Sensitivity Enhancement of Acetone Gas Sensor using Polyethylene Glycol/Multi-Walled Carbon Nanotubes Composite Sensing Film with Thermal Treatment
Abstract
:1. Introduction
2. Materials and Device Design
2.1. Materials
2.2. Preparation of Composite Sensing Film
2.3. Optimum Design of Thermostat for Gas Sensor
2.4. Gas Sensor Fabrication
- (A-1.1) The top layer of the sensor was the interdigitated electrodes (IDEs). Copper with 35 µm thickness was placed on the 50 µm thick polyimide.
- (A-1.2)–(A-1.3) The Cu IDEs were fabricated via ultraviolet (UV) lithography and wet etching. Both the width and spacing of Cu IDEs were 220 μm.
- (A-1.4) The through-hole machined wells were fabricated via UV lithography and wet etching, then placed around the IDEs, and bonded using acrylic adhesives.
- (A-2.1) The selected heating material of stainless steel (SUS304) with 30 μm thickness was placed on the 50 µm thick polyimide.
- (A-2.2)–(A-2.3) The geometry of the optimize thermostat was designated as 20 mm × 20 mm. The width and spacing of the thermostat line were 220 and 280 μm, respectively. The double-spiral shape wire was patterned on a substrate using UV lithography and wet etching
- (A-3) The fabricated IDEs were aligned and laminated onto the thermostat via acrylic adhesives.
- (1)
- In the first step, the MWCNTs was sonicated for 10 min in an ultrasonic bath at room temperature. A 4 μL solution of the MWCNTs dispersion was then deposited on the IDEs by a microjet and then placed in an oven to furnish the MWCNTs film at 80 °C for 6 hours to form the conductive layer.
- (2)
- In the second step, A 8 μL solution of PEG was then deposited on the MWCNTs layer to form the film. The fabricated device was placed in the oven to completely evaporate the solvent at 80 °C for 12 h.
3. Experiment
4. Results and Discussion
