Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Techniques
2.4. Biofilm Development and Bacterial Growth
3. Results and Discussion
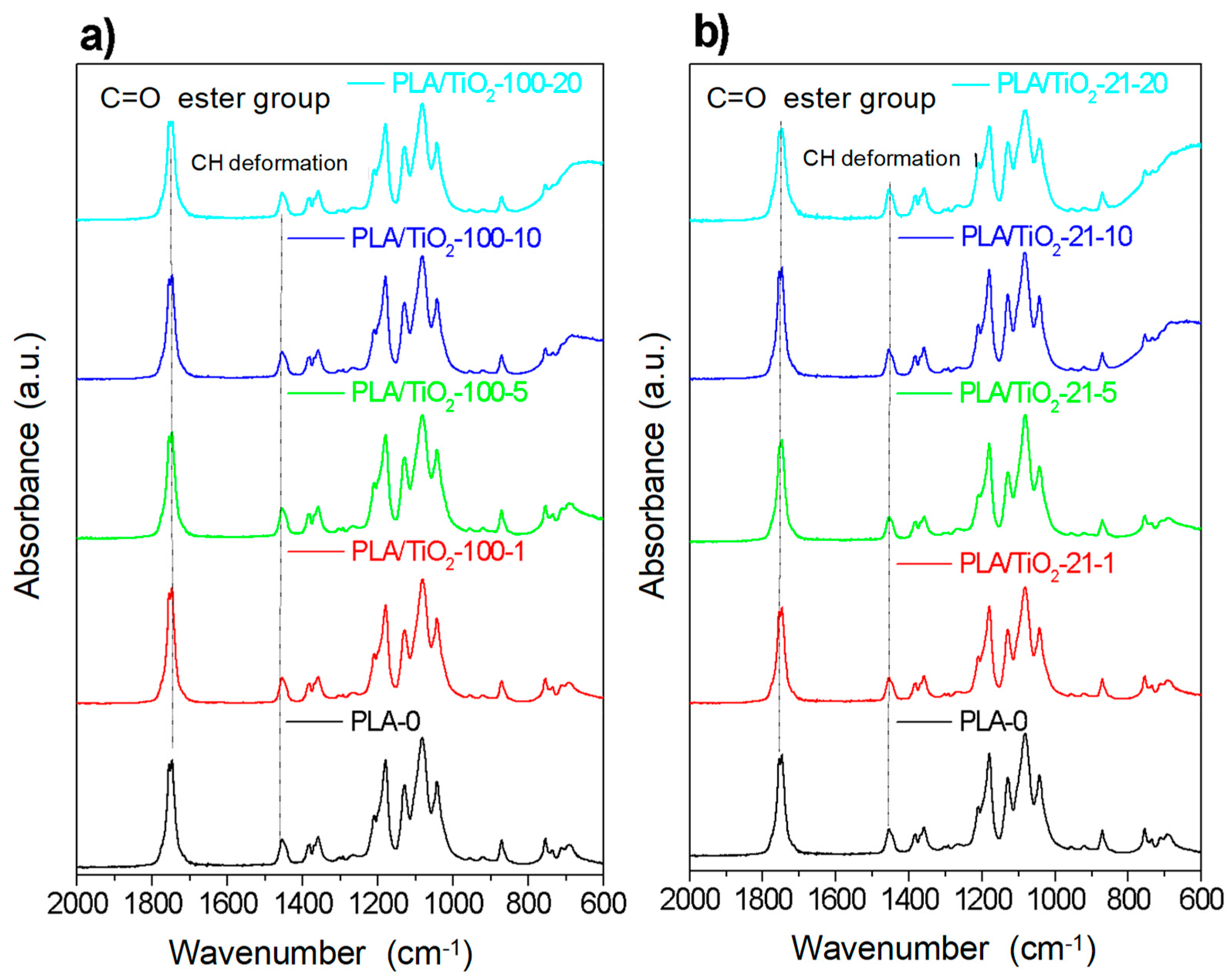
3.1. Structural Characterization
3.2. Thermal Characterization
3.3. Antimicrobial Behaviour
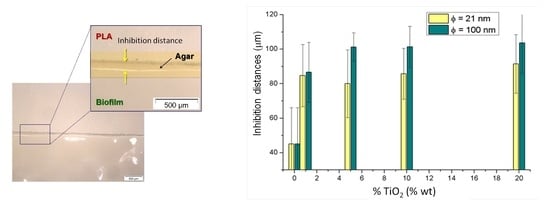
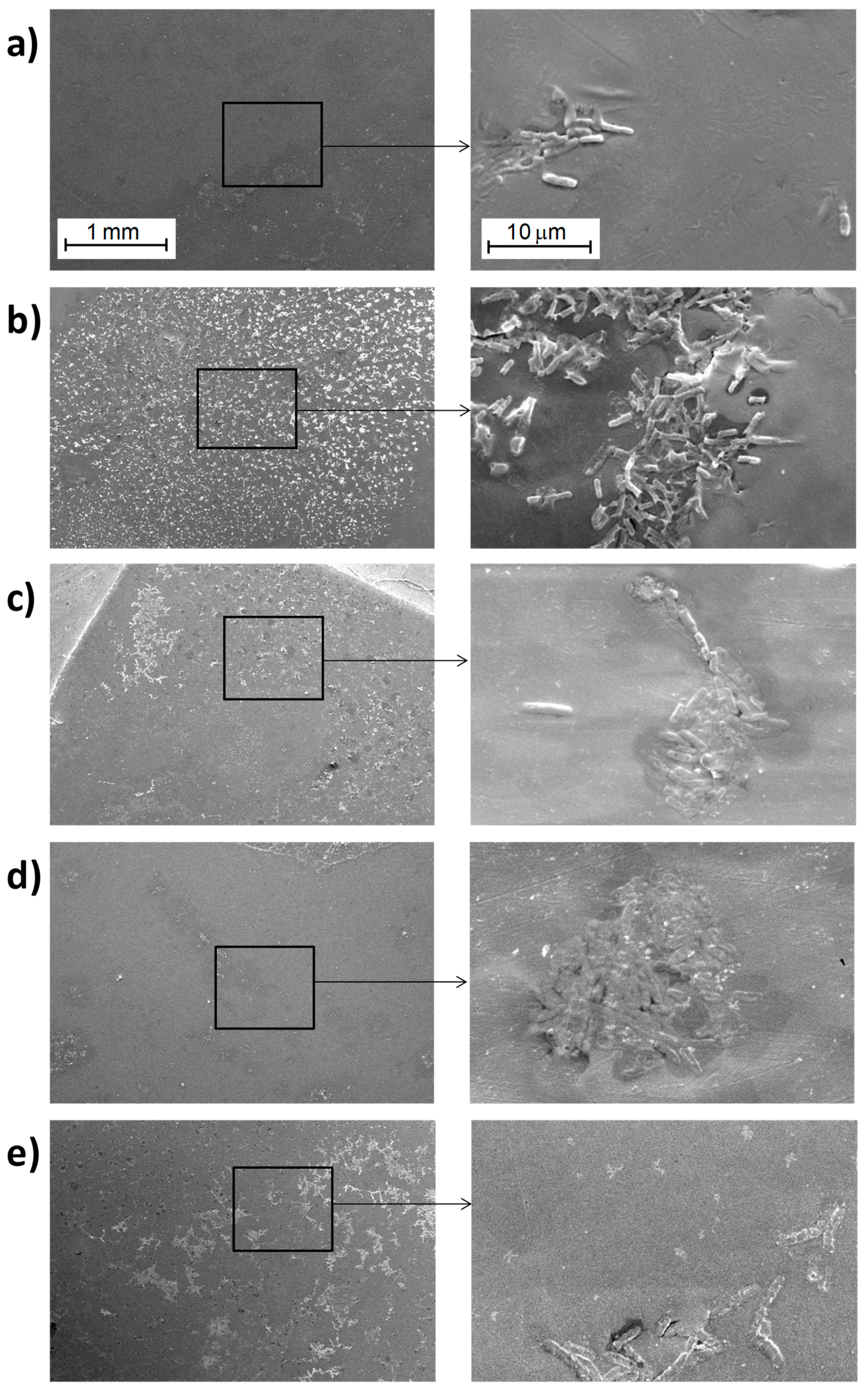
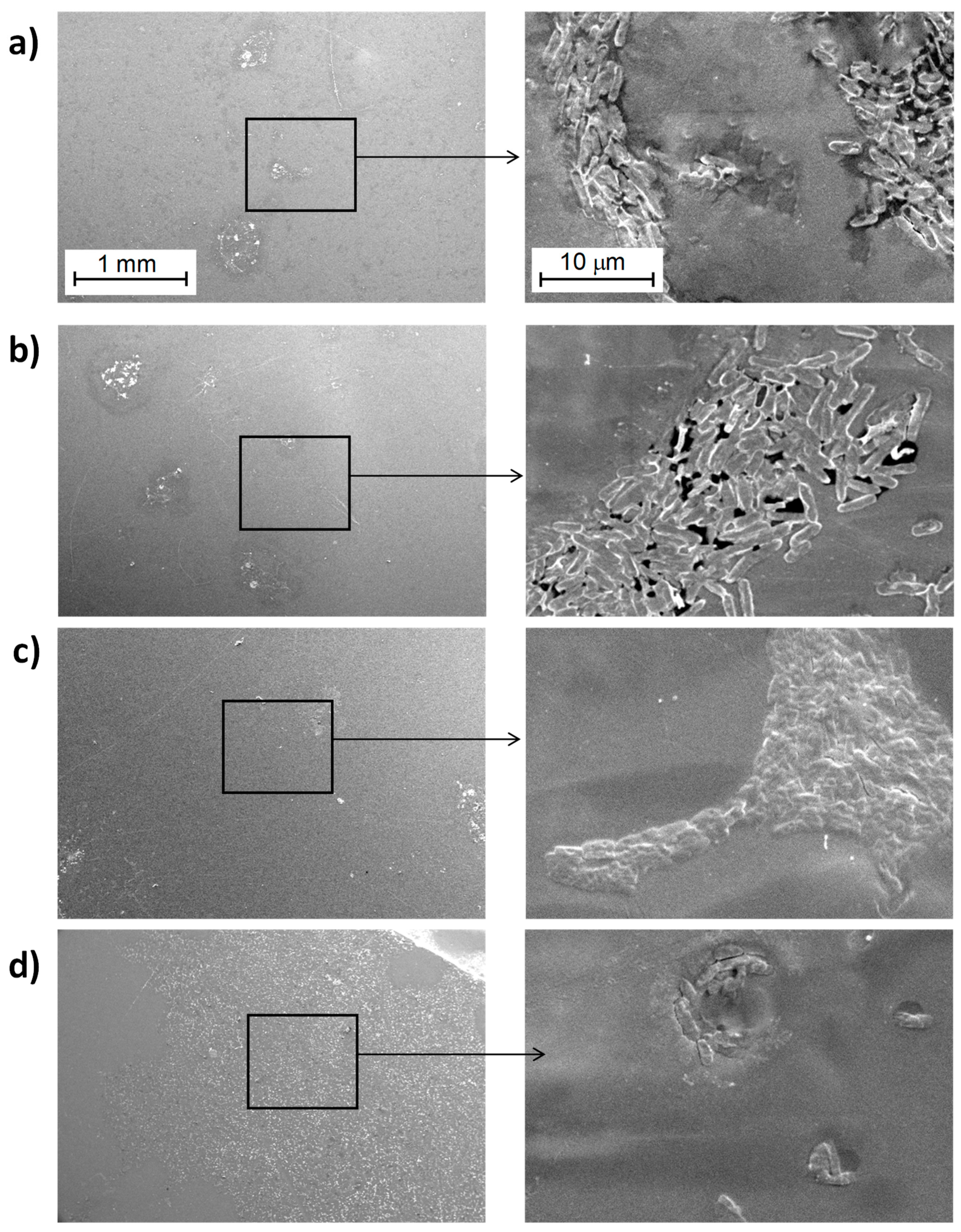
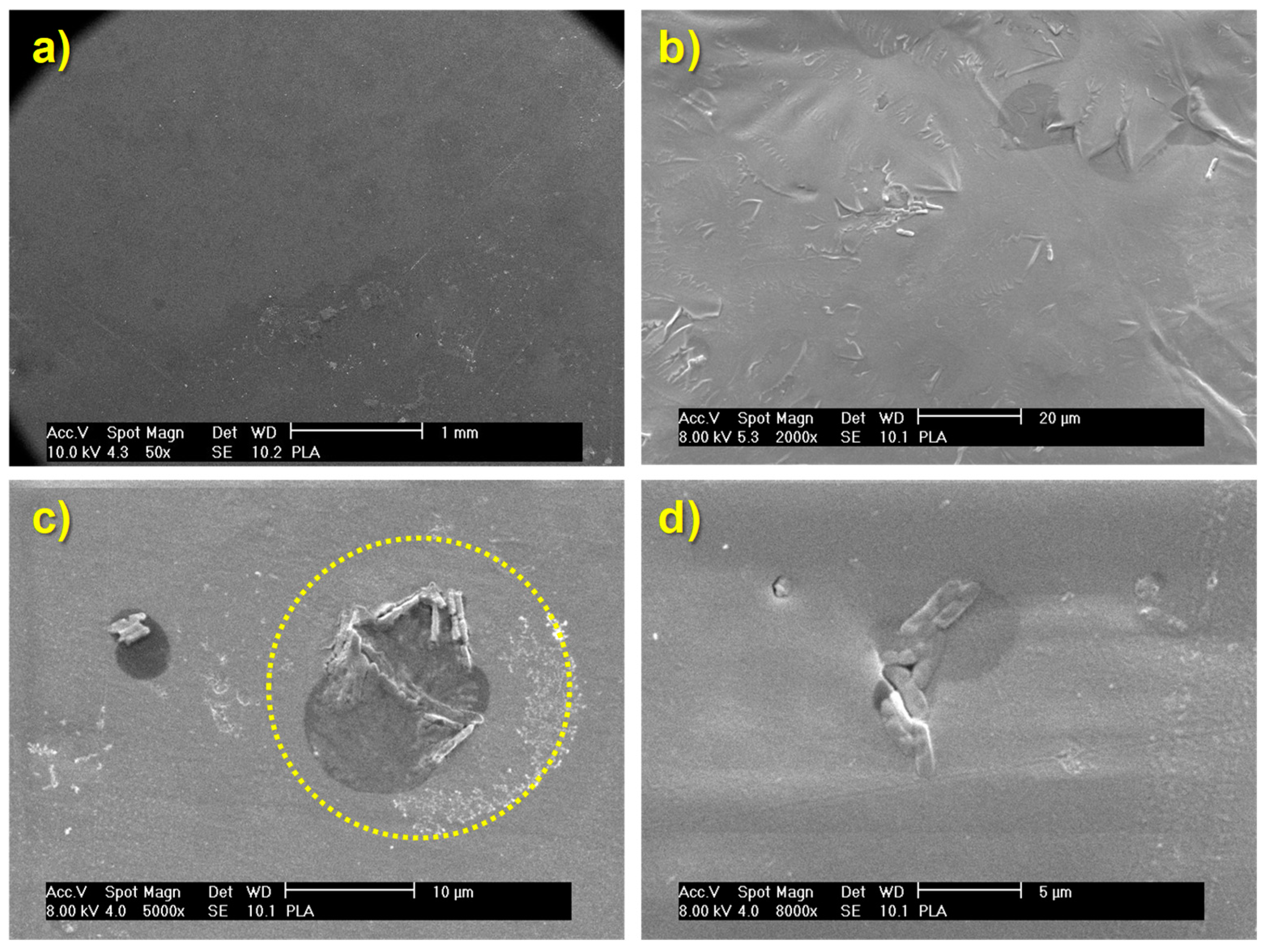
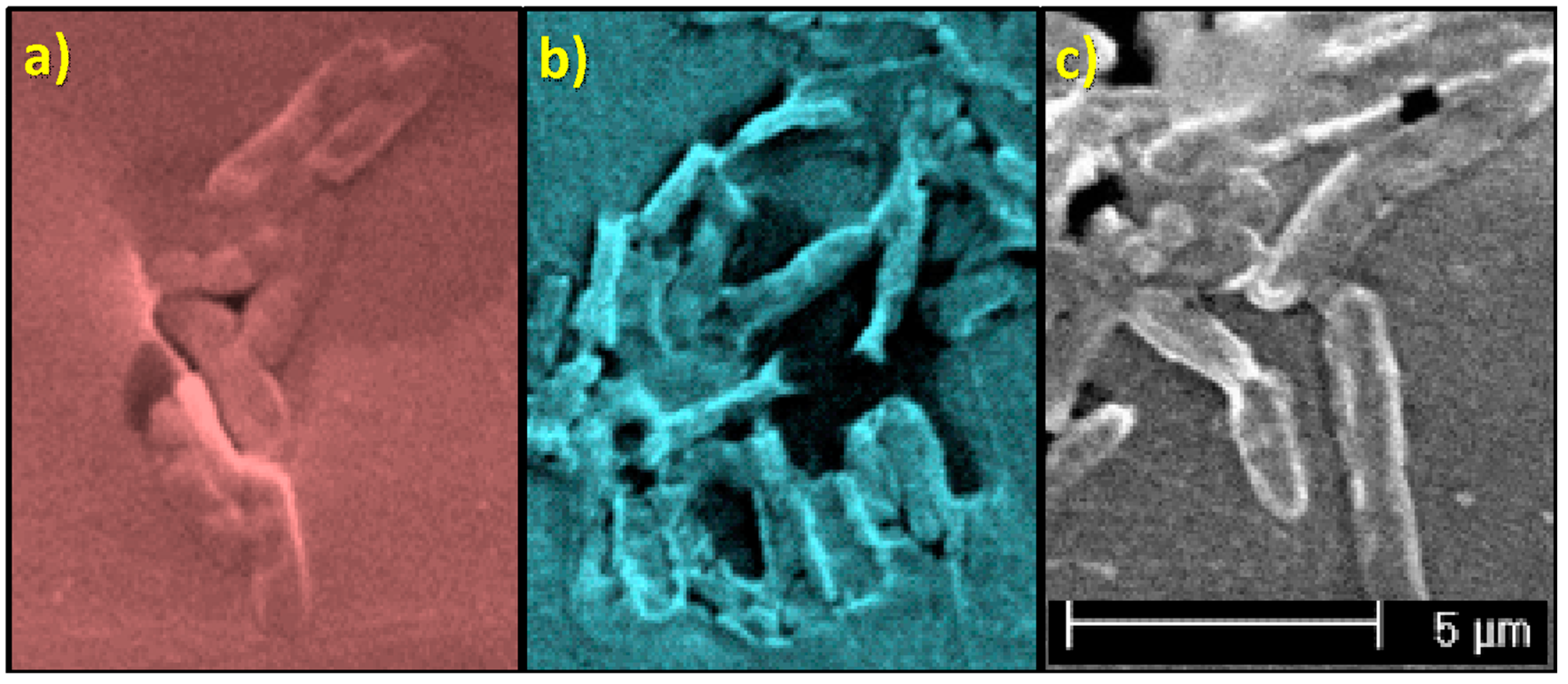
3.3.1. Study of Biofilm Development on the Surface of the Materials
3.3.2. Kirby-Bauer Diffusion Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Buzarovska, A.; Grozdanov, A. Biodegradable poly(L-lactic acid)/TiO2 nanocomposites: Thermal properties and degradation. J. Appl. Polym. Sci. 2012, 123, 2187–2193. [Google Scholar] [CrossRef]
- Nieto Pozo, I.; Olmos, D.; Orgaz, B.; Božanić, D.K.; González-Benito, J. Titania nanoparticles prevent development of Pseudomonas fluorescens biofilms on polystyrene surfaces. Mater. Lett. 2014, 127, 1–3. [Google Scholar] [CrossRef]
