Recent Progress of Carbon Dot Precursors and Photocatalysis Applications
Abstract
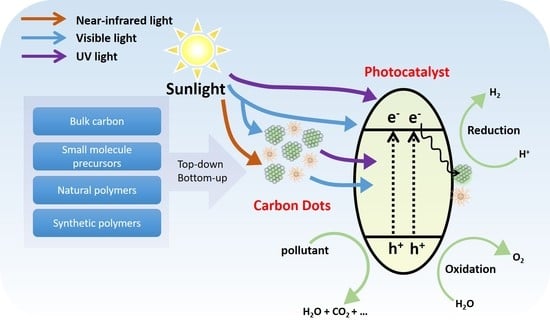
:1. Introduction
2. Synthesis Methods of Carbon Dots
2.1. Top-Down Methods
2.1.1. Laser Ablation
2.1.2. Ultrasonic Treatment
2.1.3. Electrochemical Oxidation
2.2. Bottom-Up Methods
2.2.1. Microwave Approach
2.2.2. Thermal Decomposition
2.2.3. Ultrasonic Treatment
2.2.4. Hydrothermal Approach
2.3. Factors Influencing PL Properties of CDs
3. Materials for Carbon Dot Synthesis
3.1. Small Precursors
3.2. Natural Polymers and Biomass
3.3. Synthetic Polymers
4. Synthesis of the CDs/Photocatalyst Composite
5. Photocatalysis Applications of CDs
5.1. Pure CDs as Photocatalyst
5.2. CD-Containing Composite Photocatalyst
5.2.1. Metal/Carbon Dot (CD)
5.2.2. Metal Sulfide/CD
5.2.3. Metal Oxide/CD
5.2.4. Bismuth-Based Semiconductor/CD
5.2.5. Others
6. Summary and Perspectives
- Fundamental understanding of the fluorescence mechanism. Exact mechanism of CDs’ photoluminescence phenomenon is still unclear and requires further investigation.
- Better control of CD size and homogeneity. Large size distribution leads to broad PL spectrum, complicates the mechanistic studies of CDs, and may impede the condition optimization for the relevant applications.
- CDs with both high quantum yield and high photostability. Quantum yield of CDs is generally low compared to that of quantum dots except a few cases. The apparently highest QY of CDs up to date, generated from precursors of citric acid and ethylenediamine, originates largely from the molecular fluorophores attached to the CDs instead of CDs themselves, which causes photobleaching [52,53].
- Increase pool of polymer precursors and elucidate structure-property correlations. Synthetic polymers that have been used to make CDs represent only a very small portion among all. There is a lack of rational design of precursor structures and understanding of their correlations with CD properties.
- Comparative studies of CDs for photocatalysis. The research of CDs as photocatalysts so far is somewhat qualitative. Systematic work is largely missing to investigate the effect of CD properties (e.g., particle size, concentration, composition, and PL properties) on photocatalysis efficiency and compare different CDs.
- Photocatalysis applications in real environment. Current literature mostly targets only a few model contaminants (e.g., methylene blue, methylene orange) in pure water. Real-world problems in complex environment have rarely been tackled, for example, degradation of multiple antibiotics and pesticides in river water, lake water, or even soil.
- CDs with dispersion stability in controlled and complex environment. Carbonaceous aggregation during the synthesis process of CDs is a major setback. CDs also must remain dispersed for practical applications. Particularly for the photocatalysis applications, aggregation of CDs would decrease the surface contact area and increase the recombination rate of electron-hole pairs, causing decreased catalysis efficiency. Surface properties of CDs are thus critical to keep CDs stable (i.e., no aggregation) not only in controlled environment (e.g., water and organic solvents) but also in complex real environment. Stable CDs also potentially increase the reusability of photocatalysts with more cycles.
