3.2. The Extended DLVO Theory and Calculation of the Relevant Parameters
The total interaction energy between two interfaces
VT can be written as follows:
in which VA and VR are the Lifshitz–van der Waals interaction energy and the electrostatic interaction energy, respectively. VH refers to the hydrophobic interaction or hydrophilic repulsive potential energy.
The potential energy between a spherical particle and a larger particle, which may be assumed flat depending on the geometric size ratio, can be calculated by Equations (6)–(8) [
24,
25,
26]:
where
A (J) is the Hamaker constant;
R (m) is the radius of the spherical particle;
h (nm) is the separation distance;
ε (C
2/(J·m) or F/m) is the dielectric constant of the medium;
ψ01 and
ψ02 (mV) are the Stern potentials of particle 1 and particle 2;
κ−1 (m) is the Debye length;
h0 (nm) is the attenuation length, which is between 1 and 10 nm;
(mJ/m
2) is the interaction energy parameter, which is related to the surface wettability;
H0 (nm) is the closest separation distance between two particles.
The Hamaker constant of
A132 is calculated following Equation (9) [
27,
28]:
where
A132 is the Hamaker constant for the particle 1 interacting with particle 2 in a medium 3,
A11 and
A22 are the Hamaker constants for particle 1 and particle 2 in a vacuum, respectively, and
A33 is the Hamaker constant for the medium (water) in a vacuum.
For the Hamaker constant for the substance i and j in a vacuum (i.e., Aij), it can be shown that .
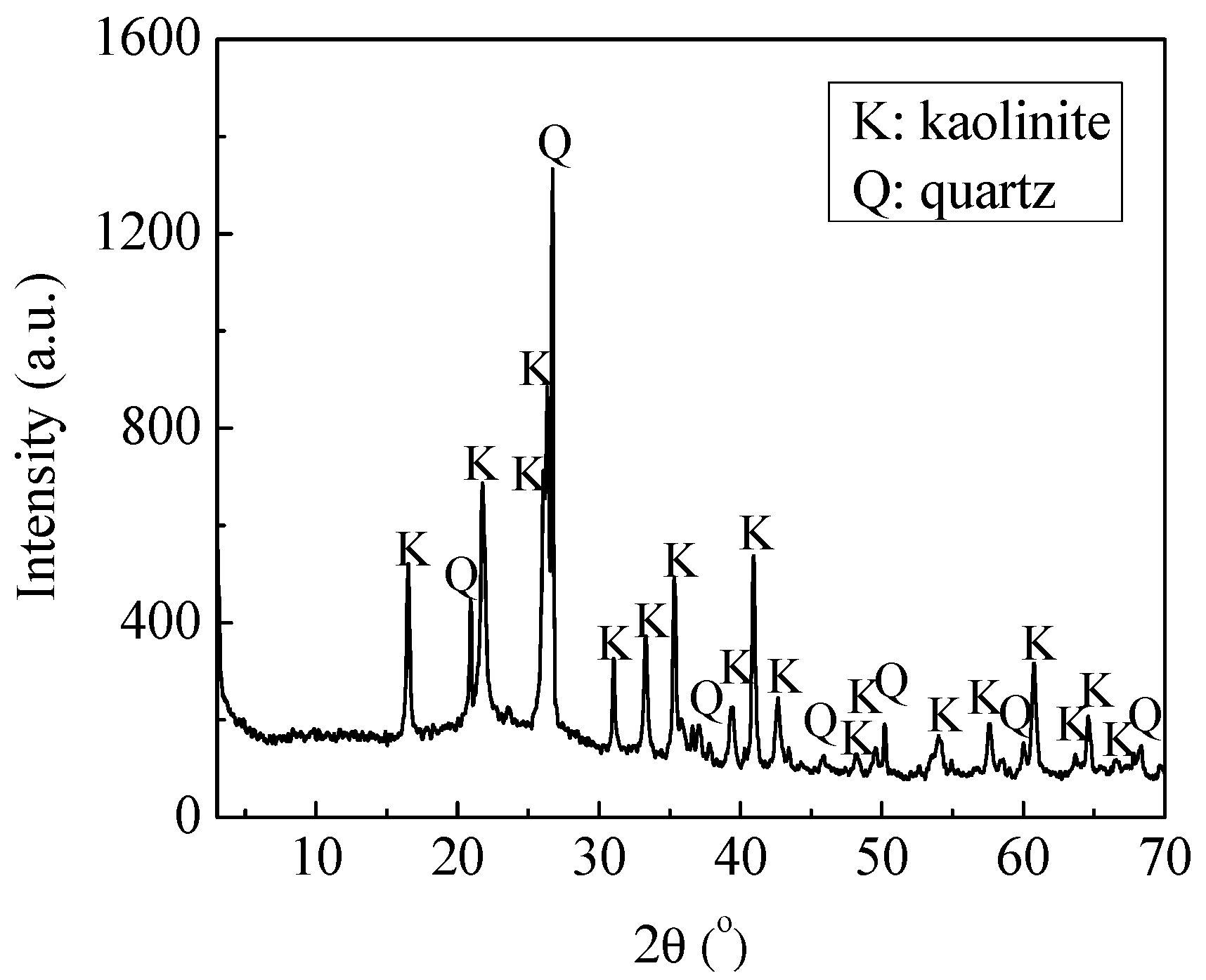
In selective flocculation flotation of fine coal, the Hamaker constants are seen in
Table 4 and
Table 5.
