A Three-Dimensional Nickel(II) Framework from a Semi-Flexible Bipyrimidyl Ligand Showing Weak Ferromagnetic Behavior
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Ni(Br)2(bpym)2]n (1)
2.3. X-ray Crystallography
2.4. Physical Measurements
3. Results and Discussion
3.1. Syntheses and Characterization of Compound 1
3.2. Description of Structure
3.2.1. Crystal Structures of Compound 1
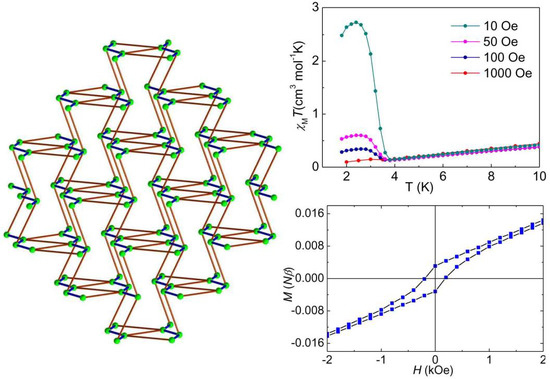
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiang, S.; Wu, X.; Zhang, J.; Zhang, J.; Fu, R.; Hu, S.; Zhang, X.Z.X. A 3D Canted Antiferromagnetic Porous Metal−Organic Framework with Anatase Topology through Assembly of an Analogue of Polyoxometalate. J. Am. Chem. Soc. 2005, 127, 16352–16353. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-F.; Hu, X.; Liu, F.-C.; Bu, X.-H. Azido-mediated systems showing different magnetic behaviors. Chem. Soc. Rev. 2009, 38, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Kahn, O.; Miller, J.S.; Palacio, F. Magnetic Molecular Materials; Kluwer Academic: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Willet, R.D.; Gatteschi, D.; Kahn, O. Magneto-Structural Correlations in Exchange Coupled Systems; Reidel Publishing: Dordrecht, The Netherlands, 1985. [Google Scholar]
- Coronado, E.; Mínguez Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Kabir, N.A.; Zhou, K.; Verpoort, F. Metal–organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy. Chem. Soc. Rev. 2013, 42, 9304. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.-F.; Wang, Z.-M.; Gao, S. Framework-structured weak ferromagnets. Chem. Soc. Rev. 2011, 40, 3157. [Google Scholar] [CrossRef]
- Wriedt, M.; Näther, C. Directed synthesis of μ-1,3,5 bridged dicyanamides by thermal decomposition of μ-1,5 bridged precursor compounds. Dalton Trans. 2011, 40, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Masciocchi, N.; Cairati, P.; Carlucci, L.; Ciani, G.; Mezzaban, G.; Sironi, A. Structural Characterization of Pyridazine (pydz) Adducts of MX, (M = Mn, Fe, Co, Ni, Cu or Zn; X = CI or Br). Ab-initio X-Ray Powder Diffraction Determination of Polymeric [NiX2(pydz)] Complexes. J. Chem. Soc. Dalton Trans. 1994, 3009–3015. [Google Scholar] [CrossRef]
- Richardson, H.W.; Hatfield, W.E. On the Superexchange Mechanism in Polymeric, Pyrazine-Bridged Copper(II) Complexes. J. Am Chem. Soc. 1976, 98, 835–839. [Google Scholar] [CrossRef]
- Chao, T.-L.; Yang, C.-I. Three new homochiral coordination polymers involving two three-dimensional structural architectures: Syntheses, structures and magnetic properties. J. Solid State Chem. 2014, 211, 25–31. [Google Scholar] [CrossRef]
- Wriedt, M.; Sellmer, S.; Näther, C. Thermal Decomposition Reactions as Tool for the Synthesis of New Metal Thiocyanate Diazine Coordination Polymers with Cooperative Magnetic Phenomena. Inorg. Chem. 2009, 48, 6896–6903. [Google Scholar] [CrossRef] [PubMed]
