Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Flame-Retardant Rigid Polyurethane Foams
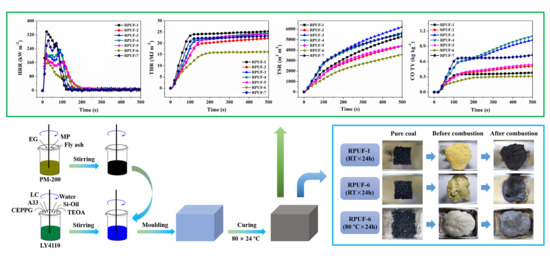
2.3. Inhibition of Coal Combustion
2.4. Instruments and Measurements
3. Results and Discussion
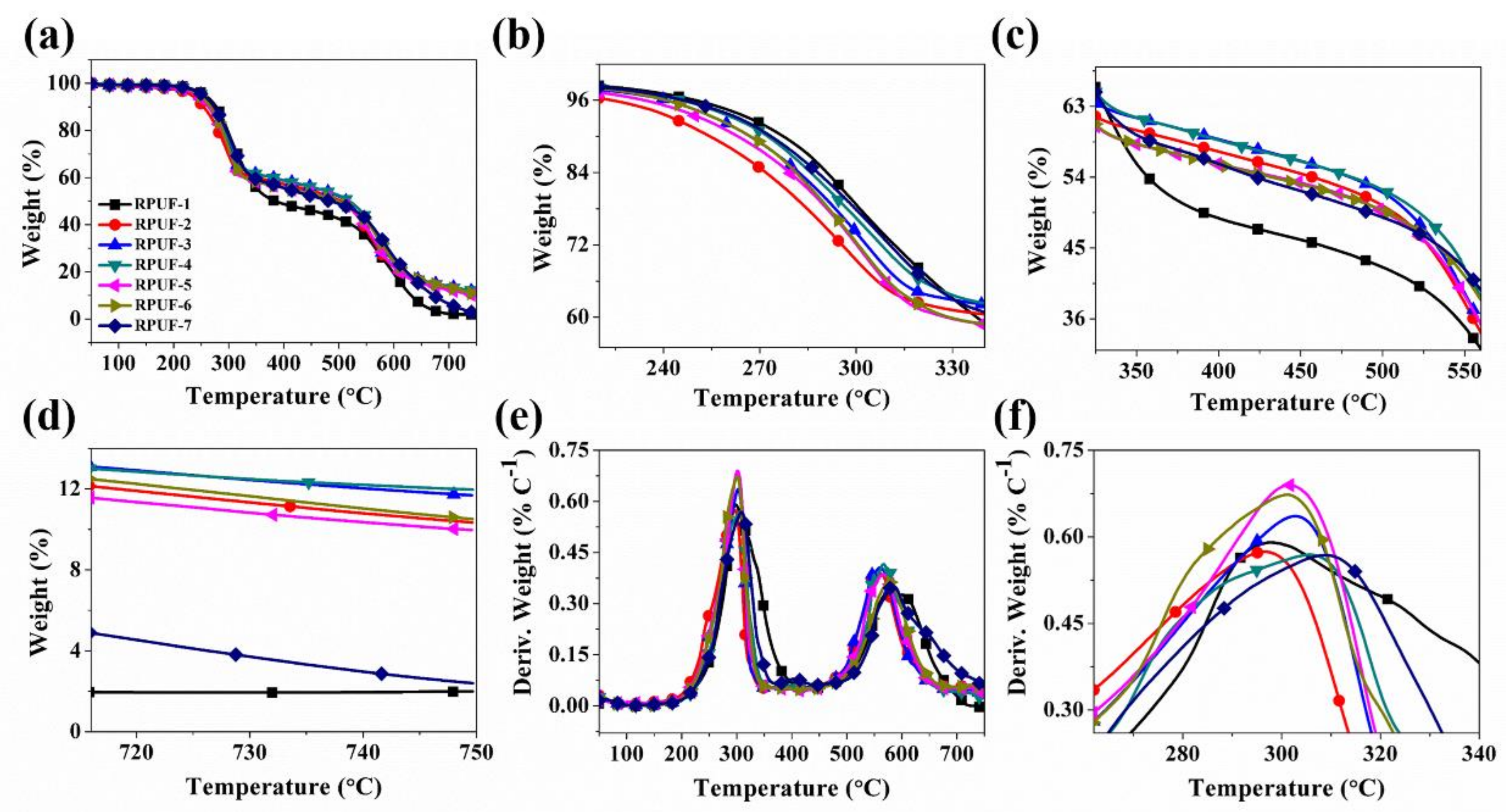
3.1. Thermal Degradation Behavior Analysis
3.2. Flame-Retardant Performance Evaluation
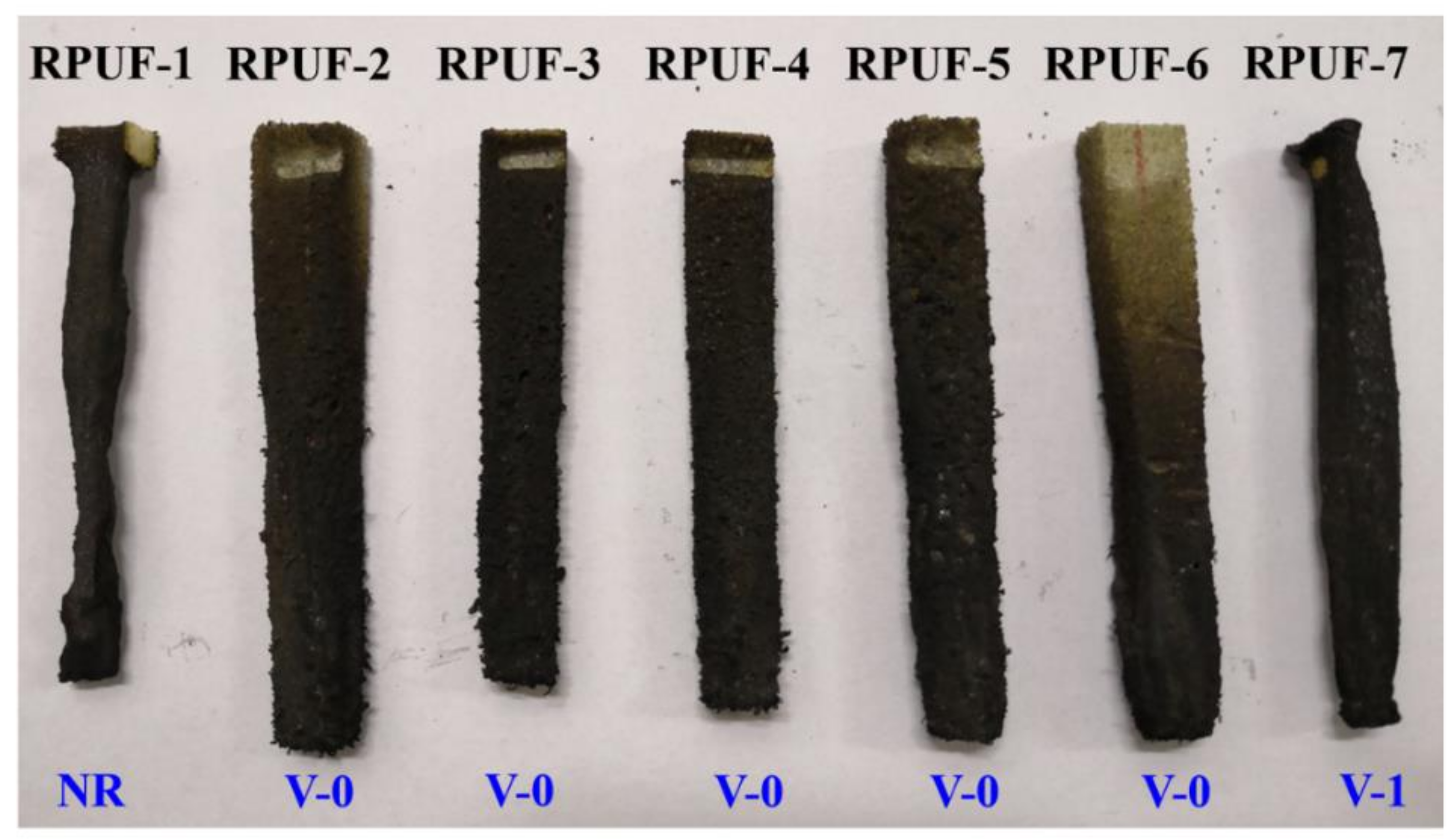
3.2.1. Vertical Combustion Testing
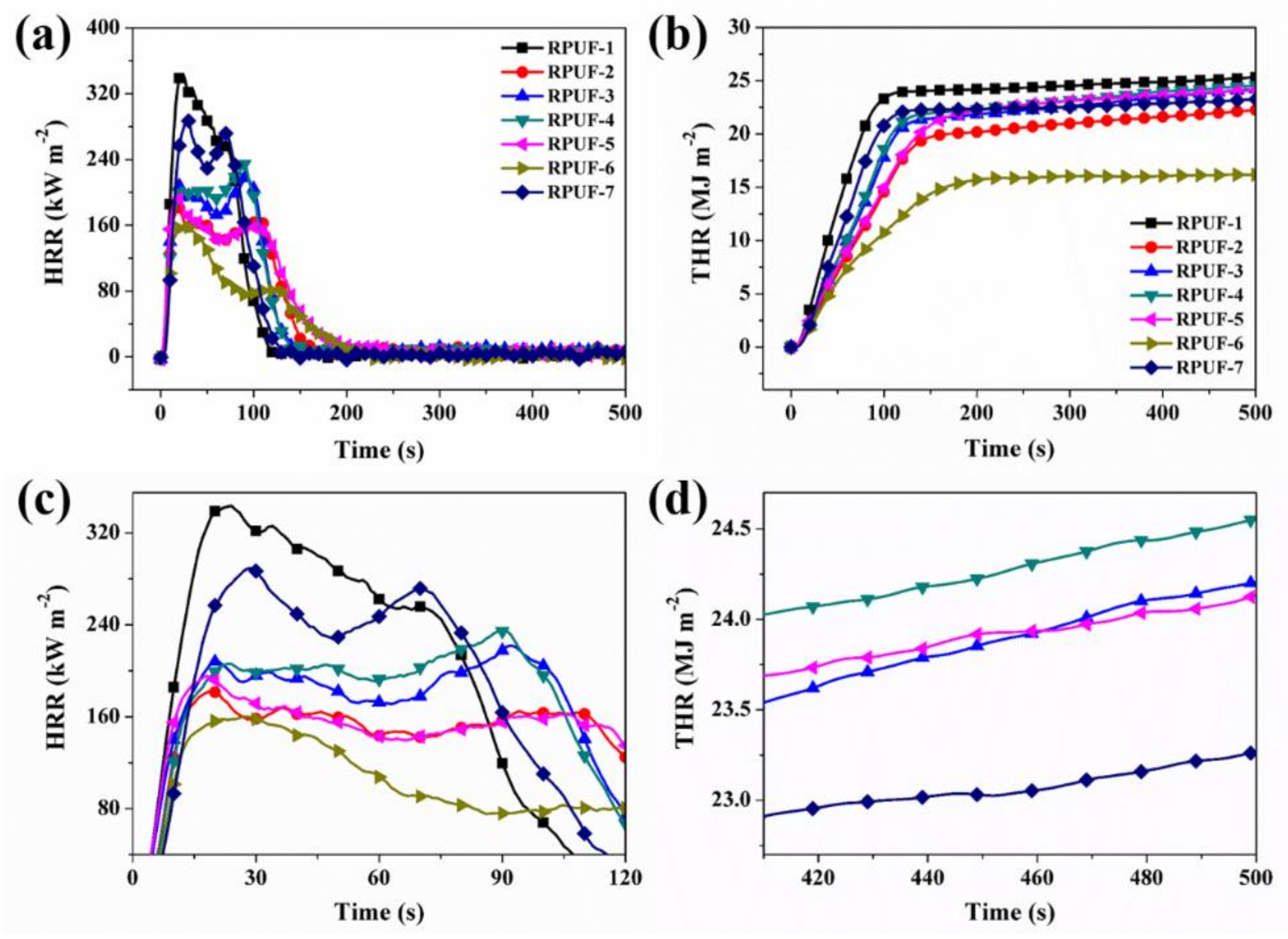
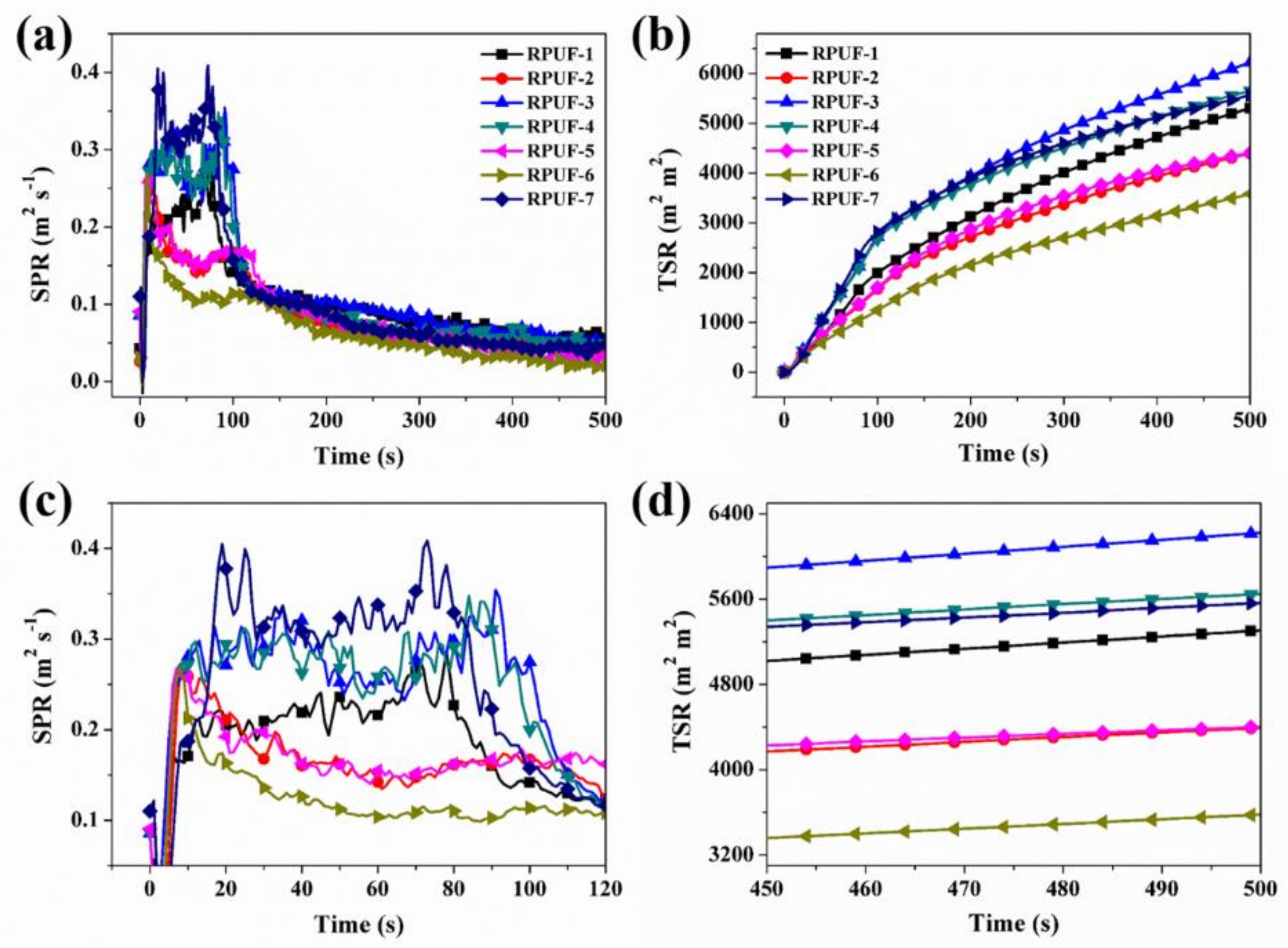
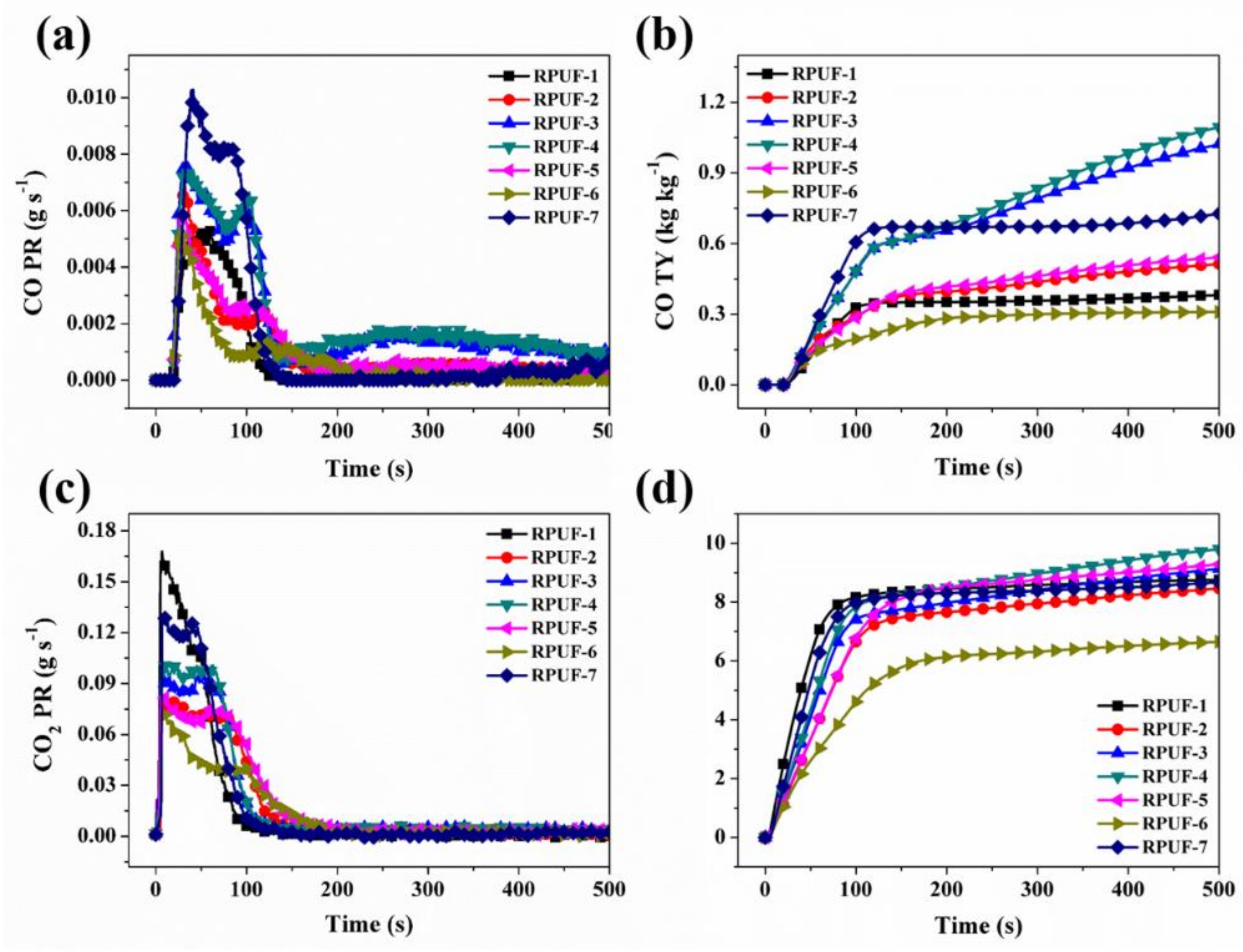
3.2.2. Cone Calorimeter Testing
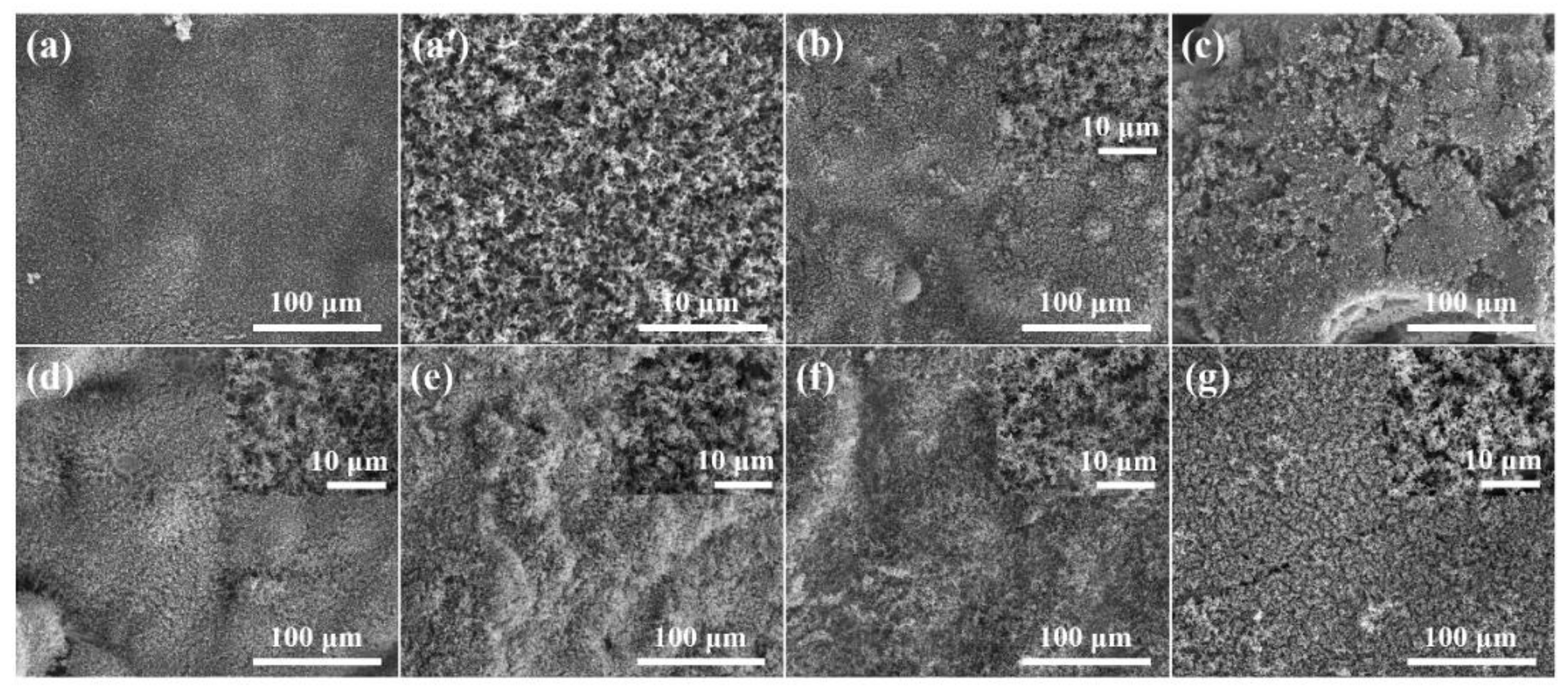
3.3. Flame-Retardant Mode of Action
3.4. Retarding Function of Coal Combustion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Santerre, J.; Woodhouse, K.; Laroche, G.; Labow, R. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials 2005, 26, 7457–7470. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Lai, J.Y. Structure-tensile properties of polyurethanes. Eur. Polym. J. 1997, 33, 1563–1567. [Google Scholar] [CrossRef]
- Cooper, S.L.; Tobolsky, A.V. Properties of linear elastomeric polyurethanes. J. Appl. Polym. Sci. 1966, 10, 1837–1844. [Google Scholar] [CrossRef]
- Chen, H.B.; Shen, P.; Chen, M.J.; Zhao, H.B.; Schiraldi, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Patra, D.; Vangal, P.; Cain, A.A.; Cho, C.; Regev, O.; Grunlan, J.C. Inorganic nanoparticle thin film that suppresses flammability of polyurethane with only a single electrostatically-assembled bilayer. ACS Appl. Mater. Interfaces 2014, 6, 16903–16908. