Synergistic Flame-Retardant Mechanism of Dicyclohexenyl Aluminum Hypophosphite and Nano-Silica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Sample Preparation
2.3. Flame-Retardant Performance Test
3. Results and Discussion
3.1. Formulation and Combustion Properties of PA66 and Its Composites
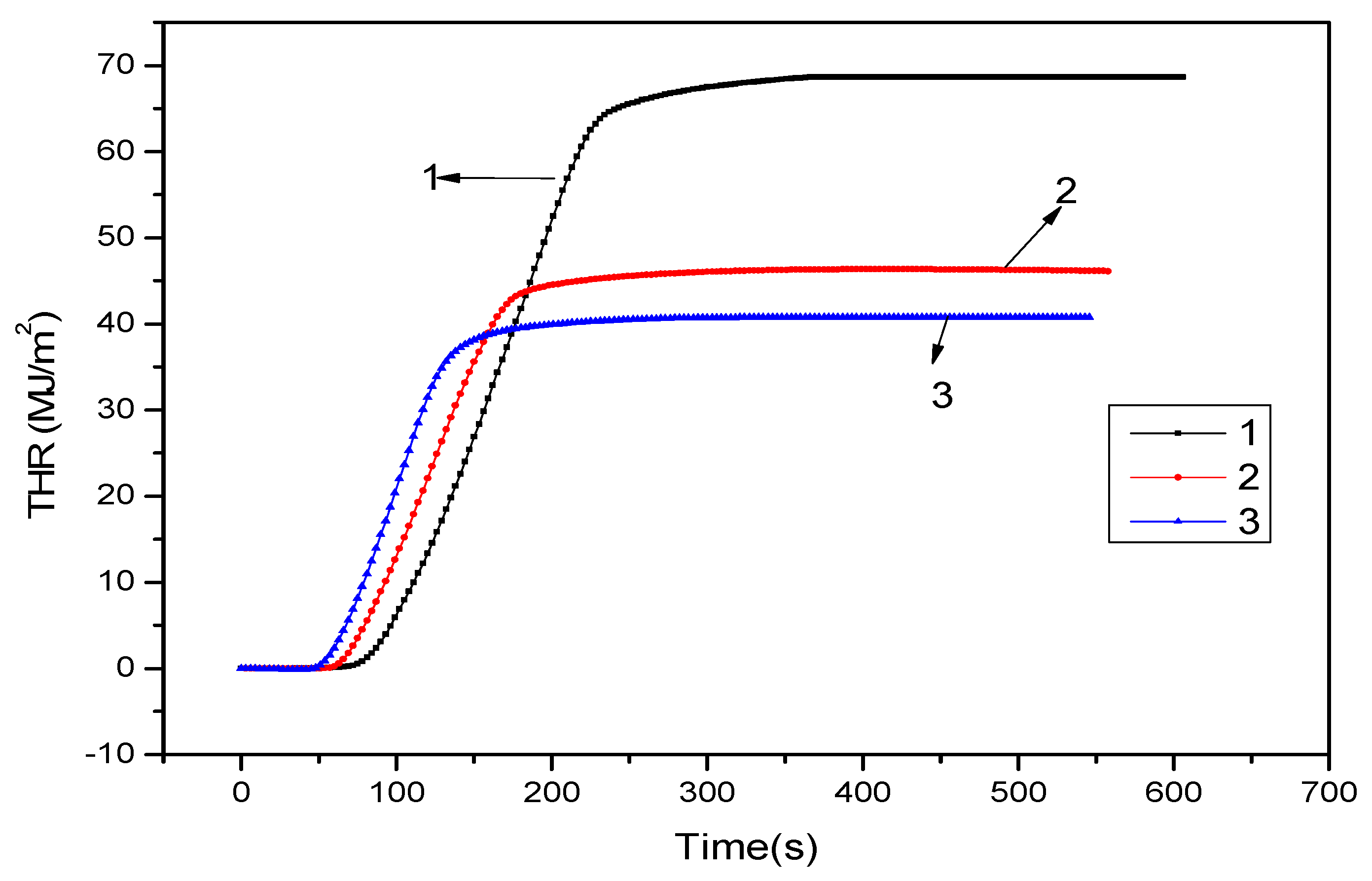
3.2. Cone Calorimeter Analysis
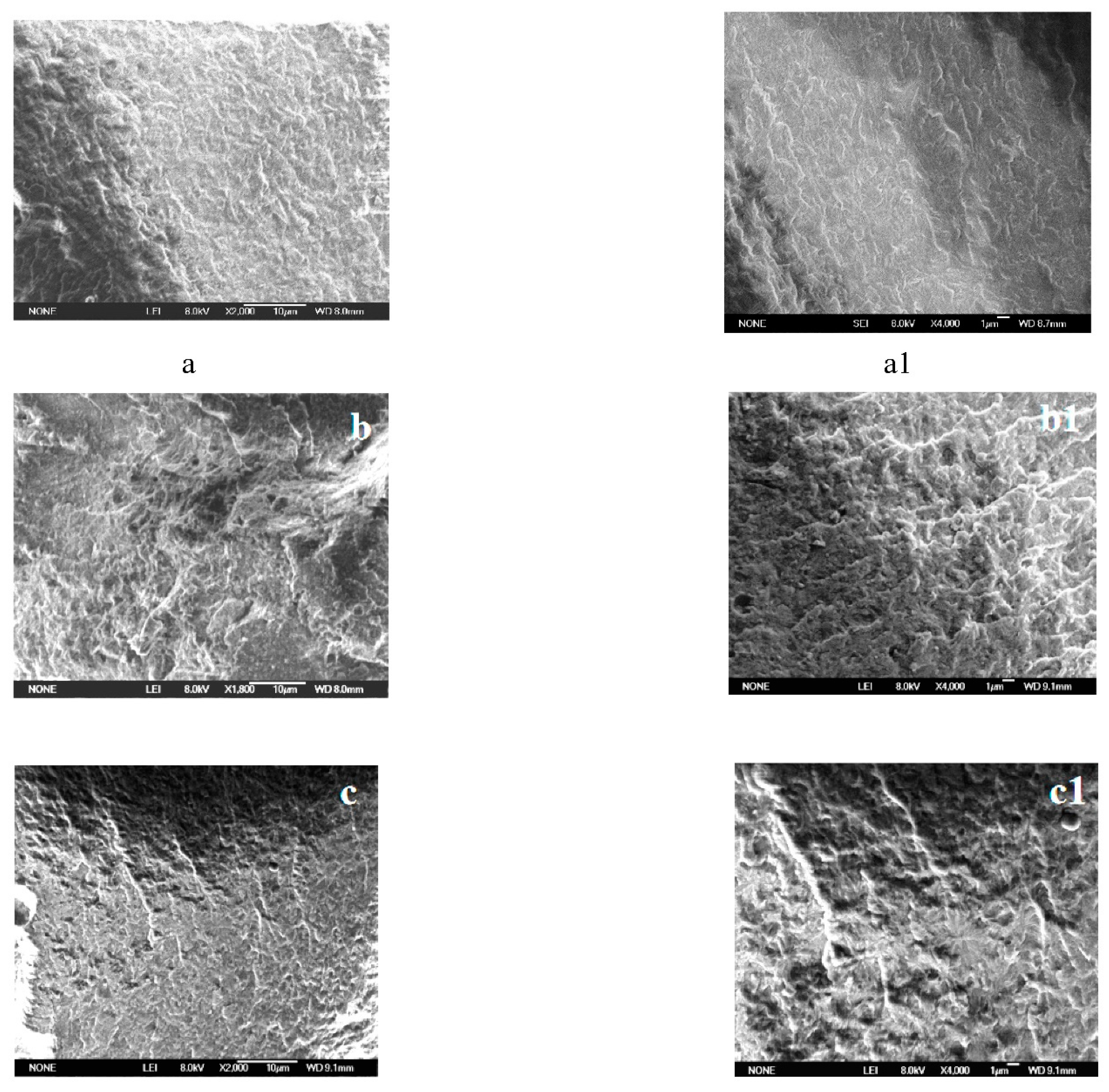
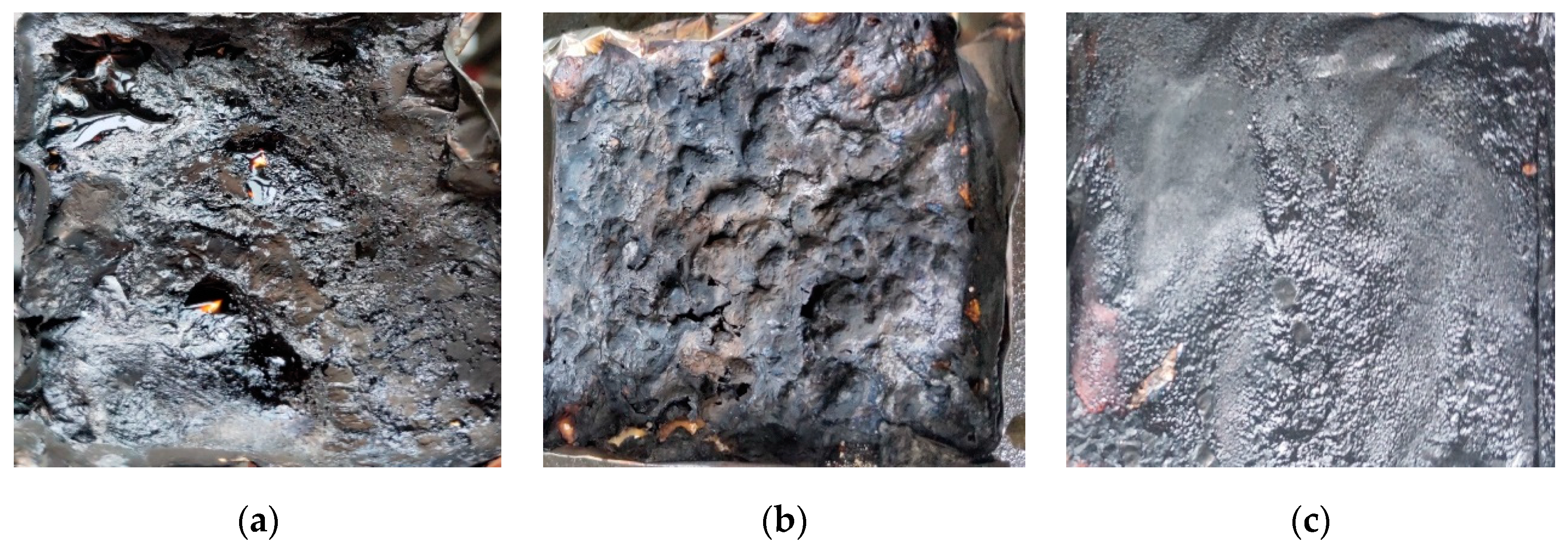
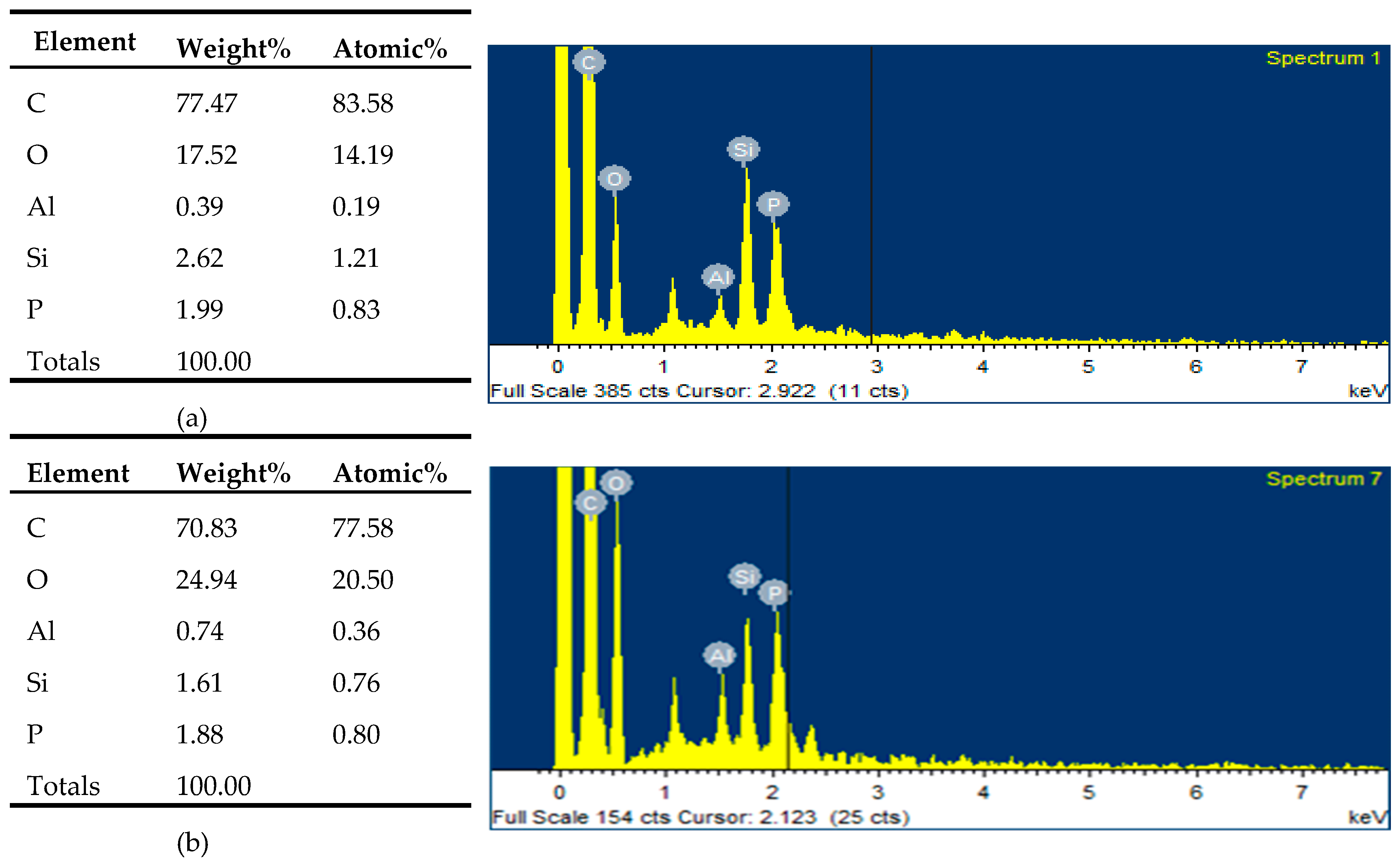
3.3. SEM and EDSanalysis after Combustion
3.4. Fourier Infrared Analysis and Detection
3.5. Analysis of Cooperative Flame-Retardant Mechanism
HPO · + H · → H2 + PO ·
PO · + OH · → HPO + · O ·
SiO2(s) + 2C(s) → Si(I) + 2CO(g) + 644.3
SiO2(s) + 3C(s) → SiC(s) + 2CO(g) + 512.6
SiC(s) + 2SiO2(s) → 3SiO(g) + CO(g) + 1372.9
SiO2(s) + Si(I) → 2SiO(s) + 614.7
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Wazarkar, K.; Kathalewar, M.; Sabnis, A. Reactive modification of thermoplastic and thermoset polymers using flame retardants: An Overview. Polym.-Plast. Technol. 2016, 55, 71–91. [Google Scholar] [CrossRef]
