RAFT Polymerization of Tert-Butyldimethylsilyl Methacrylate: Kinetic Study and Determination of Rate Coefficients
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Polymerization Procedures
2.3. PLP Investigations
2.4. Characterization Methods
2.5. Simulation
3. Results and Discussion
3.1. Determination of kp and kt
3.2. Validation of the PREDICI® Model and Assessment of the Rate Coefficients via Simulations
3.3. Effect of Initiator Concentration
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mody, N.; Agrawal, U.; Sharma, R.; Vyas, S.P. Structured Biodegradable Polymers for Drug Delivery in Handbook of Polymers for Pharmaceutical Technologies: Biodegradable Polymers; Thakur, V.K., Thakur, M.K., Eds.; John Wiley & Sons, Inc.: New York, NY, USA; Scrivener Publishing LLC: Beverly, MA, USA, 2015; Volume 3, pp. 243–273. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Bressy, C.; Nguyen, M.N.; Tanguy, B.; Ngo, V.G.; Margaillan, A. Poly(trialkylsilyl methacrylate)s: A family of hydrolysable polymers with tuneable erosion profiles. Polym. Degrad. Stab. 2010, 95, 1260–1268. [Google Scholar] [CrossRef]
- Bressy, C.; Hellio, C.; Nguyen, M.N.; Tanguy, B.; Maréchal, J.P.; Margaillan, A. Optimized silyl ester diblock methacrylic copolymers: A new class of binders for chemically active antifouling coatings. Prog. Org. Coat. 2014, 77, 665–673. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Bressy, C.; Margaillan, A. Synthesis of novel random and block copolymers of tert-butyldimethylsilyl methacrylate and methyl methacrylate by RAFT polymerization. Polymer 2009, 50, 3086–3094. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT process—A second update. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Perrier, S.; Barner-Kowollik, C.; Quinn, J.F.; Vana, P.; Davis, T.P. Origin of inhibition effects in the reversible addition–fragmentation chain transfer (RAFT) polymerization of methyl acrylate. Macromolecules 2002, 35, 8300–8306. [Google Scholar] [CrossRef]
- Chong, Y.K.; Krstina, J.; Le, T.P.T.; Moad, G.; Postma, A.; Rizzardo, E.; Thang, S.H. Thiocarbonylthio compounds [S=C(Ph)S−R] in free radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization). Role of the free-radical leaving group (R). Macromolecules 2003, 36, 2256–2272. [Google Scholar] [CrossRef]
- Peklak, A.D.; Butte, A.; Storti, G.; Morbidelli, M. Gel effect in the bulk reversible addition–fragmentation chain transfer polymerization of methyl methacrylate: Modeling and experiments. J. Polym. Sci. A 2006, 44, 1071–1085. [Google Scholar] [CrossRef]