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Thirumal, M.; Singha, N.K.; Khastgir, D.; Manjunath, B.; Naik, Y. Halogen-free flame-retardant rigid polyurethane foams: Effect of alumina trihydrate and triphenylphosphate on the properties of polyurethane foams. J. Appl. Polym. Sci. 2010, 116, 2260–2268. [Google Scholar] [CrossRef]
- Meng, X.Y.; Ye, L.; Zhang, X.G.; Tang, P.M.; Tang, J.H.; Ji, X.; Li, Z.M. Effects of expandable graphite and ammonium polyphosphate on the flame-retardant and mechanical properties of rigid polyurethane foams. J. Appl. Polym. Sci. 2009, 114, 853–863. [Google Scholar] [CrossRef]
- Qian, L.; Feng, F.; Tang, S. Bi-phase flame-retardant effect of hexa-phenoxy-cyclotriphosphazene on rigid polyurethane foams containing expandable graphite. Polymer 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Xing, W.; Yuan, H.; Yang, H.; Song, L.; Hu, Y. Functionalized lignin for halogen-free flame retardant rigid polyurethane foam: Preparation, thermal stability, fire performance and mechanical properties. J. Polym. Res. 2013, 20, 234. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Song, P.; Maluk, C.; Wang, H. Surface-coating engineering for flame retardant flexible polyurethane foams: A critical review. Compos. Part B Eng. 2019, 176, 107185. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Shi, Y.; Song, L.; Ma, C.; Hu, Y. The influence of highly dispersed Cu2O-anchored MoS2 hybrids on reducing smoke toxicity and fire hazards for rigid polyurethane foam. J. Hazard. Mater. 2019, 382, 121028. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, Y.; Yu, B.; Zhan, J.; Zhang, Y.; Song, L.; Ma, C.; Hu, Y. Facile synthesis of aluminum branched oligo(phenylphosphonate) submicro-particles with enhanced flame retardance and smoke toxicity suppression for epoxy resin composites. J. Hazard. Mater. 2019, 381, 121233. [Google Scholar] [CrossRef]