- Schmitt, E. Phosphorus-based flame retardants for thermoplastics. Plast. Addit. Compd. 2007, 9, 26–30. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perezc, R.; Sandler, J.K.W.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Lewin, M.; Weil, E.D. Mechanism and modes of action in flame retardancy of polymers. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 31–68. [Google Scholar]
- Chen, M.J.; Xu, Y.J.; Rao, W.H.; Huang, J.Q.; Wang, X.L.; Chen, L.; Wang, Y.Z. Influence of valence and structure of phosphorus-containing melamine salts on the decomposition and fire behaviors of flexible polyurethane foams. Ind. Eng. Chem. Res. 2014, 53, 8773–8783. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Jian, R.K.; Wang, Y.Z. Synergistic effect between aluminum hypophosphite and alkyl-substituted phosphinate in flame-retarded polyamide 6. Ind. Eng. Chem. Res. 2013, 48, 17162–17170. [Google Scholar] [CrossRef]
- Cao, Y.F.; Qian, L.J.; Chen, Y.J.; Wang, Z. Synergistic flame-retardant effect of phosphaphenanthrene derivative and aluminum diethylphosphinate in glass fiber reinforced polyamide 66. J. Appl. Polym. Sci. 2017, 134, 45126. [Google Scholar] [CrossRef]
- He, W.T.; Zhu, H.; Xiang, Y.S.; Long, L.J.; Qin, S.H.; Yu, J. Enhancement of flame retardancy and mechanical properties of polyamide 6 by incorporating an aluminum salt of diisobutylphosphinic combined with organoclay. Polym. Degrad. Stab. 2017, 144, 442–453. [Google Scholar] [CrossRef]