- Buback, M.; Meiser, W.; Vana, P. Mechanism of CPDB-mediated RAFT polymerization of methyl methacrylate: Influence of pressure and RAFT agent concentration. Aust. J. Chem. 2009, 62, 1484–1487. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Buback., M.; Charleux, B.; Coote, M.L.; Drache, M.; Fukuda, T.; Goto, A.; Klumperman, B.; Lowe, A.B.; McLeary, J.B.; et al. Mechanism and kinetics of dithiobenzoate-mediated RAFT polymerization. I. The current situation. J. Polym. Sci. A 2006, 44, 5809–5831. [Google Scholar] [CrossRef]
- Moad, G. Mechanism and kinetics of dithiobenzoate-mediated RAFT polymerization—Status of the dilemma. Macromol. Chem. Phys. 2014, 215, 9–26. [Google Scholar] [CrossRef]
- Nguyen, M.N.; Bressy, C.; Margaillan, A. Controlled radical polymerization of a trialkylsilyl methacrylate by reversible addition–fragmentation chain transfer polymerization. J. Polym. Sci. A. 2005, 43, 5680–5689. [Google Scholar] [CrossRef]
- Vana, P.; Davis, T.P.; Barner-Kowollik, C. Kinetic analysis of reversible addition–fragmentation chain transfer (RAFT) polymerizations: Conditions for inhibition, retardation, and optimum living polymerization. Macromol. Theory Simul. 2002, 11, 823–835. [Google Scholar] [CrossRef]
- Wulkow, M.; Busch, M.; Davis, T.P.; Barner-Kowollik, C. Implementing the reversible addition–fragmentation chain transfer process in PREDICI. J. Polym. Sci. A 2004, 42, 1441–1448. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Morsley, D.R.; Davis, T.P. Modeling the reversible addition–fragmentation chain transfer process in cumyl dithiobenzoate-mediated styrene homopolymerizations: Assessing rate coefficients for the addition–fragmentation equilibrium. J. Polym. Sci. A 2001, 39, 1353–1365. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Nguyen, T.L.U.; Heuts, J.P.A.; Davis, T.P. Kinetic investigations of reversible addition–fragmentation chain transfer polymerizations: Cumyl phenyldithioacetate mediated homopolymerizations of styrene and methyl methacrylate. Macromolecules 2001, 34, 7849–7857. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M.; Davis, T.P.; Gilbert, R.G.; Hutchinson, R.A.; Olaj, O.F.; Russell, G.T.; Schweer, J.; van Herk, A.M. Critically evaluated rate coefficients for free-radical polymerization, 2. Propagation rate coefficients for methyl methacrylate. Macromol. Chem. Phys. 1997, 198, 1545–1560. [Google Scholar] [CrossRef]