- Chen, M.J.; Shao, Z.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Halogen-free flame-retardant flexible polyurethane foam with a novel nitrogen-phosphorus flame retardant. Ind. Eng. Chem. Res. 2012, 51, 9769–9776. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Green, J. A review of phosphorus-containing flame retardants. J. Fire Sci. 1992, 10, 470–487. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.; Naik, Y.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W. A brief review on fire retardants for polymeric foams. J. Appl. Polym. Sci. 2005, 97, 366–376. [Google Scholar] [CrossRef]
- Duquesne, S.; Le Bras, M.; Bourbigot, S.; Delobel, R.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T.; Vezin, H. Mechanism of fire retardancy of polyurethanes using ammonium polyphosphate. J. Appl. Polym. Sci. 2001, 82, 3262–3274. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Zhou, Y.; Hu, L. The study of mechanical behavior and flame retardancy of castor oil phosphate-based rigid polyurethane foam composites containing expanded graphite and triethyl phosphate. Polym. Degrad. Stab. 2013, 98, 2784–2794. [Google Scholar] [CrossRef]
- Price, D.; Liu, Y.; Milnes, G.J.; Hull, R.; Kandola, B.K.; Horrocks, A.R. An investigation into the mechanism of flame retardancy and smoke suppression by melamine in flexible polyurethane foam. Fire Mater. 2002, 26, 201–206. [Google Scholar] [CrossRef]