- Ma, J.J.; Yang, J.X.; Huang, Y.W.; Ke, C. Aluminum-organo phosphorus hybrid nanorods for simultaneously enhancing the flame retardancy and mechanical properties of epoxy resin. J. Mater. Chem. 2012, 22, 2007–2017. [Google Scholar]
- Si, M.M.; Feng, J.; Hao, J.W.; Xu, L.S.; Du, J.X. Synergistic flame retardant effects and mechanisms of nano-Sb2O3 in combination with aluminum phosphinate in poly (ethylene terephthalate). Polym. Degrad. Stab. 2014, 100, 70–78. [Google Scholar] [CrossRef]
- Gallo, E.; Schartel, B.; Braun, U.; Russo, P.; Acierno, D. Fire retardant synergisms between nanometric Fe2O3 and aluminum phosphinate in poly(butylene terephthalate). Polym. Adv. Technol. 2011, 22, 2382–2391. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Xue, L.; Mu, K.J.; Tian, M.G.; Qingdao University of Science & Technology, College of Marine Science and Biological Engineering; Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control; Scien Green (Shandong) Environmental Technology Co. Ltd. Study on synthesis process and properties of flame retardant of dicyclohexenyl aluminum hypophosphite at normal pressure. Polym. Bull. 2018, 3, 59–66. [Google Scholar]