Author Contributions
Funding
Conflicts of Interest
References
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Hu, L.; Sun, Y.; Li, S.; Wang, X.; Hu, K.; Wang, L.; Liang, X.J.; Wu, Y. Multifunctional carbon dots with high quantum yield for imaging and gene delivery. Carbon 2014, 67, 508–513. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Kim, H.; Singha, K.; Kim, W.J. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-pDNA. Biomaterials 2013, 34, 7168–7180. [Google Scholar] [CrossRef]
- Dai, H.; Shi, Y.; Wang, Y.; Sun, Y.; Hu, J.; Ni, P.; Li, Z. A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sens. Actuators B Chem. 2014, 202, 201–208. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Liu, B.; Liu, S.; Wang, S. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.Q.; Tang, Z.R.; Xu, Y.J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Liang, Z.; Zeng, L.; Cao, X.; Wang, Q.; Wang, X.; Sun, R. Sustainable carbon quantum dots from forestry and agricultural biomass with amplified photoluminescence by simple NH4OH passivation. J. Mater. Chem. C 2014, 2, 9760–9766. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, W.; Shi, Y.; Li, Z.; Yang, X. Easy synthesis of highly fluorescent carbon quantum dots from gelatin and their luminescent properties and applications. Carbon 2013, 60, 421–428. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Chen, C.; Yang, X.; Li, C. Facile preparation and upconversion luminescence of graphene quantum dots. Chem. Commun. 2011, 47, 2580–2582. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Yang, B. Bioimaging based on fluorescent carbon dots. RSC Adv. 2014, 4, 27184–27200. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.B. Review on carbon dots and their applications. Chin. J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- Zhou, Y.; Sharma, S.K.; Peng, Z.; Leblanc, R.M. Polymers in carbon dots: A review. Polymers 2017, 9, 67. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, N.; Qiao, S.; Liu, R.; Huang, H.; Liu, Y. Silver modified carbon quantum dots for solvent-free selective oxidation of cyclohexane. New J. Chem. 2015, 39, 2815–2821. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Niu, F.; Gooding, J.J.; Liu, J. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Simek, P.; Jankovsky, O.; Klı´mova´, K.; Bakardjieva, S. Synthesis of strongly fluorescent graphene quantum dots by cage-opening buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Thongpool, V.; Asanithi, P.; Limsuwan, P. Synthesis of carbon particles using laser ablation in ethanol. Procedia Eng. 2012, 32, 1054–1060. [Google Scholar] [CrossRef]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser ablated carbon nanodots for light emission. Nanoscale Res. Lett. 2016, 11, 424. [Google Scholar] [CrossRef]
- Liu, R.; Huang, H.; Li, H.; Liu, Y.; Zhong, J.; Li, Y.; Zhang, S.; Kang, Z. Metal nanoparticle/carbon quantum dot composite as a photocatalyst for high-efficiency cyclohexane oxidation. ACS Catal. 2014, 4, 328–336. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Zhou, Y.; Fu, Y.; Guo, S.; Li, H.; Zhao, S.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots enhance the stability of CdS for visible-light-driven overall water splitting. Appl. Catal. B Environ. 2017, 216, 114–121. [Google Scholar] [CrossRef]
- So, R.C.; Sanggo, J.E.; Jin, L.; Diaz, J.M.A.; Guerrero, R.A.; He, J. Gram-scale synthesis and kinetic study of bright carbon dots from citric acid and Citrus japonica via a microwave-assisted method. ACS Omega 2017, 2, 5196–5208. [Google Scholar] [CrossRef]
- Huo, P.; Guan, J.; Zhou, M.; Ma, C.; Liu, X.; Yan, Y.; Yuan, S. Carbon quantum dots modified CdSe loaded reduced graphene oxide for enhancing photocatalytic activity. J. Ind. Eng. Chem. 2017, 50, 147–154. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Xu, M.; He, G.; Li, Z.; He, F.; Gao, F.; Su, Y.; Zhang, L.; Yang, Z.; Zhang, Y. A green heterogeneous synthesis of N-doped carbon dots and their photoluminescence applications in solid and aqueous states. Nanoscale 2014, 6, 10307–10315. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon 2015, 82, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.Y.; Yang, Z.; Liu, Z.G.; Wang, H.X. Ionic liquid-assisted thermal decomposition synthesis of carbon dots and graphene-like carbon sheets for optoelectronic application. RSC Adv. 2016, 6, 61292–61300. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Lee, Y.C.; Choi, S.; Cho, D.H.; Kim, H.S.; Bang, S.; Seo, S.; Lee, S.C.; Won, J.; et al. Eco-friendly carbon-nanodot-based fluorescent paints for advanced photocatalytic systems. Sci. Rep. 2015, 5, 12420. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; He, X.; Liu, Y.; Huang, H.; Lian, S.; Lee, S.T.; Kang, Z. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon 2011, 49, 605–609. [Google Scholar] [CrossRef]