In the calculation of
VR,
ψ01 and
ψ02 are often replaced with the ζ-potential as an approximation. From the zeta potential measurement, the changes are seen in
Table 6.
for material 1 interacting with material 2 in a medium 3 can be calculated from Equation (10):
where
is the free energy of two surfaces in contact,
(mJ·m
−2) is the acid part,
(mJ·m
−2) is the base part.
Thus, of coal–coal was −3.51 mJ/m2, of coal–coal after adsorption of PAM A401 was −0.92 mJ/m2, of kaolinite–kaolinite was 45.73 mJ/m2, of coal–kaolinite was 16.49 mJ/m2, of coal after adsorption of PAM A401–kaolinite was 20.48 mJ/m2.
3.3. Calculation of the Interaction Energies
(1) Interaction energies between coal particles
The dielectric constant of water in a vacuum
ε0 = 8.854 × 10
−12 C
2/(J·m) and the relative dielectric constant of water
εr = 78.5, there is
ε = 6.95 × 10
−10 C
2/(J·m). In a KCl solution (1 mM), there is
κ = 1.04 × 10
8 m
−1 [
24]. From the measurement of the zeta potential,
ψ01 =
ψ02 = −20 mV = −0.02 J/C.
R = 5 × 10
−6 m,
H0 = 0 nm. According to the literature [
24],
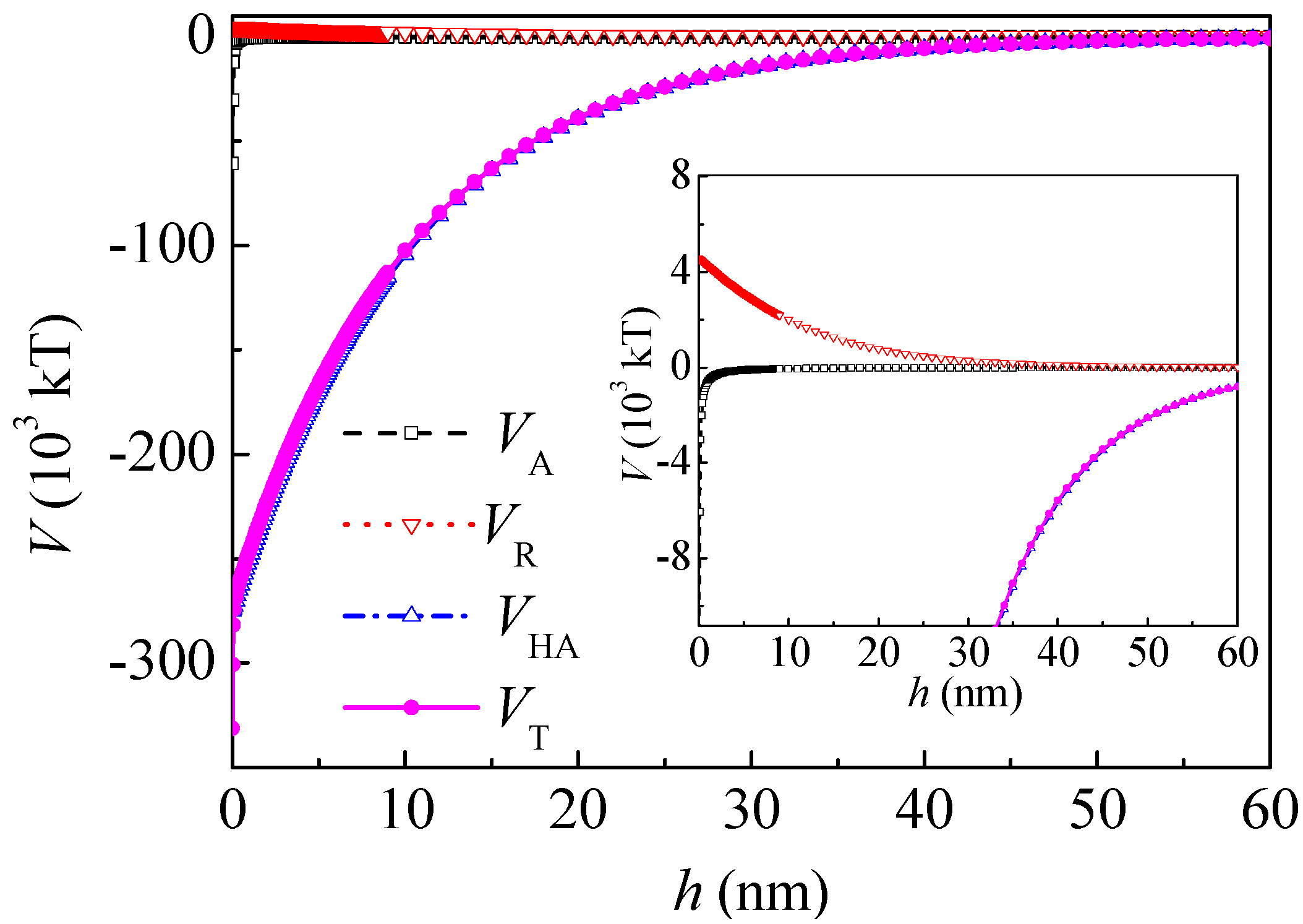
