P.; Herman, J.; Harmata, P.; Czerwiński, M. NIR and MWIR transparent liquid crystals. In Proceedings of the 39th International Conference IRMMW-THz, Tucson, AZ, USA, 9–14 September 2014. [Google Scholar]
- Skordeva, E. Photoelectret properties and high-field polarization in chalcogenide glasses and thin films. J. Optoelectron. Adv. Mater. 2001, 3, 437–442. [Google Scholar]
- Jazbinsek, M.; Mutter, L.; Gunter, P. Photonic applications with the organic nonlinear optical crystal DAST. IEEE J. Select. Top. Quantum Electron. 2008, 14, 1298–1311. [Google Scholar] [CrossRef]
- Namnabat, S.; Griebel, J.; Pyun, J.; Norwood, R.; Dereniak, E. Sulfar copolymer for IR optics. SPIE Newsroom 2014, 1–3. [Google Scholar]
- Zhang, L.; Kang, T.; Zhao, F.; Wang, D.; Shen, C.; Wang, J. Growth and property investigations of two organic–Inorganic hybrid molecular crystals with high thermal stability: 4-Iodoanilinium perchlorate 18-grown-6 and 4-Iodoanilinium borofluorate 18-crown-6. Crystals 2019, 9, 207. [Google Scholar] [CrossRef]
- Peng, F.; Lee, Y.H.; Chen, H.; Li, Z.; Bostwick, A.E.; Twieg, R.J.; Wu, S.T. Low absorption chlorinated liquid crystals for IR applications. Opt. Mater. Express 2015, 5, 1281–1288. [Google Scholar] [CrossRef]
- Speck, J.S.; Brewer, M.A.; Beltz, G. Scaling laws for the reduction of threading dislocation densities in homogeneous buffer layers. J. Appl. Phys. 1996, 80, 3808. [Google Scholar] [CrossRef]
- Ponce, F.A.; Bour, D.P. Nitride-based semiconductors for blue and green light-emitting devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Moustakas, T.D. The role of extended defects on the performance of optoelectronic devices in nitride semiconductors. Phys. Stat. Solidi 2013, 210, 169–174. [Google Scholar] [CrossRef]
- Poeppelmeier, K.R.; Rondinelli, J.M. Mismatched lattices patched up. Nat. Chem. 2016, 8, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Trampert, A.; Brandt, O.; Ploog, K.N. Crystal structure of group III Nitrides. Semicond. Semimet. 1997, 50, 167–192. [Google Scholar]
- O’Sullivan, M.; Hadermann, J.; Dyer, M.S.; Turner, S.; Alaria, J.; Abakumov, A.M.; Claridge, J.B.; Rosseinsky, M.J. Interface control by chemical and dimensional matching in an oxide heterostructure. Nat. Chem. 2016, 8, 347–353. [Google Scholar] [CrossRef] [PubMed]
































| Properties | GaAs | GaP |
|---|---|---|
| Transmission window (μm) | 1–16 [88] | 0.5–12 [88] |
| Nonlinear optical coefficient d14 (pm·aV−1; for GaP is for 1.064 μm) | 94 [89] | 71 [89] |
| Index of refraction n (allows wider gratings) | 3.34 [88] | 3.03 [88] |
| Thermal conductivity at 300 K (W·m−1·K−1) | 55 [88] | 110 [88] |
| Thermal expansion coefficient at 300 K (10−6·K−1) | 5.7 [88] | 4.6 [88] |
