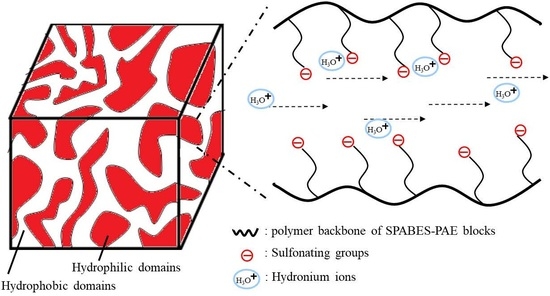
1. Introduction
Polymer electrolyte fuel cells (PEFCs) are attracting immense attention as alternative power sources for portable and stationary electronics as they allow H
2 and O
2 gases to generate energy with low/zero production of poisonous gases. Among the working components in proton exchange membrane fuel cells (PEMFCs), the proton exchange membrane (PEM) is a pivotal module, which selectively transfers the protons from the anode to the cathode. Commercial perfluorinated ionomers, such as Nafion and Flemion, are being extensively exploited as PEMs because of their excellent electrochemical properties, elevated proton conductivity and good mechanical modulus [
1,
2,
3,
4]. However, the decrease of proton conductivity at high temperature under low humidity, high methanol crossover and low glass transition temperature are the inevitable confines of perfluorosulfonic acid (PFSA) membranes that complicate the practical PEMFC operation. Accordingly, wide research efforts and activities in the fabrication of alternative PEMs are thriving.
Aromatic hydrocarbon polymers, such as sulfonated polyketones (SPKs) [
5], sulfonated polyimides (SPIs) [
6], sulfonated poly(arylene sulfone)s (SPASs) [
7,
8], sulfonated poly(arylene ether sulfone)s (SPAESs) [
9] are highlighted for their exclusive attributes such as low cost, high mechanical modulus resulting from their extended polymer chains, and flexible proton conductivity due to their adjustable degree of sulfonation (DS). The direct sulfonation method has some advantage such as avoiding cross-linking, other side reactions, and also providing good mechanical properties. Recently, investigators have attempted to improve the performance of proton conductivity through controlled morphology using directly sulfonated monomers rather than using a post sulfonation method. Therefore, the exploitation of aforementioned polymers for PEM preparation is flourishing. However, the polymeric membranes are susceptible to distortion due to volumetric changes caused by the water drift in the membranes. Such type of distortion can lead to increased pressure between the bipolar plates and membrane electrode assembly (MEA), resulting in the formation of crack, pinholes and perforations in the membrane. On the other side, chemical degradation of polymeric chains caused by the hydroxyl (·OH) and hydroperoxyl (·OOH) radical attacks in the cathode side, further exacerbate the cracks in the membranes. Altogether, these issues drastically decline the overall performance of PEMFC. It has been shown that incorporation of fluorinated segments into the polymer chains is an ultimate strategy in improving the mechanical modulus, chemical stability, and fuel barrier properties of PEMs, which enhance the membrane durability during PEMFC operation [
10,
11,
12].
Among the methods designed to solve the drawbacks of aromatic hydrocarbon membranes, block copolymers having controlled morphology and a similar ion exchange capacity (IEC) value showed not only excellent mechanical behaviors but also high ionic conductivity when compared with the random copolymer.
Numerous studies related to the characterization of block copolymers have continuously improved the performance of electrolyte membranes applicable to PEM. Furthermore, the increase in repeat units of hydrophilic segments in the polymer affects the IEC value and effectively governs the aggregation of ion clusters. In another report, McGrath et al. [
13] designed and synthesized multi-block copolymers containing sulfonated and fluorinated segments. They reported that the membranes have good thermomechanical stabilities and high ion conductivity in relation to commercial Nafion membrane under low humidity condition. Although the synthesis of block copolymer with controlled morphology has been found to be the most suitable strategy for producing a good performance of a fuel cell, still it has drawbacks such as difficult in controlling the molecular weight and limited application to commercialization.