- Zhang, H.; Yang, H.Y.; Yang, H.; Lu, J.L.; Mu, K.J.; Guo, Z.S. Pyrolysis dynamics of dicyclohexyl phosphonic acid aluminium flame retardant polyamide. Plast. Rubber Compos. 2019, 48, 240–247. [Google Scholar] [CrossRef]
- Plastics Combustion Performance Test Method Oxygen Index Method. China, GB/T 2406.1-2008. Available online: http://www.bzwxw.com/find?page=1&key=GB%2FT+2406.1-2008&Submit=%E6%A0%87%E5%87%86%E6%9F%A5%E8%AF%A2 (accessed on 18 July 2019).
- Klatt, M.; Heitz, T.; Gareiss, B. Flame-Proof Thermoplastic Moulding Materials. U.S. Patent 6,306,941, 12 September 2003. [Google Scholar]
- Gosens, J.C.; Wit, D.G. Flame Retardant Polyester Compositions. U.S. Patent 3,873,496, 25 March 1975. [Google Scholar]
- Test Method for Plastic Burning Performance Horizontal Method and Vertical Method. China, GB/T 2408-2008. Available online: http://www.bzwxw.com/find?page=1&key=GB%2FT+2408-2008&Submit=%E6%A0%87%E5%87%86%E6%9F%A5%E8%AF%A2 (accessed on 18 July 2019).
- Deng, Y.; Wang, Y.Z.; Zong, Z.J.; Liu, X.H.; Du, X.H. Study on the effects of TLCP on pyrolysis of PET and its retardant mechanism. Chin. J. Polym. Sci. 2011, 26, 111–116. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]



| Sample | PA66 | ADCP | Nano-Silica | LOI | UL-94 | |