| Maximum temperature of usage (°C) | 460 [88] | 640 [88] |
| Band gap energy (eV) | 1.42 [88] | 2.26 [88] |
| 2PA coefficient β (cm/G−1·W−1) at 1.06 μm | 28 [9] | 0.02 [11] |
| Etch pits density (EPD; EPD·cm−2; data from wafer suppliers) | 1500–5000 | 8000–100000 |
| Price | GaAs | GaP |
| Price per 2-inch wafer in USD (data from wafer suppliers) | 80–90 | 585–685 |
| Heteroepitaxy | Mismatch | III–Gas | V–Gas (Hydride) | Chemistry Paths |
|---|---|---|---|---|
| GaP/GaAs | –3.574% | HCl | PH3 | 2Ga + 6HCl → 2GaCl3 + 3H2 → GaCl3 + PH3 → GaP + 3HCl |
| GaAsP/GaAs | < –3.574% | HCl | AsH3 + PH3 | 2Ga+ 6HCl → 2GaCl3 + 3H2 → GaCl3 + AsH3 + PH3 → GaAsP + 3HCl |
| GaAsP/GaP | < +3.574% | HCl | AsH3 + PH3 | 2Ga+ 6HCl → 2GaCl3 + 3H2 → GaCl3 + AsH3 + PH3 → GaAsP + 3HCl |
| ZnSe/GaAs | +0.238% | HCl | H2Se | Zn + 2HCl →ZnCl2 + H2 → ZnCl2 + H2Se → ZnSe + 2HCl |
| ZnSe/GaAs | +0.238% | H2 | H2Se | ZnCl2 + H2 → ZnCl2 + H2Se → ZnSe + 2HCl |
| α-GaSe/GaP | –0.607% | HCl | H2Se | 2Ga + 6HCl →2GaCl3 + 3H2 → 2GaCl3 + 2H2Se → 2GaSe + 4HCl + Cl2 |
| α-GaSe/GaAs | –4.181% | HCl | H2Se | 2Ga + 6HCl →2GaCl3 + 3H2 → 2GaCl3 + 2H2Se → 2GaSe + 4HCl + Cl2 |
| β-GaSe/GaN | +17.74% | HCl | H2Se | 2Ga + 6HCl →2GaCl3 + 3H2 → 2GaCl3 + 2H2Se → 2GaSe + 4HCl + Cl2 |
| ZnTe/GaSb | +0.083% | HCl | H2Te | Zn + 2HCl →ZnCl2 + H2 → ZnCl2 + H2Te → ZnTe + 2HCl |
| Properties | GaAs | GaP | ZnSe | GaSe | ZnTe |
|---|---|---|---|---|---|
| Optical | |||||
| Transmission window (μm) | 1–16 [88] | 0.55–12 [88] | 0.6–18 [88] | 0.6–19 [88] | 0.7–20 [88] |
| Band gap energy (eV) | 1.42 [88] | 2.26 [88] | 2.82 [88] | 2.1 [88] | 2.26 [88] |
| Index of refraction n | 3.34 [88] | 3.03 [88] | 2.67 [88] | 2.93 [88] | 3.56 [88] |
| NLO coefficient d14 at 1.064 μm (pm·V−1) | 94 [89] | 71 [89] | 27 [89] | 86 [89] | 90 [89] |
| 2PA coefficient (cm/G−1·W−1) at 1.06 μm | 28 [9] | 0.02 [11] | 5.5 [89] | 6 [89] | 4.5 [89] |
| Thermal | |||||
| Thermal conductivity at 300 K (W·m−1·K−1) | 55 [88] | 110 [88] | 18 [88] | 5.0 [88] | 10.8 [88] |
| Thermal expansion coeff. at 300 K (10−6·K−1) | 5.7 [88] | 4.6 [88] | 7.1 [88] | 8.9 [88] | 8.19 [88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassev, V.L.; Vangala, S.R. Thick Hydride Vapor Phase Heteroepitaxy: A Novel Approach to Growth of Nonlinear Optical Materials. Crystals 2019, 9, 393. https://doi.org/10.3390/cryst9080393
Tassev VL, Vangala SR. Thick Hydride Vapor Phase Heteroepitaxy: A Novel Approach to Growth of Nonlinear Optical Materials. Crystals. 2019; 9(8):393. https://doi.org/10.3390/cryst9080393
Chicago/Turabian StyleTassev, Vladimir L., and Shivashankar R. Vangala. 2019. "Thick Hydride Vapor Phase Heteroepitaxy: A Novel Approach to Growth of Nonlinear Optical Materials" Crystals 9, no. 8: 393. https://doi.org/10.3390/cryst9080393