- Bahloul, W.; Mélis, F.; Bounor-Legaré, V.; Cassagnau, P. Structural characterisation and antibacterial activity of PP/TiO2 nanocomposites prepared by an in situ sol-gel method. Mater. Chem. Phys. 2012, 134, 399–406. [Google Scholar] [CrossRef]
- Robertson, J.M.C.; Robertson, P.K.J.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A Chem. 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Trapalis, C.C.; Keivanidis, P.; Kordas, G.; Zaharescu, M.; Crisan, M.; Szatvanyi, A.; Gartner, M. TiO2(Fe3+) nanostructured thin films with antibacterial properties. Thin Solid Films 2003, 433, 186–190. [Google Scholar] [CrossRef]
- Arroyo, J.M.; Olmos, D.; Orgaz, B.; Puga, C.H.; San José, C.; González-Benito, J. Effect of the presence of titania nanoparticles in the development of Pseudomonas fluorescens biofilms on LDPE. RSC Adv. 2014, 4, 51451–51458. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2(anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- De Falco, G.; Porta, A.; Petrone, A.M.; Del Gaudio, P.; El Hassanin, A.; Commodo, M.; Minutolo, P.; Squillace, A.; D’Anna, A. Antimicrobial activity of flame-synthesized nano-TiO2 coatings. Environ. Sci. Nano 2017, 4, 1095–1107. [Google Scholar] [CrossRef]
- Wang, R.M.; Wang, B.Y.; He, Y.F.; Lv, W.H.; Wang, J.F. Preparation of composited Nano-TiO2 and its application on antimicrobial and self-cleaning coatings. Polym. Adv. Technol. 2010, 21, 331–336. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, L.; Lv, J. Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym. Polym. Compos. 2013, 21, 449–456. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. (Nat. Sci.) 2008, 42, 357–361. [Google Scholar]
- González-Benito, J.; Castillo, E.; Caldito, J.F. Coefficient of thermal expansion of TiO2 filled EVA based nanocomposites. A new insight about the influence of filler particle size in composites. Eur. Polym. J. 2013, 49, 1747–1752. [Google Scholar] [CrossRef]