- Tao, S.; Song, Y.; Zhu, S.; Shao, J.; Yang, B. A new type of polymer carbon dots with high quantum yield: From synthesis to investigation on fluorescence mechanism. Polymer 2017, 116, 472–478. [Google Scholar] [CrossRef]
- Tao, S.; Lu, S.; Geng, Y.; Zhu, S.; Redfern, S.A.T.; Song, Y.; Feng, T.; Xu, W.; Yang, B. Design of metal-free polymer carbon dots: A new class of room-temperature phosphorescent materials. Angew. Chem. Int. Ed. 2018, 57, 2393–2398. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Y.; Sun, H.; Chen, N. Highly fluorescent carbon dots from peanut shells as potential probes for copper ion: The optimization and analysis of the synthetic process. Mater. Today Chem. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Zhou, L.; He, B.; Huang, J. Amphibious fluorescent carbon dots: One-step green synthesis and application for light-emitting polymer nanocomposites. Chem. Commun. 2013, 49, 8078–8080. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhang, W.L.; Zhou, Z.Q. Sodium hydroxide-mediated hydrogel of citrus pectin for preparation of fluorescent carbon dots for bioimaging. Colloids Surf. B Biointerfaces 2014, 123, 493–497. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Z.; Tang, W.; Wu, H.; Pan, J.; Hu, J.; Zeng, H. Surface states engineering carbon dots as multi-band light active sensitizers for ZnO nanowire array photoanode to boost solar water splitting. Carbon 2017, 121, 201–208. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Isnaeni; Herbani, Y.; Suliyanti, M.M. Concentration effect on optical properties of carbon dots at room temperature. J. Lumin. 2018, 198, 215–219. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.B.; Wei, J.S.; Xiong, H.M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Li, H.; Liu, R.; Lian, S.; Liu, Y.; Huang, H.; Kang, Z. Near-infrared light controlled photocatalytic activity of carbon quantum dots for highly selective oxidation reaction. Nanoscale 2013, 5, 3289–3297. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; Von Seckendorff, M.; Susha, A.S.; Kasak, P.; Rogach, A.L. Molecular fluorescence in citric acid-based carbon dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Huang, C.; Liu, G.; Leung, K.C.F.; Wáng, Y.X.J. High performance photoluminescent carbon dots for in vitro and in vivo bioimaging: Effect of nitrogen doping ratios. Langmuir 2015, 31, 8063–8073. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Zhai, X.; Liu, C.; Dai, L.; Liu, W. A facile and versatile approach to biocompatible “fluorescent polymers” from polymerizable carbon nanodots. Chem. Commun. 2012, 48, 10431–10433. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.F.; Chen, S. Amphiphilic egg-derived carbon dots: Rapid plasma fabrication, pyrolysis process, and multicolor printing patterns. Angew. Chem. Int. Ed. 2012, 51, 9297–9301. [Google Scholar] [CrossRef]
- Chen, W.; Hu, C.; Yang, Y.; Cui, J.; Liu, Y. Rapid synthesis of carbon dots by hydrothermal treatment of lignin. Materials 2016, 9, 184. [Google Scholar] [CrossRef]
- Ko, N.R.; Nafiujjaman, M.; Cherukula, K.; Lee, S.J.; Hong, S.J.; Lim, H.N.; Park, C.H.; Park, I.K.; Lee, Y.K.; Kwon, I.K. Microwave-assisted synthesis of biocompatible silk fibroin-based carbon quantum dots. Part. Part. Syst. Charact. 2018, 35, 1700300. [Google Scholar] [CrossRef]
- Pires, N.R.; Santos, C.M.W.; Sousa, R.R.; de Paula, R.C.M.; Cunha, P.L.R.; Feitosa, J.P.A. Novel and fast microwave-assisted synthesis of carbon quantum dots from raw cashew gum. J. Braz. Chem. Soc. 2015, 26, 1274–1282. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C 2017, 76, 856–864. [Google Scholar] [CrossRef]