- Beuermann, S.; Buback, M.; Davis, T.P.; Gilbert, R.G.; Hutchinson, R.A.; Kajiwara, A.; Klumperman, B.; Russell, G.T. Critically evaluated rate coefficients for free-radical polymerization, 3. Propagation rate coefficients for alkyl methacrylates. Macromol. Chem. Phys. 2000, 201, 1355–1364. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Barner-Kowollik, C.; Buback, M.; Egorov, M.; Fukuda, T.; Goto, A.; Olaj, O.F.; Russell, G.T.; Vana, P.; Yamada, B.; Zetterlund, P.B. Critically evaluated termination rate coefficients for free-radical polymerization: Experimental methods. Prog. Polym. Sci. 2005, 30, 605–643. [Google Scholar] [CrossRef]
- Taylor, D.R.; van Berkel, K.Y.; Alghamdi, M.M.; Russell, G.T. Termination rate coefficients for radical homopolymerization of methyl methacrylate and styrene at low conversion. Macromol. Chem. Phys. 2010, 211, 563–579. [Google Scholar] [CrossRef]
- Buback, M.; Egorov, M.; Gilbert, R.G.; Kaminsky, V.; Olaj, O.F.; Russell, G.T.; Vana, P.; Zifferer, G. Critically evaluated termination rate coefficients for free-radical polymerization, 1. The current situation. Macromol. Chem. Phys. 2002, 203, 2570–2582. [Google Scholar] [CrossRef]
- Olaj, O.F.; Vana, P.; Zoder, M.; Kornherr, A.; Zifferer, G. Is the rate constant of chain propagation kp in radical polymerization really chain-length independent? Macromol. Rapid Commun. 2000, 21, 913–920. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Vana, P.; Quinn, J.F.; Davis, T.P. Long-lived intermediates in reversible addition–fragmentation chain-transfer (RAFT) polymerization generated by γ radiation. J. Polym. Sci. A 2002, 40, 1058–1063. [Google Scholar] [CrossRef]
- Nicolas, J.; Mueller, L.; Dire, C.; Matyjaszewski, K.; Charleux, B. Comprehensive modeling study of nitroxide-mediated controlled/living radical copolymerization of methyl methacrylate with a small amount of styrene. Macromolecules 2009, 42, 4470–4478. [Google Scholar] [CrossRef]








| Entry | Temperature (°C) | [TBDMSMA] (mol·L−1) | [CPDB] (mol·L−1) | [AIBN] (mol·L−1) | Monomer conversion (%) | Mn (g·mol−1) | Đ (Mw/Mn) |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 1.5 | - | 6.0 × 10−3 | 13.9 | - | - |
| 2 | 70 | 1.5 | - | 6.0 × 10−3 | 13.9 | - | - |
| 3 | 80 | 1.5 | - | 6.0 × 10−3 | 15.2 | - | - |
| 4 | 70 | 1.5 | 1.0 × 10−2 | 6.0 × 10−3 | 97.1 | 24,300 | 1.10 |
| 5 | 70 | 1.5 | 1.5 × 10−2 | 6.0 × 10−3 | 96.9 | 17,700 | 1.12 |
| 6 | 70 | 1.5 | 3.0 × 10−2 | 6.0 × 10−3 | 99.3 | 9600 | 1.11 |