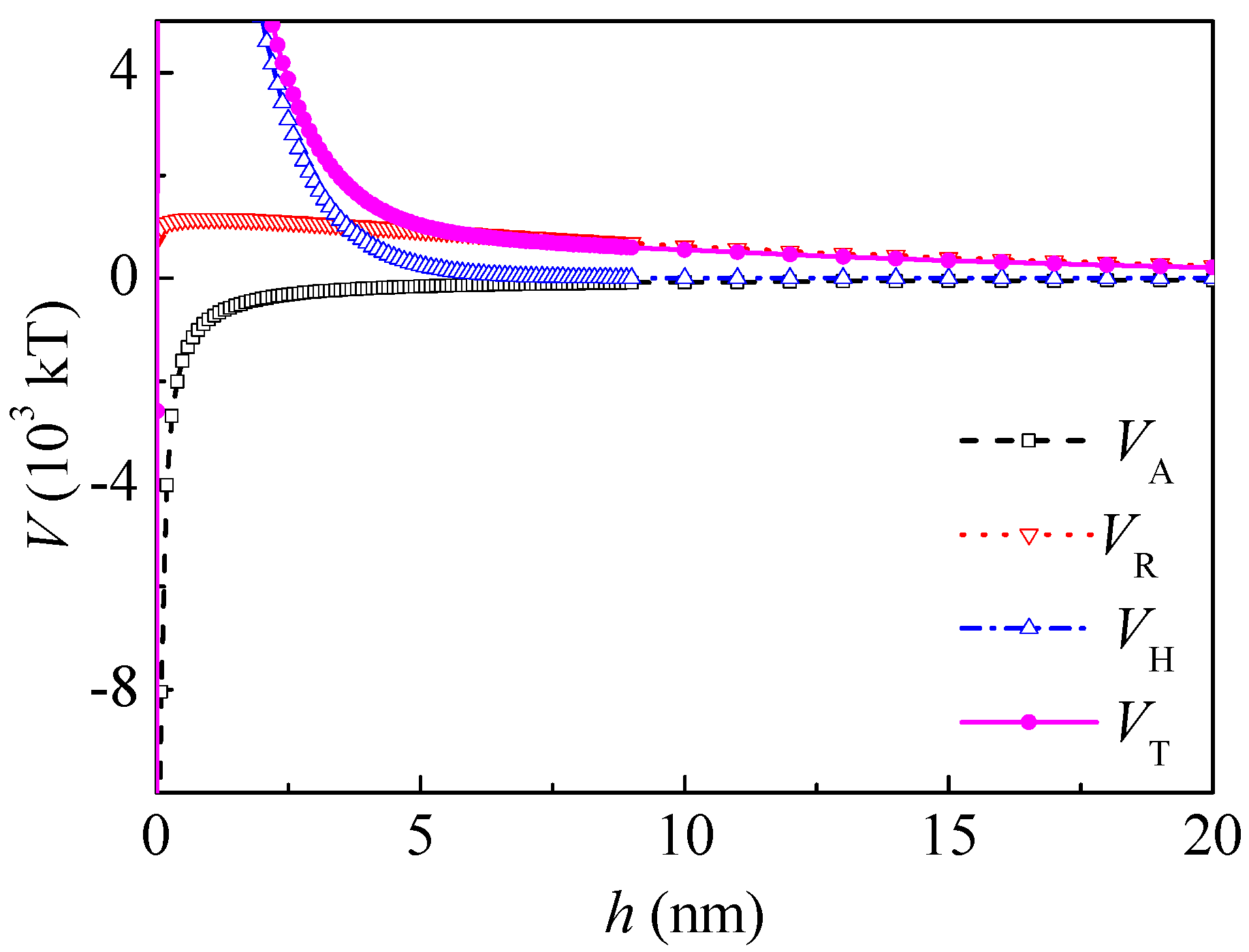
h0 = 10.3 nm. From Equations (5)–(8), the curves of the interaction between the coal particles calculated by the extended DLVO theory are shown in
Figure 3. The total interaction energy was negative thoroughly in the range of 0–60 nm and minimums occurred, which indicate that the agglomeration of the coal particles was thermodynamically preferred. This coincides well with the literature [
16]. The hydrophobic interaction energy
VHA was about two magnitudes higher than that of the Lifshitz–van der Waals and electrostatic interactions. The
VHA was relatively long-range, ranging from 0 to 60 nm. When
h was 5 nm and 20 nm,
VHA was −1.70 × 10
5 and −0.395 × 10
5 kT, and
VT was −1.66 × 10
5 and −0.388 × 10
5 kT, respectively.
After adsorption of PAM A401,
ψ was substituted by the zeta potential as an approximation
ψ = −0.029 J/C, and the thickness of the adsorption layer was
δ = 10 nm according to the literature [
21]; therefore,
VA of the coal particles with PAM A401 layer can be calculated as follows:
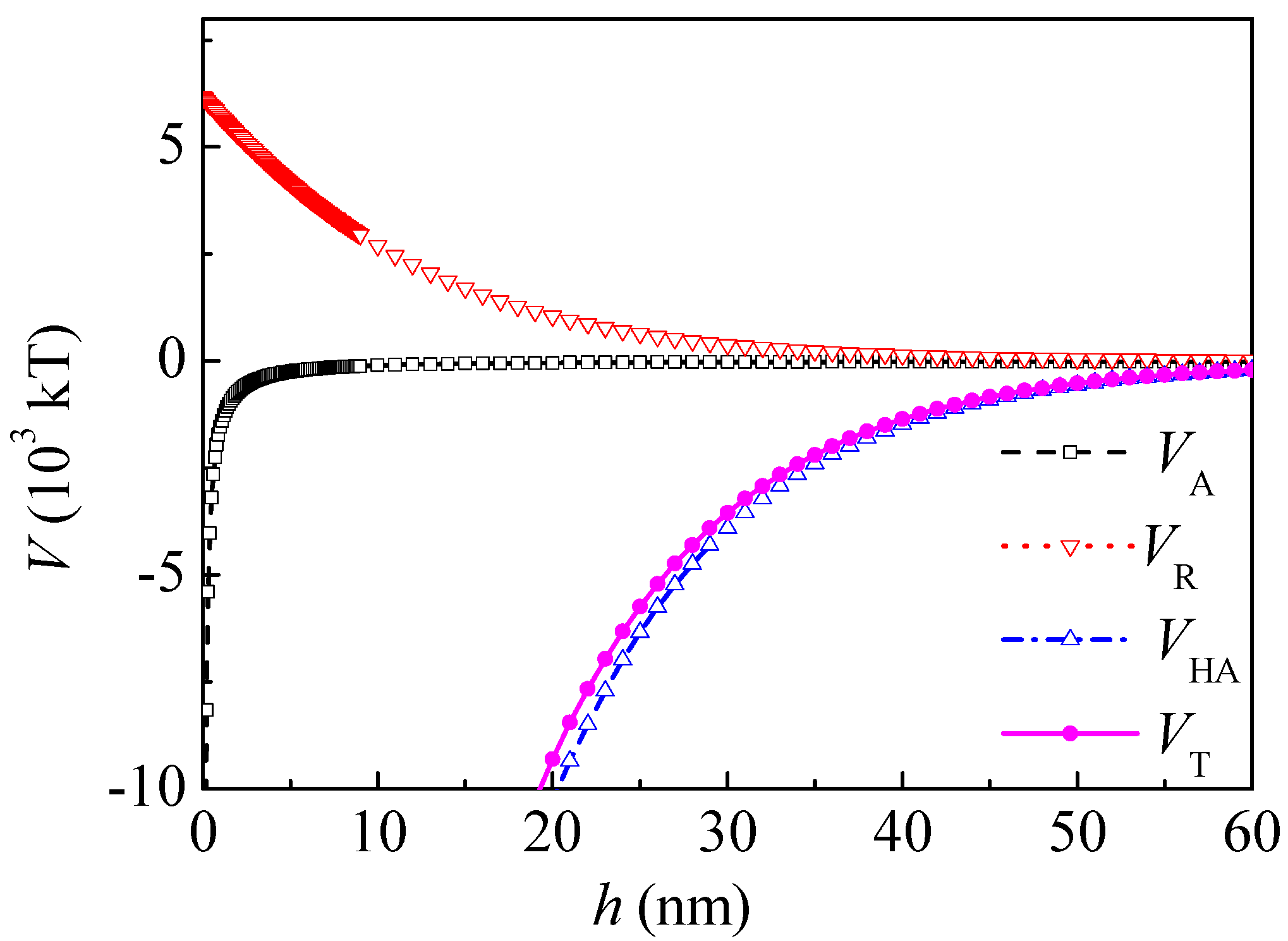