- González, E.A.S.; Teno, J.; González-Benito, J.; Olmos, D. Accurate Evaluation of Dynamics and Specific Interactions in PLA/TiO2 Nanocomposites. Sci. J. Mol. Phys. 2017, 1, 1–13. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 36, 49–52. [Google Scholar] [CrossRef]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M. Single-disk antibiotic-sensitivity testing of staphylococci. AMA Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Olmos, D.; Pontes-Quero, G.; Corral, A.; González-Gaitano, G.; González-Benito, J. Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries. Nanomaterials 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Buasri, A.; Chaiyut, N.; Kristsanakun, C.; Phatkun, C.; Khunsri, T. Preparation and properties of nanocomposites based ond poly(lactic acid) and modified TiO2. Adv. Mater. Res. 2012, 463–464, 519–522. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Xu, D.Y.; Wang, Y.Z. Preparation and characterization of poly(lactic acid)-grafted TiO2 nanoparticles with improved dispersions. Appl. Surf. Sci. 2009, 255, 6795–6801. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar] [CrossRef]
- Pillin, I.; Montrelay, N.; Bourmaud, A.; Grohens, Y. Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polym. Degrad. Stab. 2008, 93, 321–328. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Luo, Y.B.; Li, W.D.; Wang, X.L.; Xu, D.Y.; Wang, Y.Z. Preparation and properties of nanocomposites based on poly(lactic acid) and functionalized TiO2. Acta Mater. 2009, 57, 3182–3191. [Google Scholar] [CrossRef]
- Wang, W.W.; Man, C.Z.; Zhang, C.M.; Jiang, L.; Dan, Y.; Nguyen, T.P. Stability of poly(l-lactide)/TiO2 nanocomposite thin films under UV irradiation at 254 nm. Polym. Degrad. Stab. 2013. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, Z.; Yan, J.; Qiang, L.; Ru, X.; Liu, F.; Ding, D.; Li, J. Preparation and characterization of TiO2 nanofibers via using polylactic acid as template. J. Appl. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Czaczyk, K.; Myszka, K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A. review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Liang, Z.; Li, W.; Yang, S.; Du, P. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge. Chemosphere 2010, 81, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.J.; Fang, F.; Xie, W.M.; Sun, M.; Sheng, G.P.; Li, W.H.; Yu, H.Q. Characterization of extracellular polymeric substances produced by mixed microorganisms in activated sludge with gel-permeating chromatography, excitation-emission matrix fluorescence spectroscopy measurement and kinetic modeling. Water Res. 2009, 43, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, L.V.; Kiwi, J.; Nikandrov, V.V. TiO2 nanoparticles suppress Escherichia coli cell division in the absence of UV irradiation in acidic conditions. Colloids Surf. Biointerfaces 2012, 97, 240–247. [Google Scholar] [CrossRef]

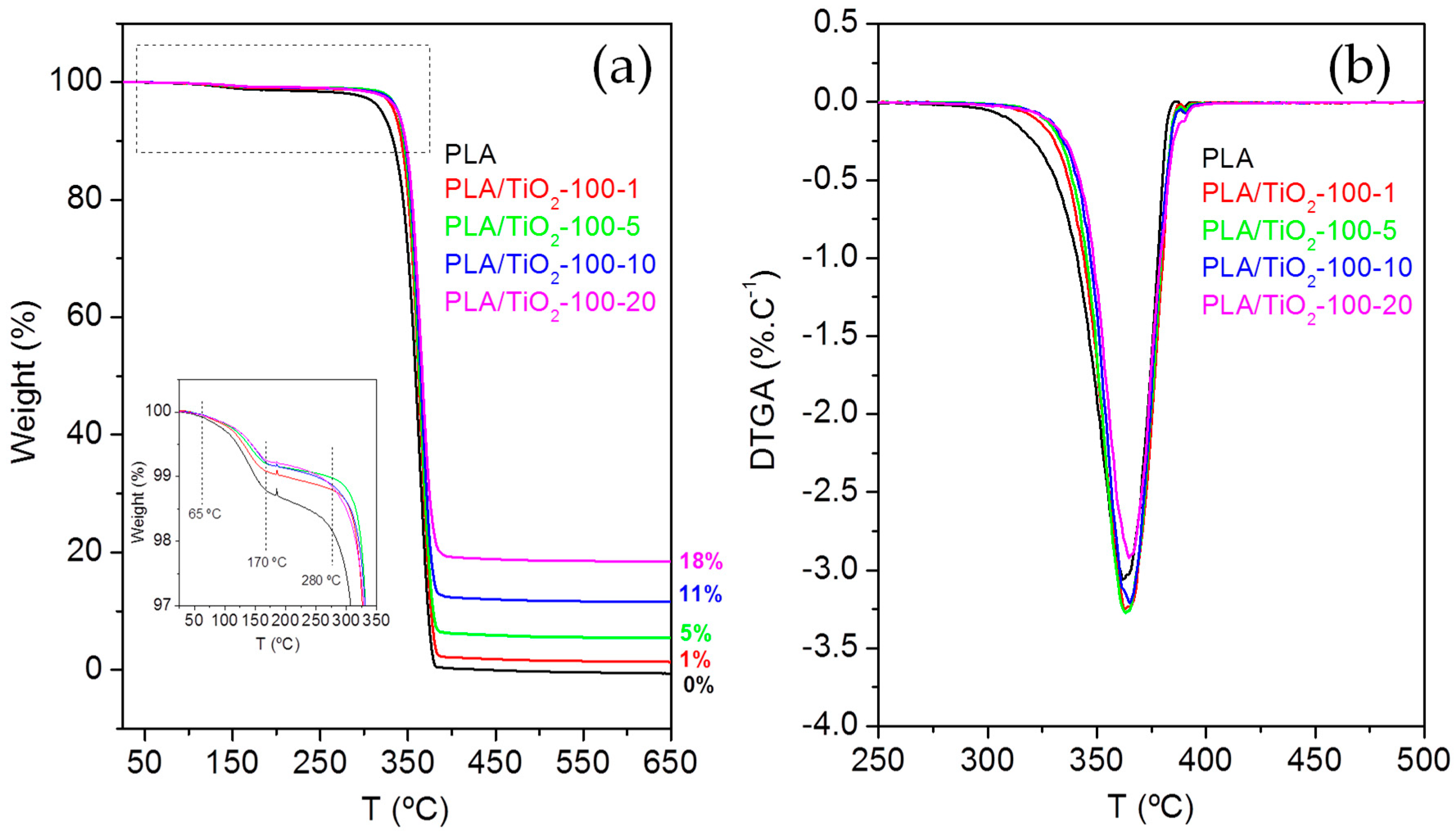


| Sample | T5 (°C) | T95 (°C) | Tp (°C) |
|---|---|---|---|
| PLA-0 | 331.9 | 382.9 | 361.8 |
| PLA/TiO2-100-1 | 337.4 | 385.9 | 363.0 |
| PLA/TiO2-100-5 | 338.9 | 385.1 | 363.1 |
| PLA/TiO2-100-10 | 341.2 | 384.6 | 364.9 |
| PLA/TiO2-100-20 | 341.2 | 385.9 | 364.7 |
| PLA/TiO2-21-1 | 335.8 | 383.3 | 359.5 |
| PLA/TiO2-21-5 | 340.7 | 385.2 | 364.0 |
| PLA/TiO2-21-10 | 341.9 | 385.1 | 366.1 |
| PLA/TiO2-21-20 | 338.5 | 390.0 | 366.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura González, E.A.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers 2018, 10, 1365. https://doi.org/10.3390/polym10121365
Segura González EA, Olmos D, Lorente MÁ, Vélaz I, González-Benito J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers. 2018; 10(12):1365. https://doi.org/10.3390/polym10121365
Chicago/Turabian StyleSegura González, Edwin A., Dania Olmos, Miguel Ángel Lorente, Itziar Vélaz, and Javier González-Benito. 2018. "Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging" Polymers 10, no. 12: 1365. https://doi.org/10.3390/polym10121365