- Feyerherm, R.; Loose, A.; Ishida, T.; Nogami, T.; Kreitlow, J.; Baabe, D.; Litterst, F.J.; Süllow, S.; Klauss, H.-H.; Doll, K. Weak ferromagnetism with very large canting in a chiral lattice: Fe(pyrimidine)2Cl2. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Barea, E.; Romero, M.A.; Navarro, J.A.R.; Salas, J.M.; Masciocchi, N.; Galli, S.; Sironi, A. Structure, Spectroscopic Properties, and Reversible Solid-to-Solid Reactions of Metal Complexes of 5-Nitro-pyrimidin-2-olate. Inorg. Chem. 2005, 44, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Diéguez, A.; Cano, J.; Kivekäs, R.; Debdoubi, A.; Colacio, E. Self-Assembled Cationic Heterochiral Honeycomb-Layered Metal Complexes with the in Situ Generated Pyrimidine-2-carboxylato Bisdidentate Ligand. Hydrothermal Synthesis, Crystal Structures, Magnetic Properties, and Theoretical Study of [M2(μ-pymca)3]OH·H2O (M = FeII, CoII). Inorg. Chem. 2007, 46, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- De Munno, G.; Poerio, T.; Julve, M.; Lloret, F.; Faus, J.; Caneschi, A. Syntheses, crystal structures and magnetic properties of one-, two-and three-dimensional 2, 2′-bipyrimidine-containing copper (II) complexes. J. Chem. Soc. Dalton Trans. 1998, 1679–1686. [Google Scholar] [CrossRef]
- Marino, N.; Armentano, D.; De Munno, G.; Lloret, F.; Cano, J.; Julve, M. Towards a better understanding of honeycomb alternating magnetic networks. Dalton Trans. 2015, 44, 11040–11051. [Google Scholar] [CrossRef] [PubMed]
- Munno, G.D.; Poerio, T.; Julve, M.; Lloretb, F.; Viau, G. Synthesis, crystal structure and magnetic properties of the cobalt(II) chain and the dinuclear compounds [Co(bipym)(H2O)2](NO3)2 and [Co2(bipym)3(H2O)4](NO3)4·2H2O [Co2(bipym)3(H2O)2(SO4)2]·12H2O. New J. Chem. 1998, 299–305. [Google Scholar] [CrossRef]
- Luo, T.-T.; Liu, Y.-H.; Chan, C.-C.; Huang, S.-M.; Chang, B.-C.; Lu, Y.-L.; Lee, G.-H.; Peng, S.-M.; Wang, J.-C.; Lu, K.-L. Toward Quartz and Cristobalite: Spontaneous Resolution, Structures, and Characterization of Chiral Silica−Mimetic Silver(I)−Organic Materials. Inorg. Chem. 2007, 46, 10044–10046. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Hsu, H.-Y.; Chan, C.-C.; Wen, Y.-S.; Tsai, C.; Lu, K.-L. Formation of Infinite Linear Mercury Metal Chains Assisted by Face-to-Face π−π(Aryl−Aryl) Stacking Interactions. Cryst. Growth Des. 2009, 9, 258–262. [Google Scholar] [CrossRef]
- Tseng, T.-W.; Luo, T.-T.; Tsai, C.-C.; Wu, J.-Y.; Tsai, H.-L.; Lu, K.-L. Crystal Engineering of Three Net-to-Net Intersecting Metal-Organic Frameworks from Two Comparable Organic Linking Squares. Eur. J. Inorg. Chem. 2010, 3750–3755. [Google Scholar] [CrossRef]
- Barnes, C.L.; Bosch, E. Self-Assembly of C-Methyl Calix[4]resorcinarene with 5,5′-Bipyrimidine. J. Chem. Crystallgr. 2007, 37, 783–786. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Chang, H.-T.; Kuo, Y.; Lee, G.-H.; Yang, C.-I. Two New Three-Dimensional Pillared-Layer Co(II) and Cu(II) Frameworks Involving a [M2(EO-N3)2] Motif from a Semi-Flexible N-Donor Ligand, 5,5′-Bipyrimidin: Syntheses, Structures and Magnetic Properties. Polymers 2018, 10, 229. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Lee, G.-H.; Yang, C.-I. The use of a semi-flexible bipyrimidyl ligand for the construction of azide-based coordination polymers: Structural diversities and magnetic properties. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd(OAc)2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1993. [Google Scholar]