Therefore, sulfonated bis[(4-chlorophenyl)sulfonyl]-biphenyl (BCPSBP) containing thiophene moiety as a hydrophilic oligomer can form steric hindrance, minimize interactions with radicals, and improve mechanical/thermal stabilities [
14]. In addition, block copolymer containing hydrophobic oligomer with perfluorinated structure and hydrophilic oligomer is deduced as a strategy for the production of ideal PEM through lowering the volume change by water drift.
In line with these facts, the sulfonated poly(arylene biphenylether sulfone)-poly(arylene ether) (SPABES-PAE) block copolymers in di- or tri-block structure were designed in order to improve membrane properties, and these copolymers were investigated for their comparison. Initially, sulfonated SPABES-PAE block copolymers were synthesized with SPABES oligomer as the hydrophilic part and PAE oligomer as the hydrophobic part via direct polymerization reaction. The chemical structure of prepared block copolymers was analyzed using 1H nuclear magnetic resonance (1H NMR), Fourier transform infrared spectroscopy (FT-IR), and gel permeation chromatography (GPC). The properties of SPABES-PAE membranes were widely studied in terms of water uptake, ion exchange capacity (IEC), thermal stability, oxidative stability, and proton conductivity.
2. Experimental
2.1. Materials
4,4’-(hexafluoroisopropylidene)diphenol (6F-BPA) and decafluorobiphenyl (DFBP) were received from Alfa Aesar. 4,4’-bis[(4-chlorophenyl)sulfonyl]-biphenyl (BCPSBP), anhydrous N,N’-dimethylacetamide (DMAc), anhydrous toluene, and dimethyl sulfoxide-d6 (DMSO-d6) were purchased from Sigma–Aldrich (Saint Louis, MI, USA). Others solvents were obtained from commercial companies.
2.2. Synthesis of Polymers
2.2.1. Synthesis of Sulfonated Poly(Arylene Biphenylehter Sulfone), Poly(Arylene Ether), and Poly(Arylene Biphenylether Sulfone)
We have prepared a di-sulfonated BCPSBP monomer (sBCPSBPms) in meta positions to the sulfone groups, and the synthetic steps of sBCPSBPms was followed from a previously reported paper [
14,
15]. The sulfonated poly(arylene ether biphenyl sulfone) (SPAEBS) was synthesized by direct nucleophilic substitution polymerization using sBCPSBPms and 6F-BPA (
Scheme S1a). In a typical polymerization, sBCPSBPms (2.00 g, 2.60 mmol), 6F-BPA (1.31 g, 2.87 mmol), K
2CO
3 (1.19 g, 5.73 mmol), 30 mL of DMAc, and 15 mL of toluene were put together into 100 mL round-bottomed flask equipped with a Dean–Stark trap under nitrogen gas. The solution was refluxed at 100 °C for 2 h, and then the solution was heated at 160 °C for 4 h to remove toluene. The solution was increased to 170 °C and kept for 24 h to complete the polymerization. The viscous solution was slowly poured into the deionized (DI) water/methanol (
v/
v = 2/5) to precipitate solid powder. The precipitated powder was washed with methanol several times, filtered, and dried at 70 °C for 24 h.
1H NMR (600 MHz, DMSO-
d6): 7.0–8.4 ppm; GPC (LiBr in dimethylformamide (DMF), refractive index (RI) detector)
Mn (number average molecular weight) = 20.9 kDa,
Mw (weight average molecular weight) = 41.3 kDa, polydispersity index (PDI) (
Mw/
Mn) = 2.0.
The fluorinated poly(arylene ether) (PAE) was prepared from 6F-BPA and DFBP (
Scheme S1b) as described below. A 100 mL round-bottomed flask equipped with condenser was charged with 6F-BPA (2.00 g, 5.95 mmol), DFBP (2.21 g, 6.50 mmol), K
2CO
3 (1.81 g, 1.31 mmol), and DMAc (25 mL) under nitrogen gas. The mixture was stirred for 2 h at 110 °C and then the reaction temperature was raised 145 °C. The polymerization reaction was maintained for 24 h. After the polymerization reaction was maintained for 24 h, the mixture was cooled at room temperature (RT). The viscous solution was directly poured into DI water/methanol (
v/
v = 2/1) to produce the polymer powder. The polymer powder was collected by washing with methanol several times, filtered, and dried at 60 °C for 12 h.