- Singh, H.; Jain, A. Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: A comprehensive review. J. Appl. Polym. Sci. 2009, 111, 1115–1143. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.; Naik, Y. Effect of expandable graphite on the properties of intumescent flame-retardant polyurethane foam. J. Appl. Polym. Sci. 2008, 110, 2586–2594. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G.; Zhang, M. Synergistic effect of expandable graphite, diethyl ethylphosphonate and organically-modified layered double hydroxide on flame retardancy and fire behavior of polyisocyanurate-polyurethane foam nanocomposite. Polym. Degrad. Stab. 2014, 101, 92–101. [Google Scholar] [CrossRef]
- Rao, W.H.; Liao, W.; Wang, H.; Zhao, H.B.; Wang, Y.Z. Flame-retardant and smoke-suppressant flexible polyurethane foams based on reactive phosphorus-containing polyol and expandable graphite. J. Hazard. Mater. 2018, 360, 651–660. [Google Scholar] [CrossRef]
- Cheng, W.; Hu, X.; Xie, J.; Zhao, Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, F.B. A comprehensive hazard evaluation system for spontaneous combustion of coal in underground mining. Int. J. Coal Geol. 2010, 82, 27–36. [Google Scholar] [CrossRef]
- Fierro, V.; Miranda, J.; Romero, C.; Andres, J.; Arriaga, A.; Schmal, D.; Visser, G. Prevention of spontaneous combustion in coal stockpiles: Experimental results in coal storage yard. Fuel Process. Technol. 1999, 59, 23–34. [Google Scholar] [CrossRef]
- Pan, R.; Cheng, Y.; Yu, M.; Lu, C.; Yang, K. New technological partition for “three zones” spontaneous coal combustion in goaf. Int. J. Min. Sci. Technol. 2013, 23, 489–493. [Google Scholar] [CrossRef]