4.1. Thermostat Performance
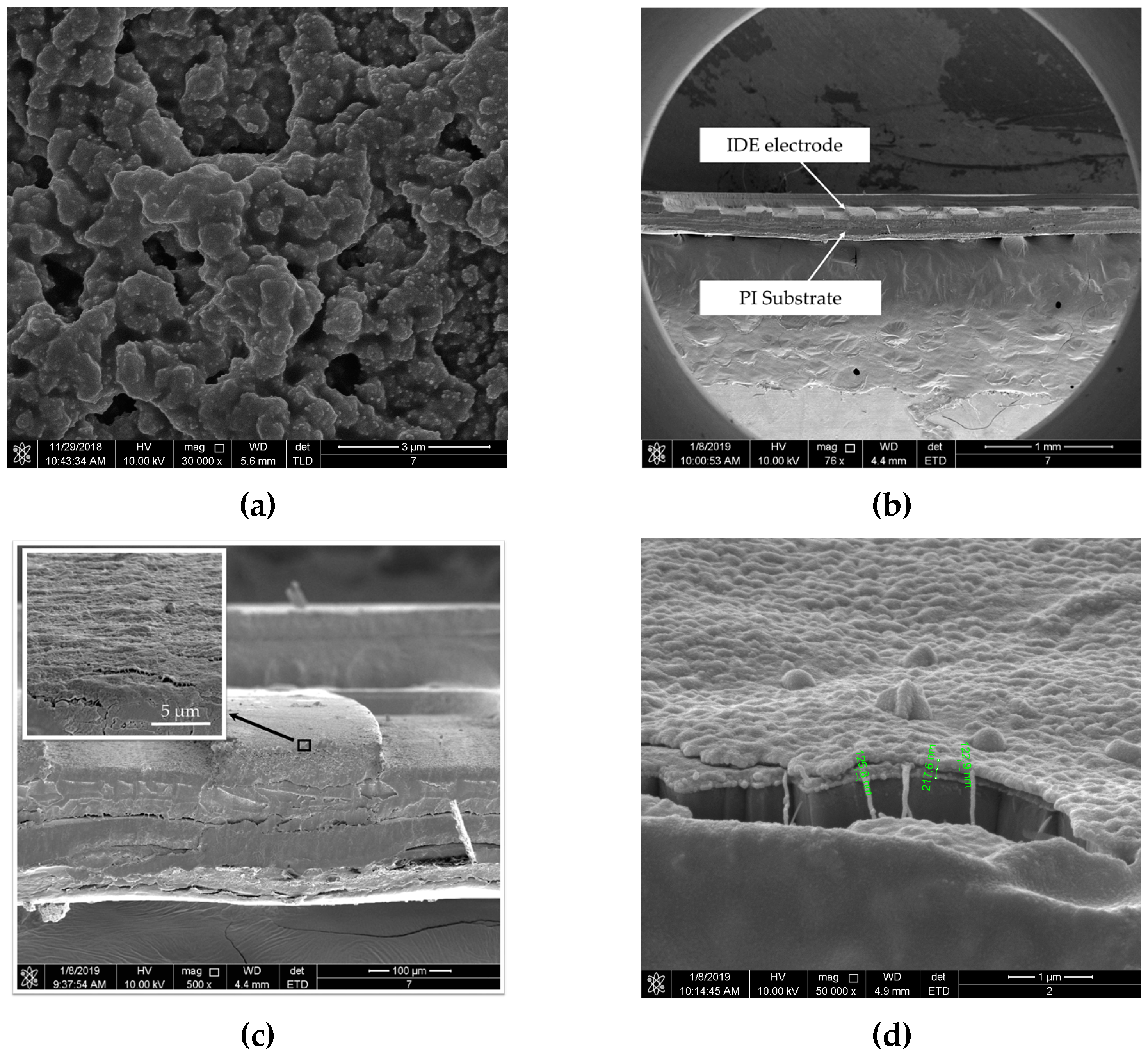
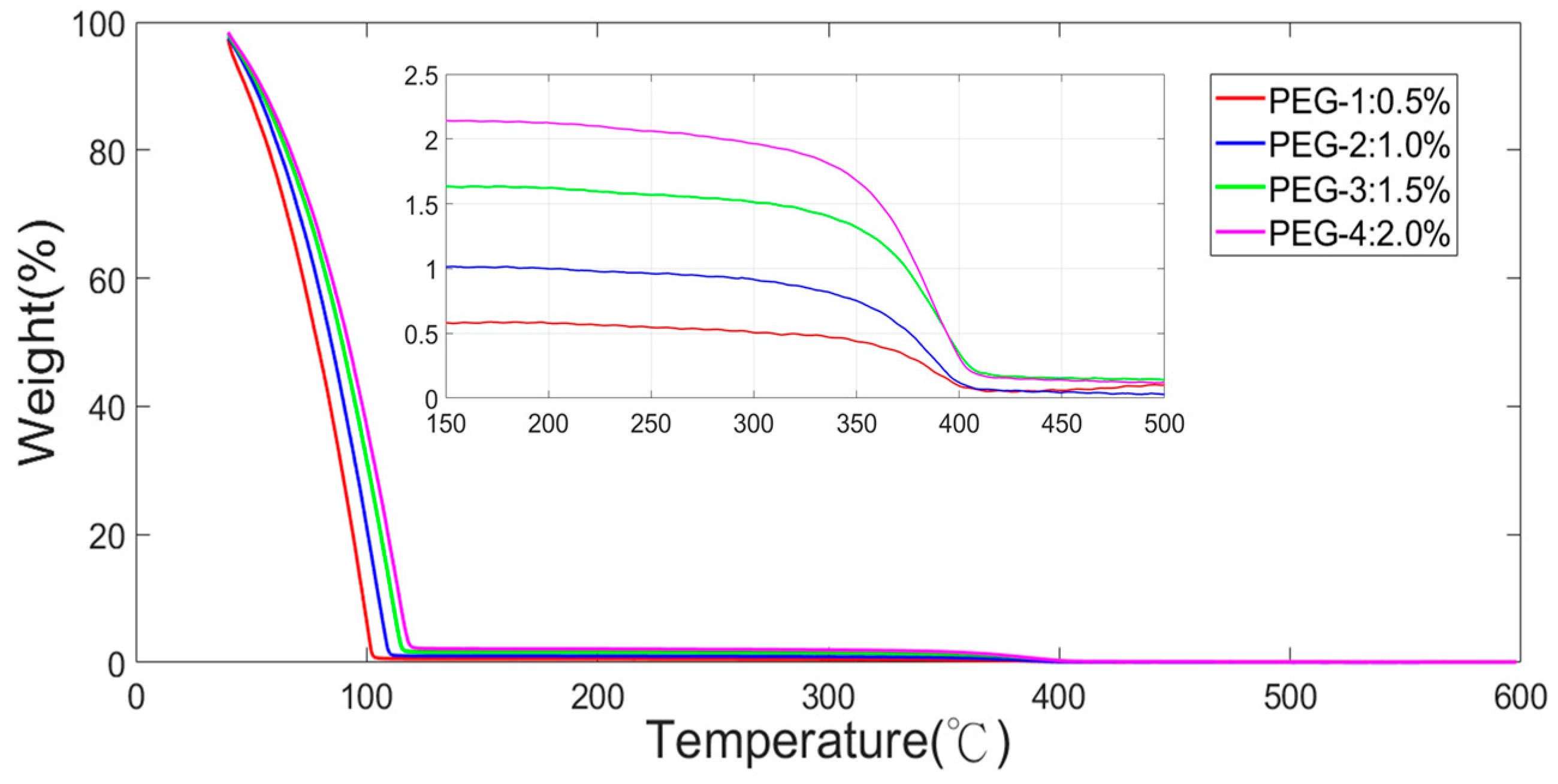
4.2. Characterizations
4.3. Response of Single Film and Composited Film to Acetone Gas
4.4. Sensor Response of Acetone with Thermal Treatment
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Ngo, J.; Blake, D.; Meinardi, S.; Pontello, A.M.; Newcomb, R.; Galassetti, P.R. Improved predictive models for plasma glucose estimation from multi-linear regression analysis of exhaled volatile organic compounds. J. Appl. Physiol. 2009, 107, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Righettoni, M.; Schmid, A.; Amann, A.; Pratsinis, S.E. Correlations between blood glucose and breath components from portable gas sensors and PTR-TOF-MS. J. Breath Res. 2013, 7, 037110. [Google Scholar] [CrossRef] [PubMed]
- Federal Advisory Council on Occupational Safety and Health. Recommendations for Consideration by the U.S. Secretary of Labor on the Adoption and Use of Occupational Exposure Limits by Federal Agencies. Available online: https://www.osha.gov/dep/facosh/Exhibit_9b.pdf (accessed on 5 December 2018).
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, C.; Guo-Guang, W. Synthesis and improved acetone sensing properties of porous α-Fe2O3 nanowires. Chin. Phys. Lett. 2013, 30, 020701. [Google Scholar] [CrossRef]
- Zhang, H.; Cen, Y.; Du, Y.; Ruan, S. Enhanced Acetone Sensing Characteristics of ZnO/Graphene Composites. Sensors 2016, 16, 1876. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.; Nadargi, D.; Mulla, I.S.; Suryavanshi, S.S. Spinel MgFe2O4 thick films: A colloidal approach for developing gas sensors. Mater. Lett. 2018, 213, 27–30. [Google Scholar] [CrossRef]