- Sheldrick, G.M. SHELXL2014; University of Goëttingen: Goëttingen, Germany, 2014. [Google Scholar]
- Mulay, L.N.; Boudreaux, E.A. Theory and Applications of Molecular Diamagnetism; Wiley-VCH: New York, NY, USA, 1976; pp. 491–494, ISBN-10: 047162358X. [Google Scholar]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. First Magnetostructural Study on a Heterodinuclear 2,2′-Bipyrimidine-Bridged Complex. Inorg. Chem. 2011, 50, 12405–12407. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lara, B.; Carabineiro, S.A.; Krishnamoorthy, P.; Rodríguez, A.M.; Mano, J.F.; Manzano, B.R.; Jalón, F.A.; Gomes, P.T. Nickel(II) complexes of bidentate N–N′ ligands containing mixed pyrazole, pyrimidine and pyridine aromatic rings as catalysts for ethylene polymerisation. J. Organomet. Chem. 2015, 799–800, 90–98. [Google Scholar] [CrossRef]
- Masaki, M.E.; Prince, B.J.; Turnbull, M.M. Transition Metal Halide Salts and Complexes of 2-Aminopyrimidine: Cobalt(II) and Nickel(II) compounds, Crystal Structures of Bis(2-Aminopyrimidinium)MX4 [M = Co, Ni; X = Cl, Br] and 2-Aminopyrimidinium(+2) [NiBr2(H2O)4]Br2. J. Coord. Chem. 2002, 55, 1337–1351. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Palacio, F.; Andrés, M.; Horne, R.; van Duyneveldt, A.J. Weak ferromagnetism in the linear chain RbMnF4⋯H2O. J. Magn. Magn. Mater. 1986, 54–57, 1487–1488. [Google Scholar] [CrossRef]
- Tsai, J.-D.; Yang, C.-I. Utilization of a ligand containing 2,2′-bipyridyl and tetrazolate groups to construct a 2D Co(II) coordination polymer: Spin canting and metamagnetism. Dalton Trans. 2014, 43, 15576–15582. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-P.; Tian, J.-L.; Li, D.-D.; Chen, G.-J.; Gu, W.; Yan, S.-P.; Liu, X.; Liao, D.-Z.; Cheng, P. Spin Canting and Slow Relaxation in a 3D Pillared Nickel−Organic Framework. Inorg. Chem. 2010, 49, 2525–2529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Gamez, P.; Kou, H.-Z.; Fan, C.; Zhang, H.; Sun, G. Spin canting and metamagnetism in the two azido-bridged 1D complexes [Ni(3,5-dmpy)2(N3)2]n and [Co1.5(3,5-dmpy)3(N3)3]n. CrystEngComm 2012, 14, 5035. [Google Scholar] [CrossRef]
- Fisher, M.E. Magnetism in One-Dimensional Systems—The Heisenberg Model for Infinite Spin. Am. J. Phys. 1964, 32, 343–346. [Google Scholar] [CrossRef]
- Gobeze, W.A.; Milway, V.A.; Moubaraki, B.; Murray, K.S.; Brooker, S. Solvent control: Dinuclear versus tetranuclear complexes of a bis-tetradentate pyrimidine-based ligand. Dalton Trans. 2012, 41, 9708. [Google Scholar] [CrossRef] [PubMed]
- Beobide, G.; Castillo, O.; Luque, A.; García-Couceiro, U.; García-Terán, J.P.; Román, P. Rational design of 1-D metal–organic frameworks based on the novel pyrimidine-4,6-dicarboxylate ligand. New insights into pyrimidine through magnetic interaction. Dalton Trans. 2007, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-N.; Xue, W.; Zhang, W.-X.; Chen, X.-M. Weak Ferromagnetism and Dynamic Magnetic Behavior of Two 2D Compounds with Hydroxy/Carboxylate-Bridged Co(II) Chains. Chem. Mater. 2008, 20, 5345–5350. [Google Scholar] [CrossRef]