Figure 4 shows the interaction potential energy between the coal particles after adsorption of PAM A401. At a separation distance
h of 5 nm and 20 nm,
VHA was −4.42 × 10
4 and −1.31 × 10
4 kT, respectively. The range of
VHA decreased and
VHA was about one magnitude higher than that of the Lifshitz–van der Waals and electrostatic interactions. At a separation distance
h of 5 and 20 nm,
VT changed to −4.03 × 10
4 and −0.931 × 10
4 kT, respectively. The interaction between the coal particles was still attractive in the range of 0–60 nm.
(2) Interaction energies between kaolinite and coal particles
For kaolinite particles,
R = 1 × 10
−6 m.
Figure 5 shows the interaction potential energy between coal and kaolinite particles before adsorption of PAM A401.
The total interaction energy was positive at the separation distance ranging from 0.05 to 47 nm, and an energy barrier can be observed, which showed that the total interaction between coal and kaolinite was mainly repulsive. The hydrophilic repulsive potential energy within the range of 0–5 nm dominated the interaction between kaolinite and coal particles. The energy barrier was 2.782 × 10
4 kT at a separation distance of 0.2 nm. The order of magnitude of the energy barrier was the same with that in the literature [
16]. When
h was 5 and 20 nm,
VH was 0.25 × 10
3 and 0 kT and
VT was 1.03 × 10
3 and 0.21 × 10
3 kT, respectively.
According to the literature [
14], the adsorption of PAM A401 to kaolinite was much less than that to coal. In order to simplify the calculation, it was assumed that PAM A401 did not adsorb onto the surface of kaolinite. The potential energy between kaolinite and coal-adsorbed PAM A401 is shown in
Figure 6. After adsorption of PAM A401, the energy barrier decreased to 2.782 × 10
4 kT at the separation distance of 0.2 nm. The electrostatic interaction increased and the hydrophilic repulsive potential energy decreased slightly. The total potential energy decreased in the range from 0 to 2.9 nm and increased in the range from 2.9 to 20 nm. When
h was 5 and 20 nm,
VH was 0.21 × 10
3 and 0 kT and
VT was 1.28 × 10
3 and 0.28 × 10
3 kT, respectively. However, the total interaction between kaolinite and coal after adsorption of PAM A401 was still repulsive at a separation distance ranging from 0.05 to 50 nm.