- Fierro, V.; Miranda, J.; Romero, C.; Andres, J.; Arriaga, A.; Schmal, D. Model predictions and experimental results on self-heating prevention of stockpiled coals. Fuel 2001, 80, 125–134. [Google Scholar] [CrossRef]
- Smith, A.C.; Miron, Y.; Lazzara, C.P. Inhibition of spontaneous combustion of coal. Rep. Investig. 1988, 9196, 1–16. [Google Scholar]
- Tosun, A. Development of a technology to prevent spontaneous combustion of coal in underground coal mining. J. S. Afr. Inst. Min. Metall. 2017, 117, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Qin, B. Development of a New Material for Mine Fire Control. Combust. Sci. Technol. 2014, 186, 928–942. [Google Scholar] [CrossRef]
- Li, L.J.; Duan, R.T.; Zhang, J.B.; Wang, X.L.; Chen, L.; Wang, Y.Z. Phosphorus-Containing Poly(ethylene terephthalate): Solid-State Polymerization and Its Sequential Distribution. Ind. Eng. Chem. Res. 2013, 52, 5326–5333. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, R.K.K. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stab. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal degradation characteristics of rigid polyurethane foam and the volatile products analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Backus, J.; Darr, W.; Gemeinhardt, P.; Saunders, J. Thermal decomposition of rigid urethane foams. J. Cell. Plast. 1965, 1, 178–186. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, D.M. Enhanced fire behavior of rigid polyurethane foam by intumescent flame retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Zheng, Y.Y.; Yang, J.; Duan, Z.P.