|---|---|---|---|---|---|---|
| (wt%) | (wt%) | (wt%) | (%) | Dripping | Rating | |
| PA66-0 | 100 | 0 | 0 | 21.5 | Y | V-2 |
| PA66-1 | 85 | 15 | 0 | 32 | N | V-0 |
| PA66-2 | 88 | 12 | 0 | 30.5 | N | V-0 |
| PA66-3 | 87 | 12 | 1 | 31.7 | N | V-0 |
| PA66-4 | 85 | 12 | 3 | 33.1 | N | V-0 |
| PA66-5 | 83 | 12 | 5 | 35.2 | N | V-0 |
| PA66 | 15%ADCP FR-PA66 | 12%ADCP/3% Nano-Silica FR-PA66 | |
|---|---|---|---|
| Peak MLR(g/s) | 0.13 | 0.08 | 0.06 |
| Peak SPR(m2/s) | 0.16 | 0.12 | 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Lu, J.; Yang, H.; Yang, H.; Lang, J.; Zhang, Q. Synergistic Flame-Retardant Mechanism of Dicyclohexenyl Aluminum Hypophosphite and Nano-Silica. Polymers 2019, 11, 1211. https://doi.org/10.3390/polym11071211
Zhang H, Lu J, Yang H, Yang H, Lang J, Zhang Q. Synergistic Flame-Retardant Mechanism of Dicyclohexenyl Aluminum Hypophosphite and Nano-Silica. Polymers. 2019; 11(7):1211. https://doi.org/10.3390/polym11071211
Chicago/Turabian StyleZhang, Heng, Junliang Lu, Hongyan Yang, Heng Yang, Jinyan Lang, and Qinqin Zhang. 2019. "Synergistic Flame-Retardant Mechanism of Dicyclohexenyl Aluminum Hypophosphite and Nano-Silica" Polymers 11, no. 7: 1211. https://doi.org/10.3390/polym11071211