- Keat, G.O.; Kefeng, Z.; Grimes, C.A. A wireless, passive carbon nanotube-based gas sensor. IEEE Sens. J. 2002, 2, 82–88. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-S.; Wang, S.-H. On the sensitivity of conductimetric acetone gas sensor based on polypyrrole and polyaniline conducting polymers. Sens. Actuators B Chem. 2013, 185, 39–46. [Google Scholar] [CrossRef]
- Varghese, O.K.; Kichambre, P.D.; Gong, D. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuators B Chem. 2001, 81, 32–41. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Leghrib, R.; Felten, A.; Demoisson, F.; Reniers, F.; Pireaux, J.-J.; Llobet, E. Room-temperature, selective detection of benzene at trace levels using plasma-treated metal-decorated multiwalled carbon nanotubes. Carbon 2010, 48, 3477–3484. [Google Scholar] [CrossRef]
- Tan, Q.; Fang, J.; Liu, W.; Xiong, J.; Zhang, W. Acetone Sensing Properties of a Gas Sensor Composed of Carbon Nanotubes Doped with Iron Oxide Nanopowder. Sensors 2015, 15, 28502–28512. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.S.; Alenezi, M.R.; Lai, K.T.; Silva, S.R.P. Inkjet printing of polymer functionalized CNT gas sensor with enhanced sensing properties. Mater. Lett. 2016, 189, 299–302. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Clément, P.; Hafaiedh, I.; Parra, E.J.; Thamri, A.; Guillot, J.; Abdelghani, A.; Llobet, E. Iron oxide and oxygen plasma functionalized Multi-walled Carbon Nanotubess for the discrimination of volatile organic compounds. Carbon 2014, 78, 510–520. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Faglia, G.; Comini, E.; Cristalli, A.; Sberveglieri, G.; Dori, L. Very low power consumption micromachined term CO sensors. Sens. Actuators B Chem. 1999, 55, 140–146. [Google Scholar] [CrossRef]
- Semancik, S.; Cavicchia, R.E.; Wheeler, M.C.; Tiffany, J.E.; Poirier, G.E.; Walton, R.M.; Suehle, J.S.; Panchapakesan, B.; DeVoe, D.L. Microhotplate platforms for chemical sensor research. Sens. Actuators B Chem. 2001, 77, 579–591. [Google Scholar] [CrossRef]