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interface Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yin Yuen, A.C.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, P.; Mou, Y.; Kang, M.; Liu, M.; Song, L.; Lu, A.; Rong, J. Synthesis of the polyethylene glycol solid-solid phase change materials with a functionalized graphene oxide for thermal energy storage. Polym. Test. 2017, 63, 494–504. [Google Scholar] [CrossRef]
- Yuan, B.; Hu, Y.; Chen, X.; Shi, Y.; Niu, Y.; Zhang, Y.; He, S.; Dai, H. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos. Part A Appl. Sci. Manuf. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- He, Y.; Zhou, M.; Wu, B.; Jiang, Z.; Nie, J. Synthesis and properties of novel polyurethane acrylate containing 3-(2-hydroxyethyl) isocyanurate segment. Prog. Org. Coat. 2010, 67, 264–268. [Google Scholar] [CrossRef]
- Ding, H.; Xia, C.; Wang, J.; Wang, C.; Chu, F. Inherently flame-retardant flexible bio-based polyurethane sealant with phosphorus and nitrogen-containing polyurethane prepolymer. J. Mater. Sci. 2016, 51, 5008–5018. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Zheng, Y.Y.; Guo, J.; Chen, B.H.; Pan, Z.M.; Hu, Y. A combination of POSS and polyphosphazene for reducing fire hazards of epoxy resin. Polym. Adv. Technol. 2018, 29, 1242–1254. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crop. Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Usta, N. Investigation of fire behavior of rigid polyurethane foams containing fly ash and intumescent flame retardant by using a cone calorimeter. J. Appl. Polym. Sci. 2012, 124, 3372–3382. [Google Scholar] [CrossRef]