1H NMR (600 MHz, DMSO-
d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector)
Mn = 9.3 kDa,
Mw = 56.0 kDa, PDI (
Mw/
Mn) = 6.0.
The poly(arylene biphenyl ether sulfone) (PABES) oligomer was prepared using BCPSBP monomer following a similar method as described above. 1H NMR (600 MHz, DMSO-d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector) Mn = 15.8 kDa, Mw = 31.5 kDa, PDI (Mw/Mn) = 2.0.
2.2.2. Synthesis of Block Copolymers (PABES-PAE and SPABES-PAE)
The PABES-PAE block copolymer was synthesized by nucleophilic substitution reaction using PABES and PAE to compare chemical properties with SPABES-PAE block copolymers, and the synthetic step is shown in
Scheme S2. The polymerization procedure of PABES-PAE is as follows. A 100 mL round-bottomed flask equipped with Dean–Stark trap was charged with PABES oligomer (1.50 g, 3.00 mmol), PAE oligomer (1.10 g, 3.30 mmol), K
2CO
3 (1.10 g, 3.30 mmol), and DMAc/toluene (30 mL/20 mL). The mixture was heated to 130 °C for 4 h to remove toluene and moisture. After finishing the azeotrope, the mixture temperature was maintained at 170 °C for 48 h. After the reaction time, the viscous solution was poured in co-solvent (methanol:DI water = 1:1) to collect the white powder. The produced powder was obtained by filtration, washed with methanol several times, dried in oven at 80 °C for 24 h.
1H NMR (600 MHz, DMSO-
d6): 7.0–8.4 ppm.
1H NMR (600 MHz, DMSO-
d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector)
Mn = 14.8 kDa,
Mw = 122.8 kDa, PDI (
Mw/
Mn) = 8.3.
The SPABES-PAE block copolymers were prepared by controlling SPABES and PAE molar ratio (1:2, 1:1, and 2:1). The polymerization of SPABES-PAE block copolymer (1:1) is as follows. SPABES oligomer (1.00 g, 0.024 mmol), PAE oligomer (1.36 g, 0.024 mmol), K2CO3 (0.006 g, 0.048 mmol), and DMAc/toluene (25 mL/20 mL) were put into 100 mL round-bottomed flask with a Dean–Stark trap under an N2 atmosphere. Next, the reaction procedure and recrystallizations were carried out in a similar procedures to the preparation of PAE oligomer to collect SPABES-PAE block copolymer (1:1). 1H NMR (600 MHz, DMSO-d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector) Mn = 13.9 kDa, Mw = 96.5 kDa, PDI (Mw/Mn) = 6.9.
SPABES-PAE block copolymer (1:2). 1H NMR (600 MHz, DMSO-d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector) Mn = 24.0 kDa, Mw = 122.7 kDa, PDI (Mw/Mn) = 5.1.
SPABES-PAE block copolymer (2:1). 1H NMR (600 MHz, DMSO-d6): 7.0–8.4 ppm; GPC (LiBr in DMF, RI detector) Mn = 16.7 kDa, Mw = 112.3 kDa, PDI (Mw/Mn) = 6.7.
2.3. Membrane Preparation
The membrane of SPABES-PAE block copolymers was prepared by solution casting method. The copolymer solutions was casted on a glass dish and then dried in an oven at 90 °C for 24h. After, all membranes were acidified in 1 M H2SO4 solution at 95 °C for 3 h and washed several times to remove the excess H2SO4 over 10 h. Before using the membranes for measurement of chemical and electric properties, SPABES-PAE block copolymer membranes were obtained by drying in an oven at 90 °C for 12 h (membrane thickness was 70 ± 10 μm).