- Bhattacharyya, P. Technological journey towards reliable microheater development for mems gas sensors: A review. IEEE. Trans. Device Mater. Reliab. 2014, 14, 589–599. [Google Scholar] [CrossRef]
- Sidek, O.; Ishak, M.Z.; Khalid, M.A.; Abu Bakar, M.Z.; Miskam, M.A. Effect of heater geometry on the high temperature distribution on a MEMS micro-hotplate. In Proceedings of the 3rd Asia Symposium on Quality Electronic Design (ASQED), Kuala Lumpur, Malaysia, 19–20 July 2011; pp. 100–104. [Google Scholar]
- Hwang, W.; Shin, K.; Roh, J.; Lee, D.; Choa, S. Development of micro-heaters with optimized temperature compensation design for gas sensors. Sensors 2010, 11, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Prasad, M. Development of double spiral MEMS hotplate using front-side etching cavity for gas sensors. NCTP 2016, 1724, 2088–2106. [Google Scholar]
- Saxena, G.; Paily, R. Analytical modeling of square microhotplate for gas sensing application. IEEE. Sens. J. 2013, 13, 4851–4858. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing mechanisms for carbon nanotube based NH3 gas detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuator B Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, T.; Dong, Y.; Wang, X. A room temperature VOCs gas sensor based on a layer by layer Multi-walled Carbon Nanotubess/poly-ethylene glycol composite. Sensors 2018, 18, 3113. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Luo, Y.; Li, Z. A highly selective chemical gas sensor based on functionalization of Multi-walled Carbon Nanotubess with poly(ethylene glycol). Sens. Actuators B Chem. 2007, 126, 361–367. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Huang, J.X.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber gas sensors: Examination of response mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Ryan, M.A.; Shevade, A.V.; Zhou, H.; Homer, M.L. Polymer-carbon black composite sensors in an electronic nose for air-quality monitoring. MRS Bull. 2004, 29, 714–719. [Google Scholar] [CrossRef] [PubMed]