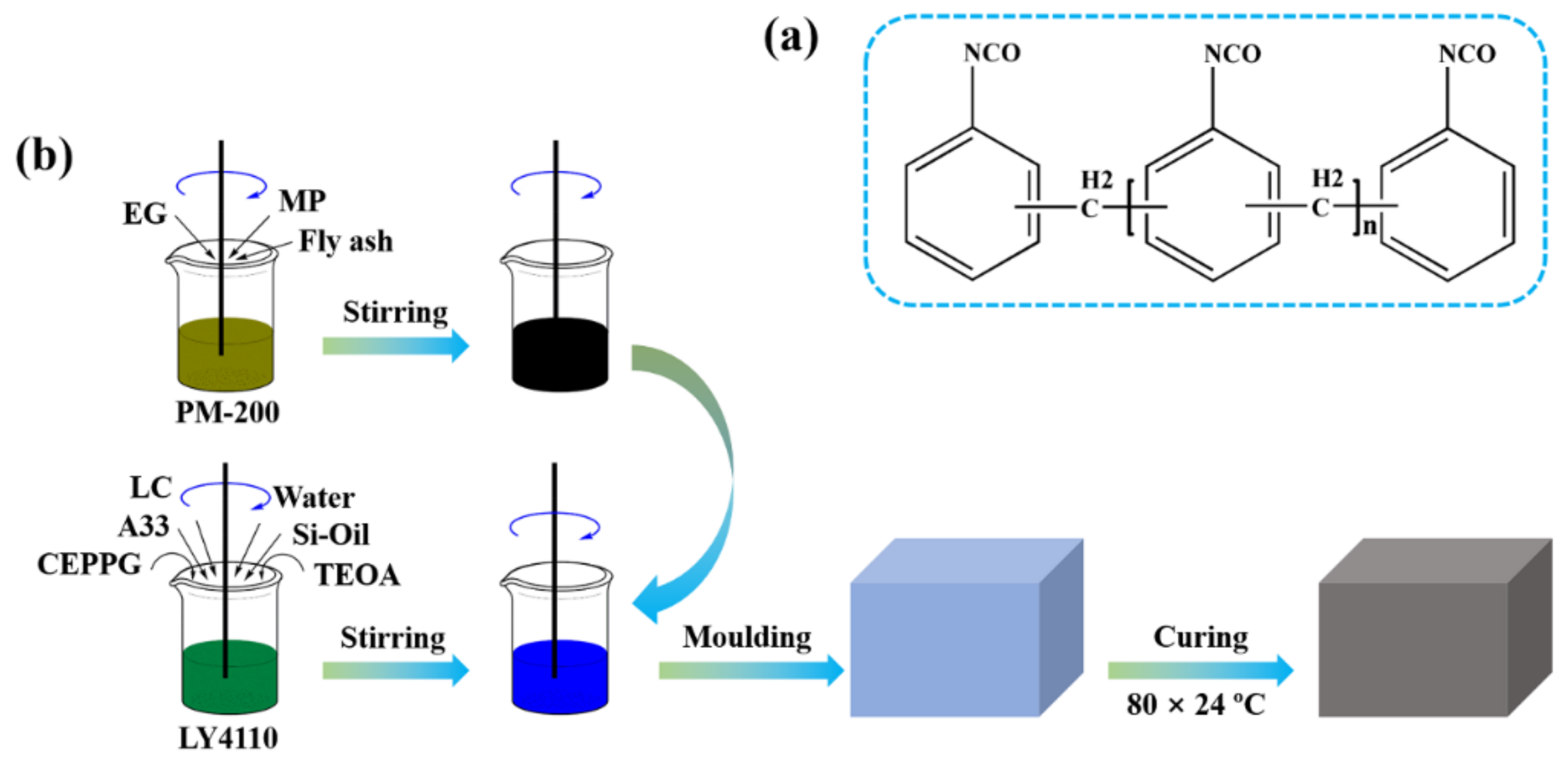


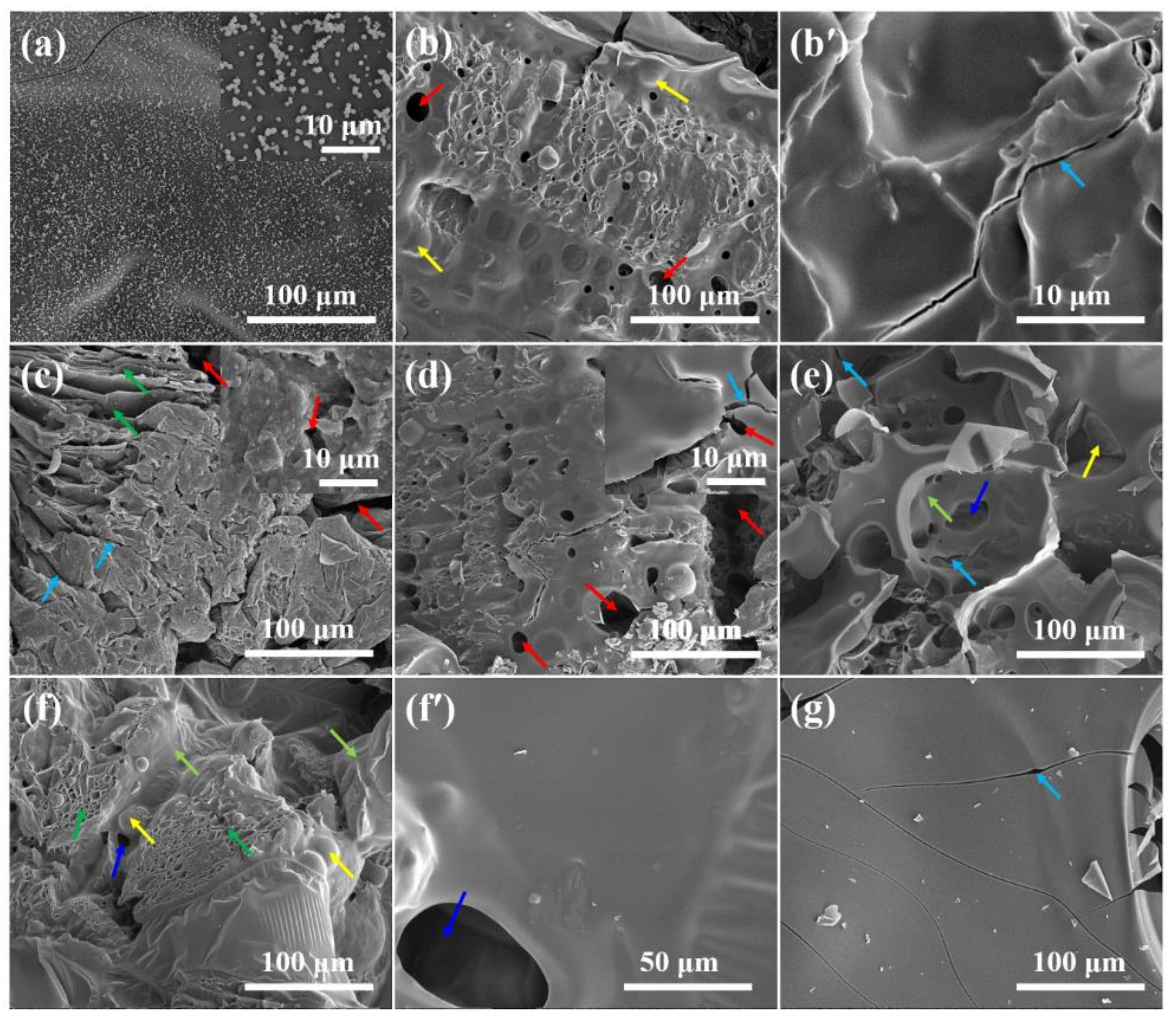

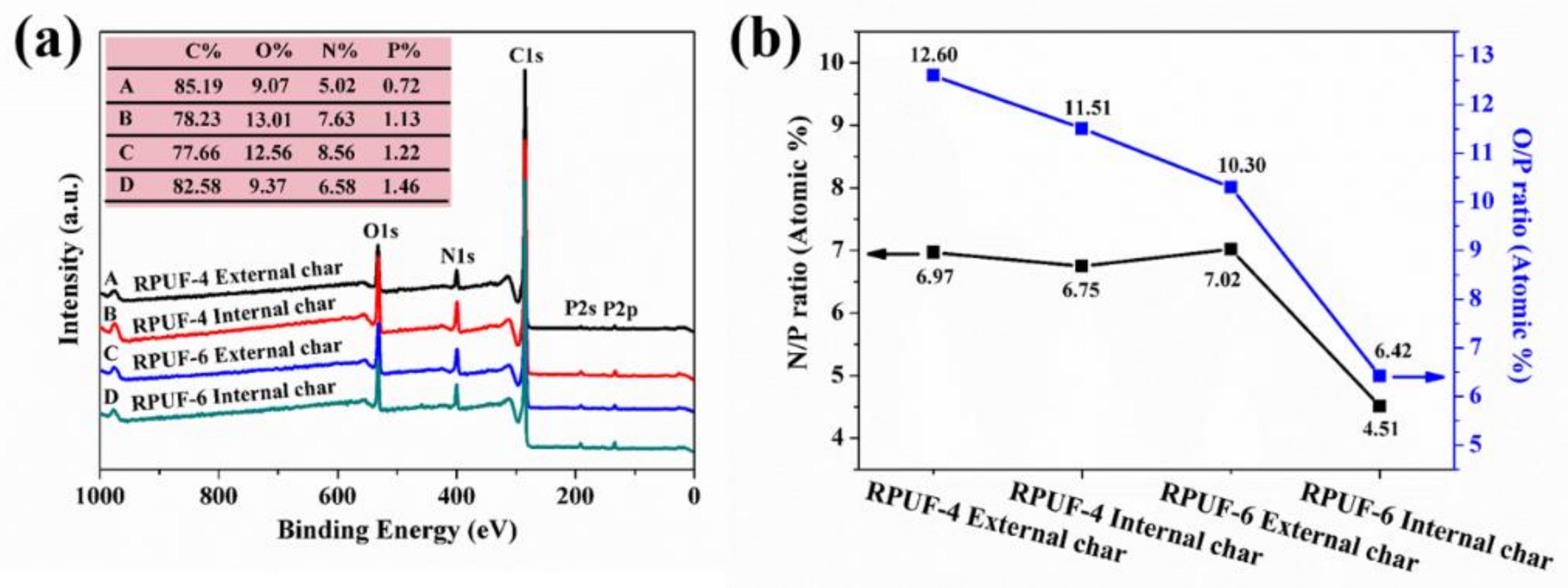
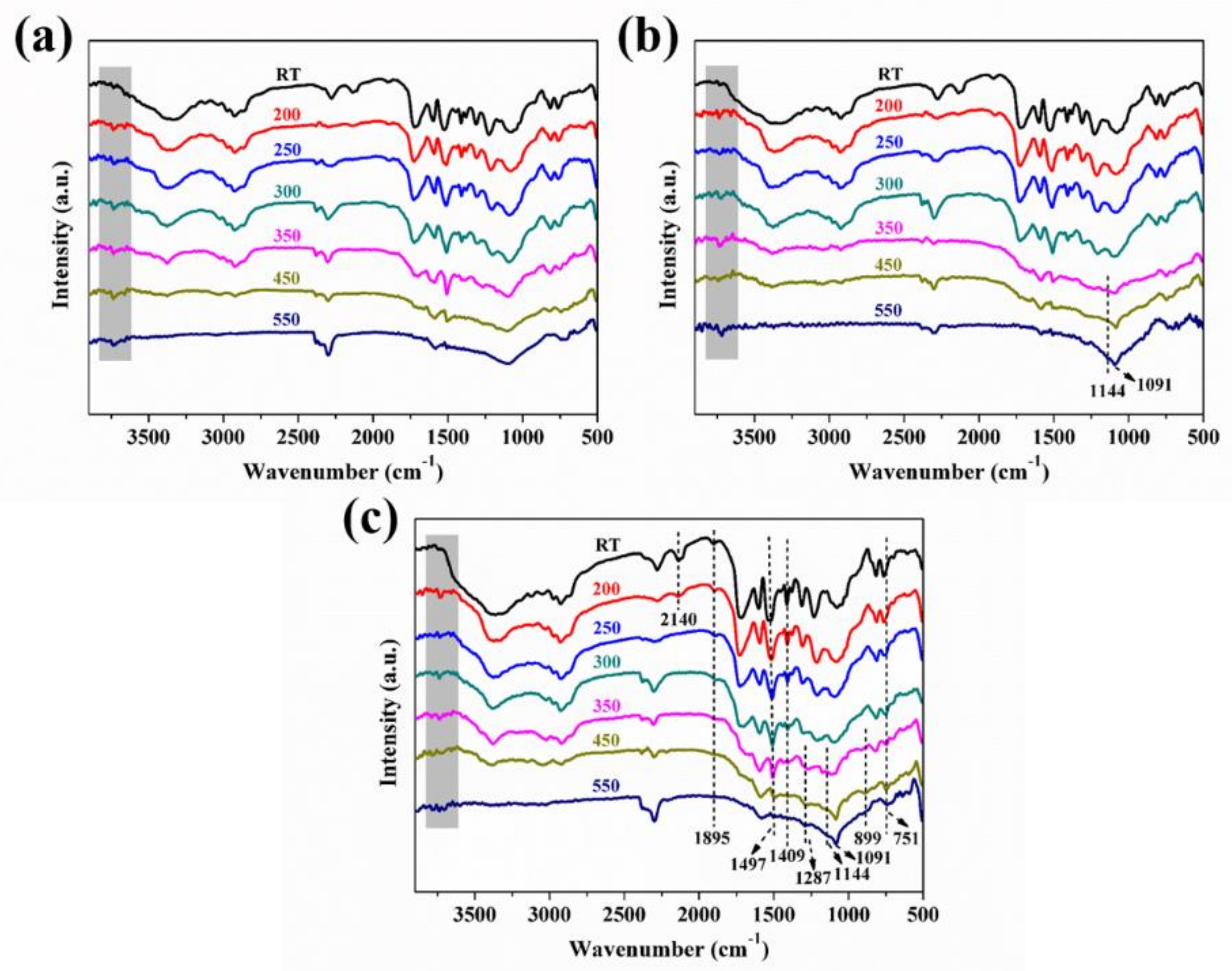
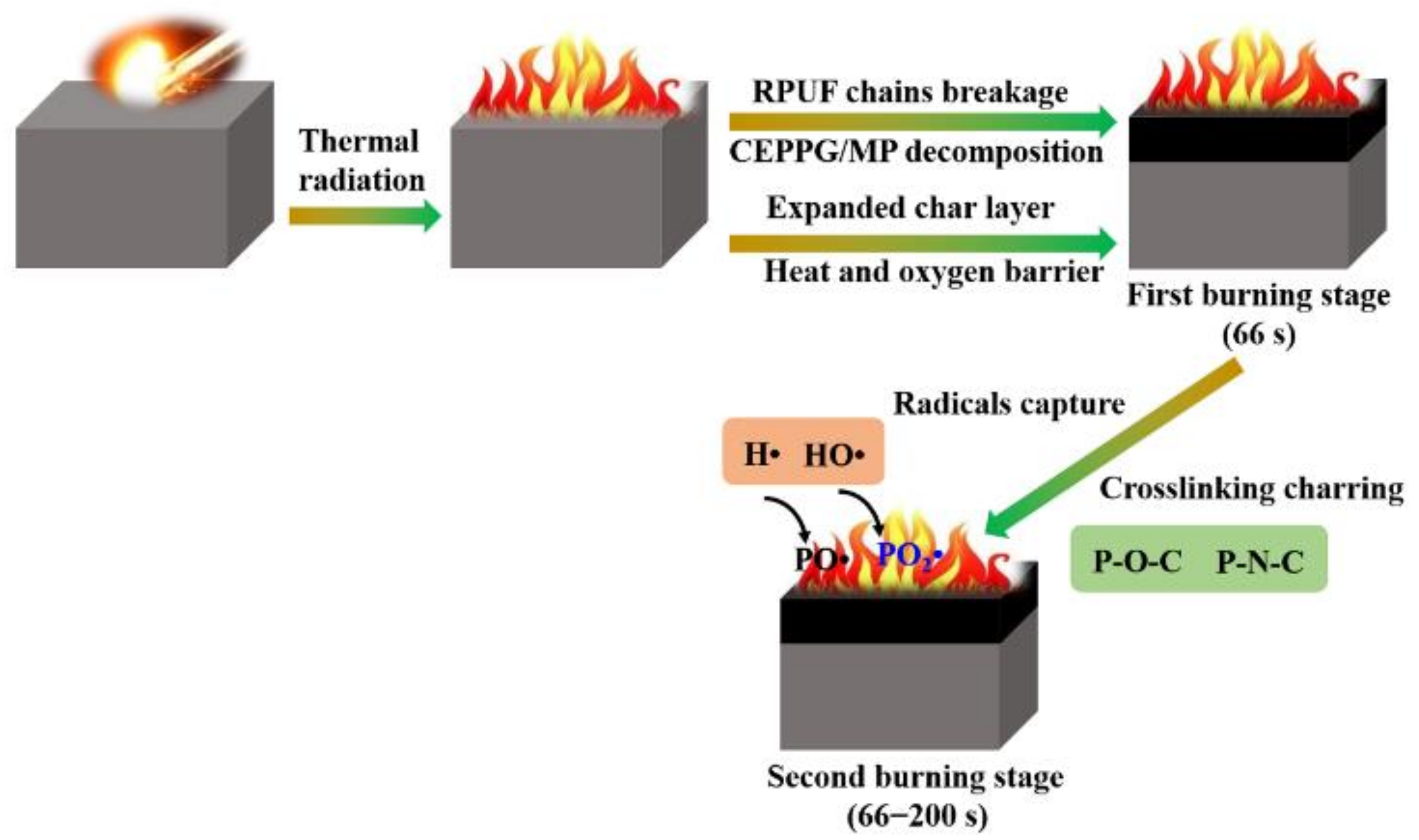

| Sample No. | RPUF-1 | RPUF-2 | RPUF-3 | RPUF-4 | RPUF-5 | RPUF-6 | RPUF-7 |
|---|---|---|---|---|---|---|---|
| LY4110 (g) | 100.00 | 77.47 | 77.47 | 77.47 | 77.47 | 77.47 | 77.47 |
| CEPPG (g) | 0.00 | 22.53 | 22.53 | 22.53 | 22.53 | 22.53 | 22.53 |
| A33 (g) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| LC (g) | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Water (g) | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Si–Oil (g) | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| TEOA (g) | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| PM-200 (g) | 151.66 | 149.12 | 149.12 | 149.12 | 149.12 | 149.12 | 149.12 |
| EG (g) | 0.00 | 13.50 | 18.00 | 20.25 | 9.00 | 6.75 | 0.00 |
| MP (g) | 0.00 | 13.50 | 9.00 | 6.75 | 18.00 | 20.25 | 0.00 |
| Fly ash (g) | 0.00 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 0.00 |
| NCO index | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| P wt % | 0.00 | 1.46% | 1.25% | 1.14% | 1.68% | 1.78% | 0.82% |
| Sample No. | T−5 (°C) | T−50 (°C) | Tmax (°C) | Residue (wt %) | |
|---|---|---|---|---|---|
| Step 1 | Step 2 | ||||
| RPUF-1 | 249.9 | 384.5 | 297.8 | 585.8 | 1.99 |
| RPUF-2 | 232.2 | 502.9 | 296.7 | 559.3 | 10.34 |
| RPUF-3 | 246.9 | 513.2 | 302.7 | 560.5 | 11.68 |
| RPUF-4 | 252.1 | 519.0 | 305.7 | 566.7 | 11.96 |
| RPUF-5 | 241.2 | 496.8 | 301.6 | 565.5 | 9.97 |