- Bellitto, C.; Federici, F.; Colapietro, M.; Portalone, G.; Caschera, D. X-ray Single-Crystal Structure and Magnetic Properties of Fe[CH3PO3)]·H2O: A Layered Weak Ferromagnet. Inorg. Chem. 2002, 41, 709–714. [Google Scholar] [CrossRef] [PubMed]










| Compound | 1 |
|---|---|
| Formula | C16H12Br2N8Ni |
| Fw | 534.83 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a/Å | 18.4662(15) |
| b/Å | 18.4523(15) |
| c/Å | 11.0947(9) |
| α/° | 90 |
| β/° | 108.5770(10) |
| γ/° | 90 |
| V/Å3 | 3535.4(5) |
| Z | 4 |
| T/K | 150(2) |
| Dc/g cm−3 | 2.010 |
| μ/mm−1 | 5.639 |
| (Δρ)max, min/e Å−3 | 0.606, −0.677 |
| Measured/independent (Rint) reflections | 14591/4786(0.0396) |
| Observed reflections [I > 2σ(I)] | 4786 |
| Goodness-of-fits on F2 | 1.046 |
| R11, wR22 (all data) | 0.0430, 0.0707 |
| R11, wR22 (I > 2σ (I)) | 0.0304, 0.0657 |
| Compound 1 | |||
|---|---|---|---|
| Br(1)-Ni(1) | 2.5925(7) | Ni(2)-Br(3)#B | 2.5723(3) |
| Br(3)-Ni(2) | 2.5723(3) | Br(2)-Ni(1) | 2.5884(6) |
| Ni(2)-N(3) | 2.101(2) | Ni(1)-N(7) | 2.100(2) |
| Ni(2)-N(3)#B | 2.101(2) | Ni(1)-N(7)#A | 2.100(2) |
| Ni(2)-N(5)#B | 2.135(2) | Ni(1)-N(6) | 2.139(2) |
| Ni(2)-N(5) | 2.135(2) | Ni(1)-N(6)#A | 2.139(2) |
| N(3)-Ni(2)-N(3)#B | 93.13(13) | N(7)-Ni(1)-N(7)#A | 178.87(13) |
| N(3)-Ni(2)-N(5)#B | 177.25(8) | N(7)-Ni(1)-N(6) | 90.93(9) |
| N(3)#B-Ni(2)-N(5)#B | 88.61(9) | N(7)#A-Ni(1)-N(6) | 89.06(9) |
| N(3)-Ni(2)-N(5) | 88.61(9) | N(7)-Ni(1)-N(6)#A | 89.06(9) |
| N(3)#B-Ni(2)-N(5) | 177.25(8) | N(7)#A-Ni(1)-N(6)#A | 90.93(9) |
| N(5)#B-Ni(2)-N(5) | 89.74(12) | N(6)-Ni(1)-N(6)#A | 179.18(12) |
| N(3)-Ni(2)-Br(3) | 87.70(6) | N(7)-Ni(1)-Br(2) | 90.57(6) |
| N(3)#B-Ni(2)-Br(3) | 87.77(6) | N(7)#A-Ni(1)-Br(2) | 90.57(6) |
| N(5)#B-Ni(2)-Br(3) | 94.51(6) | N(6)-Ni(1)-Br(2) | 90.41(6) |
| N(5)-Ni(2)-Br(3) | 90.17(6) | N(6)#A-Ni(1)-Br(2) | 90.41(6) |
| N(3)-Ni(2)-Br(3)#B | 87.77(6) | N(7)-Ni(1)-Br(1) | 89.43(6) |
| N(3)#B-Ni(2)-Br(3)#B | 87.70(6) | N(7)#A-Ni(1)-Br(1) | 89.43(6) |
| N(5)#B-Ni(2)-Br(3)#B | 90.17(6) | N(6)-Ni(1)-Br(1) | 89.59(6) |
| N(5)-Ni(2)-Br(3)#B | 94.51(6) | N(6)#A-Ni(1)-Br(1) | 89.59(6) |
| Br(3)-Ni(2)-Br(3)#B | 173.40(2) | Br(2)-Ni(1)-Br(1) | 180.000(1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.-S.; Yang, C.-I. A Three-Dimensional Nickel(II) Framework from a Semi-Flexible Bipyrimidyl Ligand Showing Weak Ferromagnetic Behavior. Polymers 2019, 11, 119. https://doi.org/10.3390/polym11010119
Dong S-S, Yang C-I. A Three-Dimensional Nickel(II) Framework from a Semi-Flexible Bipyrimidyl Ligand Showing Weak Ferromagnetic Behavior. Polymers. 2019; 11(1):119. https://doi.org/10.3390/polym11010119
Chicago/Turabian StyleDong, Shin-Shan, and Chen-I Yang. 2019. "A Three-Dimensional Nickel(II) Framework from a Semi-Flexible Bipyrimidyl Ligand Showing Weak Ferromagnetic Behavior" Polymers 11, no. 1: 119. https://doi.org/10.3390/polym11010119