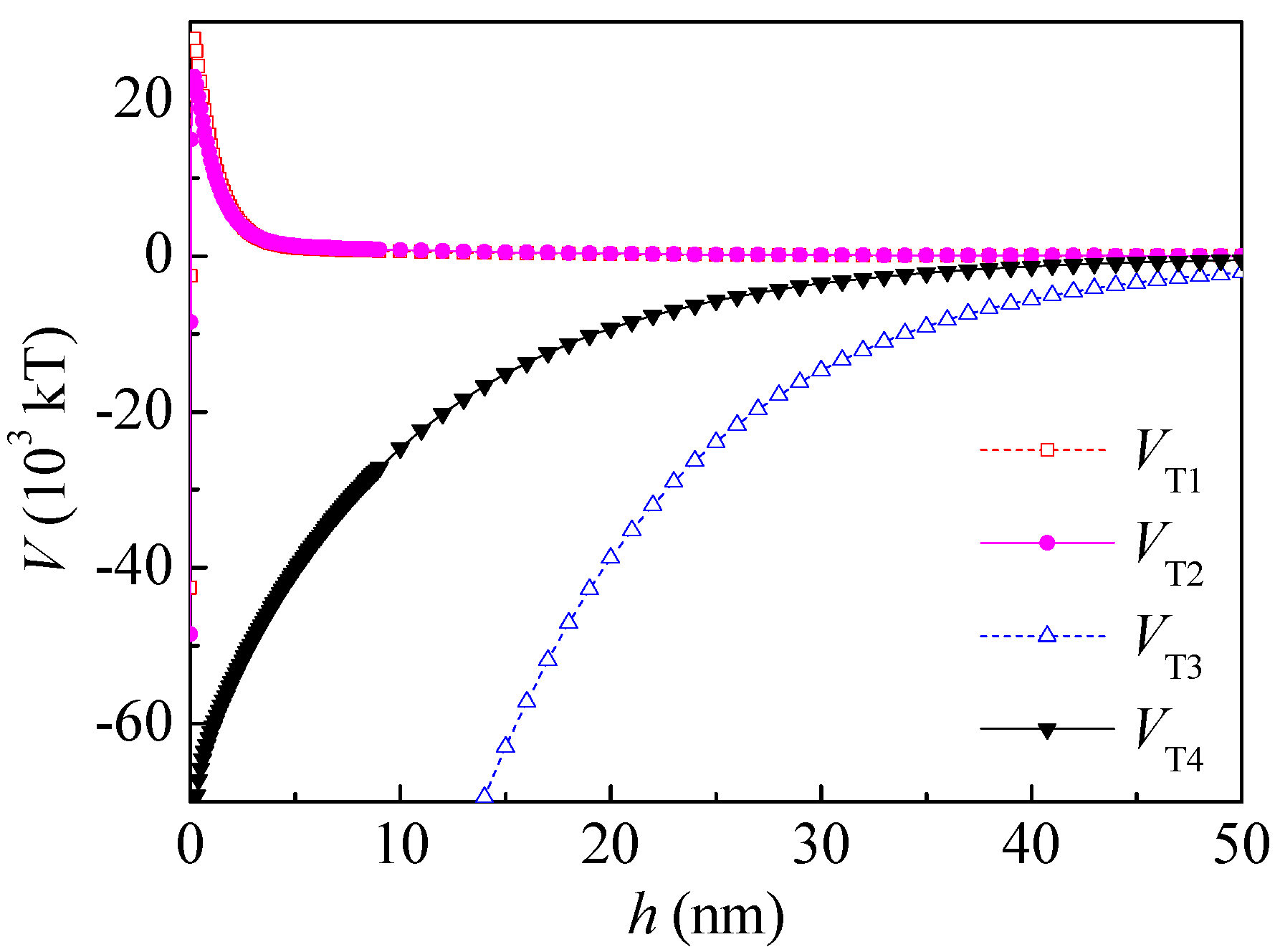
Therefore, both the surface free energy components and the interaction energies between particles changed after PAM A401 adsorption. As seen in
Figure 7, the total potential energy between the kaolinite and coal particles after PAM A401 adsorption was still repulsive, and the range of the repulsive interaction increased from ~0.05 to 47 nm to ~0.05 to 50 nm. The total potential energy of coal particles after PAM A401 adsorption was still attractive. The floc size measured by Microtrac S3500 laser diffractometer (Microtrac Inc., North Largo, FL, USA) evidenced that the
d10,
d50, and
d90 of coal flocculated by PAM A401 with the concentration of 12 mg·L
−1 at natural pH were 3.18, 2.76, and 2.59 times the corresponding levels of these parameters for kaolinite floc, respectively. The apparent size of the coal particles increased selectively with the bridging effect of flocculants, while the other particles remained in the pulp. Results of the calculation of the interaction energies coincide well with adsorption and flocculation behavior of the coal and kaolinite [
14,
15,
21]. This behavior of coal and kaolinite particles with the effect of PAM A401 demonstrates enhanced recovery of fine coal particles in selective flocculation flotation.