| RPUF-6 | 246.8 | 497.4 | 301.2 | 576.5 | 10.51 |
| RPUF-7 | 253.1 | 484.8 | 309.3 | 584.4 | 2.40 |
| Sample No. | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | PSPR (m2 s−1) | TSR (m2 m−2) | PCO PR (g s−1) | PCO2 PR (g s−1) | CO TY (kg kg−1) | CO2 TY (kg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| RPUF-1 | 4 | 339 | 25.4 | 0.285 | 5306 | 0.0053 | 0.168 | 0.38 | 8.76 |
| RPUF-2 | 5 | 183 | 22.3 | 0.275 | 4389 | 0.0069 | 0.079 | 0.51 | 8.46 |
| RPUF-3 | 4 | 221 | 24.2 | 0.354 | 6217 | 0.0076 | 0.095 | 1.02 | 9.14 |
| RPUF-4 | 5 | 235 | 24.6 | 0.348 | 5656 | 0.0075 | 0.100 | 1.10 | 9.80 |
| RPUF-5 | 4 | 195 | 24.1 | 0.268 | 4401 | 0.0058 | 0.082 | 0.54 | 9.29 |
| RPUF-6 | 5 | 160 | 16.3 | 0.268 | 3585 | 0.0054 | 0.072 | 0.31 | 6.66 |
| RPUF-7 | 6 | 289 | 23.3 | 0.410 | 5570 | 0.0103 | 0.127 | 0.73 | 8.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tawiah, B.; Shi, Y.; Cai, S.; Rao, X.; Liu, C.; Yang, Y.; Yang, F.; Yu, B.; Liang, Y.; et al. Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion. Polymers 2019, 11, 1776. https://doi.org/10.3390/polym11111776
Wang L, Tawiah B, Shi Y, Cai S, Rao X, Liu C, Yang Y, Yang F, Yu B, Liang Y, et al. Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion. Polymers. 2019; 11(11):1776. https://doi.org/10.3390/polym11111776
Chicago/Turabian StyleWang, Liancong, Benjamin Tawiah, Yongqian Shi, Suncheng Cai, Xiaohui Rao, Chuan Liu, Ye Yang, Fuqiang Yang, Bin Yu, Yuntao Liang, and et al. 2019. "Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion" Polymers 11, no. 11: 1776. https://doi.org/10.3390/polym11111776