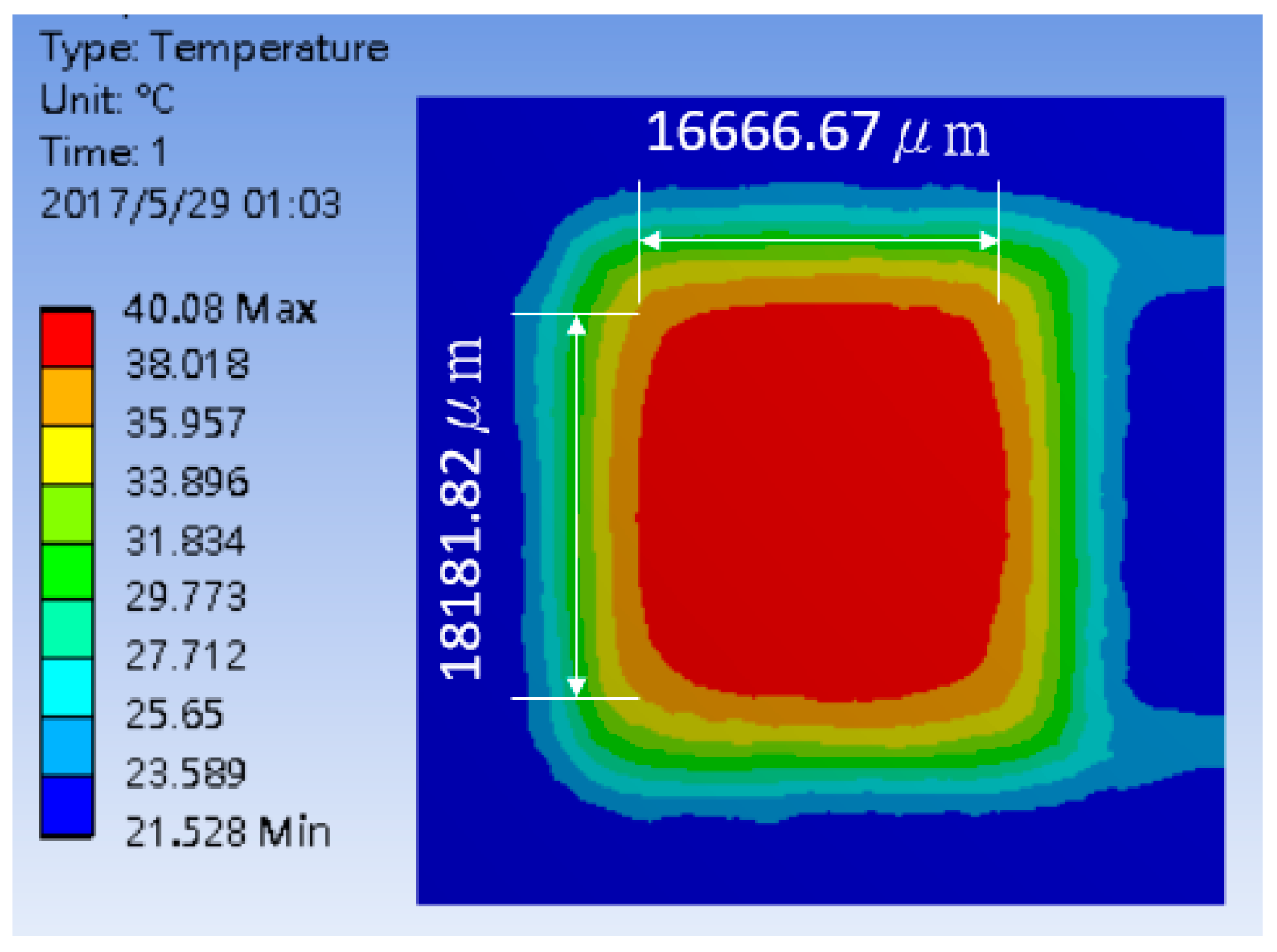







| Type | Geometry | Thermal Distribution |
|---|---|---|
| Meander |  |  |
| Double Meander |  |  |
| Double Spiral |  |  |
| Complex Double Spiral | 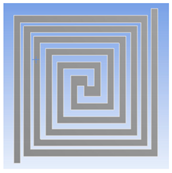 | 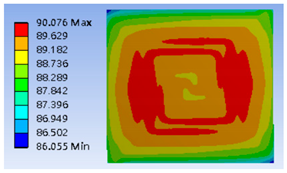 |
| 40 °C | ||||
| Material | Aluminum | Platinum | Copper | Stainless Steel |
| Power (mW) | 199.15 | 190.78 | 205.76 | 179.48 |
| Heating Zone (μm2) | L: 17,714 W: 16,571 | L: 17,777 W: 16,666 | L: 17,714 W: 16,581 | L: 18,181 W: 16,667 |
| 80 °C | ||||
| Material | Aluminum | Platinum | Copper | Stainless Steel |
| Power (mW) | 687.09 | 666.78 | 696.62 | 631.78 |
| Heating Zone (μm2) | L: 17,948 W: 17,178 | L: 17,948 W: 17,179 | L: 17,647 W: 17,058 | L: 17,948 W: 17,201 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, J.-C.; Wu, C.-C.; Lin, T.-M. Sensitivity Enhancement of Acetone Gas Sensor using Polyethylene Glycol/Multi-Walled Carbon Nanotubes Composite Sensing Film with Thermal Treatment. Polymers 2019, 11, 423. https://doi.org/10.3390/polym11030423
Chiou J-C, Wu C-C, Lin T-M. Sensitivity Enhancement of Acetone Gas Sensor using Polyethylene Glycol/Multi-Walled Carbon Nanotubes Composite Sensing Film with Thermal Treatment. Polymers. 2019; 11(3):423. https://doi.org/10.3390/polym11030423
Chicago/Turabian StyleChiou, Jin-Chern, Chin-Cheng Wu, and Tse-Mei Lin. 2019. "Sensitivity Enhancement of Acetone Gas Sensor using Polyethylene Glycol/Multi-Walled Carbon Nanotubes Composite Sensing Film with Thermal Treatment" Polymers 11, no. 3: 423. https://doi.org/10.3390/polym11030423