| 7 | 70 | 1.5 | 6.0 × 10−2 | 6.0 × 10−3 | 97.5 | 5400 | 1.12 |
| 8 | 70 | 1.5 | 3.0 × 10−2 | 3.0 × 10−3 | 91.0 | 9840 | 1.10 |
| 9 | 70 | 1.5 | 3.0 × 10−2 | 1.5 × 10−3 | 71.2 | 7950 | 1.10 |
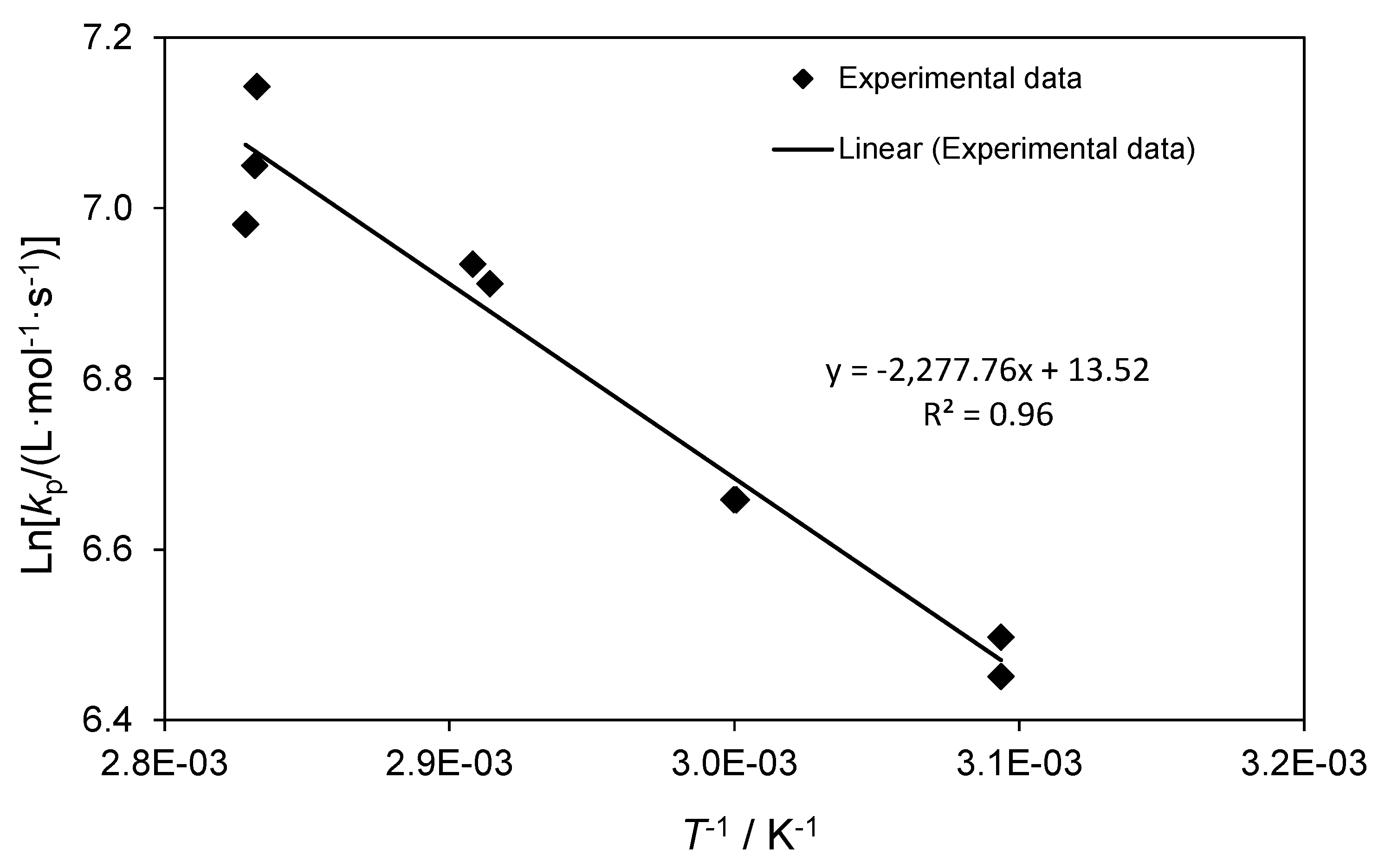
| Entry | Temperature (°C) | [TBDMSMA] (mol·L−1) | tf (s) | DP | kp (L·mol−1·s−1) | lnkp |
|---|---|---|---|---|---|---|
| 10 | 50.1 | 1.502 | 0.05 | 49.8 | 663 | 6.50 |
| 11 | 50.1 | 1.502 | 0.05 | 47.6 | 653 | 6.48 |
| 12 | 60.2 | 1.502 | 0.05 | 58.5 | 779 | 6.66 |
| 13 | 60.1 | 1.502 | 0.05 | 58.5 | 779 | 6.66 |
| 14 | 70.0 | 1.502 | 0.05 | 75.4 | 1004 | 6.91 |
| 15 | 70.7 | 1.502 | 0.05 | 77.1 | 1027 | 6.93 |
| 16 | 79.9 | 1.502 | 0.05 | 94.9 | 1264 | 7.14 |
| 17 | 80.0 | 1.502 | 0.05 | 86.5 | 1152 | 7.05 |
| 18 | 80.4 | 1.502 | 0.05 | 80.8 | 1075 | 6.98 |
| Temperature (°C) | kt (L·mol−1·s−1) |
|---|---|
| 60 | 1.49 × 107 |
| 70 | 1.68 × 107 |
| 80 | 1.90 × 107 |
| Parameter | Value |
|---|---|
| kd,eff (s−1) | 2.40 × 10−5 |
| ki (L·mol−1·s−1) | 4.86 × 103 |
| kp (L·mol−1·s−1) | 9.72 × 102 |
| kp, rein (L·mol−1·s−1) | 4.86 × 103 |
| kt (L·mol−1·s−1) | 1.68 × 107 |
| α | 1 |
| (mol·L−1) | 1.5 |
| (mol·L−1) | 1.0 × 10−2; 1.5 × 10−2; 3.0 × 10−2; 6.0 × 10−2 |
| (mol·L−1) | 6.0 × 10−3 |
| Parameter | Value |
|---|---|
| kβ (s−1) | 1.80 × 104 |
| kβ,1 (s−1) | 1.80 × 104 |
| kβ,2 (s−1) | 1.80 × 104 |
| k-β (s−1) | 2.0 × 10−2 |
| k-β,1 (s−1) | 2.0 × 10−2 |
| k-β,2 (s−1) | 2.0 × 10−2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, M.N.; Margaillan, A.; Pham, Q.T.; Bressy, C. RAFT Polymerization of Tert-Butyldimethylsilyl Methacrylate: Kinetic Study and Determination of Rate Coefficients. Polymers 2018, 10, 224. https://doi.org/10.3390/polym10020224
Nguyen MN, Margaillan A, Pham QT, Bressy C. RAFT Polymerization of Tert-Butyldimethylsilyl Methacrylate: Kinetic Study and Determination of Rate Coefficients. Polymers. 2018; 10(2):224. https://doi.org/10.3390/polym10020224
Chicago/Turabian StyleNguyen, Minh Ngoc, André Margaillan, Quang Trung Pham, and Christine Bressy. 2018. "RAFT Polymerization of Tert-Butyldimethylsilyl Methacrylate: Kinetic Study and Determination of Rate Coefficients" Polymers 10, no. 2: 224. https://doi.org/10.3390/polym10020224
APA StyleNguyen, M. N., Margaillan, A., Pham, Q. T., & Bressy, C. (2018). RAFT Polymerization of Tert-Butyldimethylsilyl Methacrylate: Kinetic Study and Determination of Rate Coefficients. Polymers, 10(2), 224. https://doi.org/10.3390/polym10020224