2.4. Chraterizations
The
1H NMR spectra were analyzed by JNM-ECA 600 instrument (JEOL, Akishima, Tokyo, Japan). The IR spectra were obtained by Frontier MIR/NIR spectrometer (PerkinElmer, Waltham, MA, USA). The molecular weights (
Mn,
Mw, and
Mz) and polydispersity indices (PDI,
Mw/
Mn) were measured by HLC-8320 (Tosoh Corporation, Minato, Tokyo, Japan). The thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were conducted by Q50 and Q20 (TA Instruments, New Castle, DE, USA), respectively. The tapping mode-atomic force microscopy images were conducted by a Veeco multimode atomic force microscope (AFM, Veeco Corporation, Plainview, NY, USA). The proton conductivity was measured by conductivity test bench (Scitech Korea, Gangbuk, Seoul, Korea) [
16,
17,
18]. The average ion domain size was recorded using EMPYREAN (Malvern Panalytical Ltd., Malvern, Grovewood, UK) [
19,
20]. The fuel cell performance was measured using a single cell test station (Scitech, Gangbuk, Seoul, Korea).
The activation energy (
Ea) of membranes was calculated using:
where R and T are the gas constant and Kelvin temperature, respectively [
21].
The solubility of the copolymers was investigated by using concentration of 10 wt % at 60 °C in variety of solvents.
The oxidative stability of membranes was measured into 3% H
2O
2 containing 4 ppm FeSO
4 solution (Fenton’s reagent) at 60 °C for 8 h [
12,
22].
The IEC of all membranes was measured by the titration method [
15,
22]. In brief, all acidified membranes were converted to salt form by immersing in 2 M NaCl solution for 36 h at room temperature. And then the solution was titrated with 0.1 N NaOH solution using phenolphthalein as an indicator.
The water uptake and swelling ratio was evaluated using dried and wet membrane [
23,
24]. The water uptake value of the membranes was reported using the following equation:
where W
dry and W
wet are the membrane weights of the dried and wetted states.
The dimensional ratio was calculated using this equation:
where S
wet and S
dry are the corresponding volumes of membrane length, width, and thickness, respectively [
25,
26].
2.5. Preparation of Membrane Electrode Assembly
The MEA was fabricated with membrane and commercial gas diffusion electrodes (GDE, loading of 0.3 mg·cm–2 Pt/C). The electrodes were sandwiched to both sides of the membrane (press condition: 110 °C, at 1400 psi for 1 min). The single cell active area was 5 cm2, and humidified H2 (anode flow rate: 0.1 L·min–1) and O2 (cathode flow rate: 0.4 L·min–1) were fed into the test station as fuel without any back pressure.
4. Conclusions
The SPABES-PAE (1:2, 1:1, and 2:1) block copolymers were synthesized by controlling the molar ratio of SPABES and PAE oligomers via a nucleophilic aromatic substitution reaction. The chemical structure of synthesized SPABES-PAE was confirmed through 1H NMR, IR, and GPC. The fabricated SPABES-PAE membranes have reasonable dimensional stabilities (water uptake and swelling ratio), and the AFM and SAXS results show well-defined ion transport channels. The proton conductivity of SPABES-PAE (2:1) membrane (IEC = 1.23 mequiv.·g−1) showed 129 mS·cm−1 (at 90 °C under 100% RH), which is similar to Nafion 212 (148.6 mS·cm−1, under the same condition). Moreover, the SPABES-PAE (2:1) membrane (333.2 mW·cm−2) showed remarkable sing cell performance at 100% RH and 60 °C, when compared with Nafion 212 (430.9 mW·cm−2). Overall, the SPABES-PAE membranes showed high oxidative/thermal stability compared with other aromatic hydrocarbon membranes, and balanced properties of PEM between water resistances and proton conductivities, which indicate that it is a promising candidate as PEM material for PEMFCs.