- Han, S.; Zhang, H.; Xie, Y.; Liu, L.; Shan, C.; Li, X.; Liu, W.; Tang, Y. Application of cow milk-derived carbon dots/Ag NPs composite as the antibacterial agent. Appl. Surf. Sci. 2015, 328, 368–373. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, W.; Wu, P. Highly photoluminescent carbon dots derived from egg white: Facile and green synthesis, photoluminescence properties, and multiple applications. ACS Sustain. Chem. Eng. 2015, 3, 1412–1418. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Wang, L.; Song, Y.; Zhang, G.; Wang, H.; Yang, B. A general route to make non-conjugated linear polymers luminescent. Chem. Commun. 2012, 48, 10889–10891. [Google Scholar] [CrossRef]
- Shen, J.; Li, Q.; Zhang, Y.; She, X.J.; Wang, C.F.; Chen, S. Nitrogen-doped carbon dots derived from polyamindoamine dendrimer. RSC Adv. 2016, 6, 59702–59707. [Google Scholar] [CrossRef]
- Li, G.; Wang, F.; Liu, P.; Chen, Z.; Lei, P.; Xu, Z.; Li, Z.; Ding, Y.; Zhang, S.; Yang, M. Polymer dots grafted TiO2 nanohybrids as high performance visible light photocatalysts. Chemosphere 2018, 197, 526–534. [Google Scholar] [CrossRef]
- Gu, J.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Synthesis of fluorescent carbon nanoparticles from polyacrylamide for fast cellular endocytosis. RSC Adv. 2013, 3, 15589–15591. [Google Scholar] [CrossRef]
- Aji, M.P.; Wati, A.L.; Priyanto, A.; Karunawan, J.; Nuryadin, B.W.; Wibowo, E.; Marwoto, P. Sulhadi Polymer carbon dots from plastics waste upcycling. Environ. Nanotechnol. Monit. Manag. 2018, 9, 136–140. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, S.; Wang, Y.; Deng, S.; Qian, G.; Wang, M.; Hu, A. Preparation of carbon nanodots from single chain polymeric nanoparticles and theoretical investigation of the photoluminescence mechanism. J. Mater. Chem. C 2013, 1, 580–586. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, Q.; Xue, H.; Cheng, G.; Jiang, S. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chem. Int. Ed. 2010, 49, 3771–3776. [Google Scholar] [CrossRef]
- Li, W.; Chu, K.; Liu, L. Zwitterionic gel coating endows gold nanoparticles with ultrastability. Langmuir 2019, 35, 1369–1378. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Yue, Z.; Liu, W.; Cao, Z. Ultrastable core–shell structured nanoparticles directly made from zwitterionic polymers. Chem. Commun. 2014, 50, 15030–15033. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, H.; Cao, Z.; Keefe, A.; Wang, J.; Jiang, S. Multifunctional and degradable zwitterionic nanogels for targeted delivery, enhanced MR imaging, reduction-sensitive drug release, and renal clearance. Biomaterials 2011, 32, 4604–4608. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Liu, L. Antifouling gold surfaces grafted with aspartic acid and glutamic acid based zwitterionic polymer brushes. Langmuir 2014, 30, 12619–12626. [Google Scholar] [CrossRef]
- Liu, Q.; Singh, A.; Liu, L. Amino acid-based zwitterionic poly(serine methacrylate) as an antifouling material. Biomacromolecules 2013, 14, 226–231. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Singh, A.; Cheng, G.; Liu, L. Two amino acid-based superlow fouling polymers: Poly(lysine methacrylamide) and poly(ornithine methacrylamide). Acta Biomater. 2014, 10, 2956–2964. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Wang, H.; Newby, B.M.Z.; Cheng, F.; Liu, L. Amino acid-based zwitterionic polymer surfaces highly resist long-term bacterial adhesion. Langmuir 2016, 32, 7866–7874. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Liu, L. Amino acid-based zwitterionic polymers: Antifouling properties and low cytotoxicity. J. Biomater. Sci. Polym. Ed. 2014, 25, 1730–1742. [Google Scholar] [CrossRef]
- Wang, W.; Ni, Y.; Xu, Z. One-step uniformly hybrid carbon quantum dots with high-reactive TiO2 for photocatalytic application. J. Alloy. Compd. 2015, 622, 303–308. [Google Scholar] [CrossRef]
- Kannan, R.; Kim, A.R.; Eo, S.K.; Kang, S.H.; Yoo, D.J. Facile one-step synthesis of cerium oxide-carbon quantum dots/RGO nanohybrid catalyst and its enhanced photocatalytic activity. Ceram. Int. 2017, 43, 3072–3079. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Huang, H.; Liu, Y.; Li, H.; Ming, H.; Kang, Z. ZnO/carbon quantum dots nanocomposites: One-step fabrication and superior photocatalytic ability for toxic gas degradation under visible light at room temperature. New J. Chem. 2012, 36, 1031–1035. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Wang, J.; Cui, H.; Li, L.; Zhu, Z. Pure carbon nanodots for excellent photocatalytic hydrogen generation. RSC Adv. 2015, 5, 21332–21335. [Google Scholar] [CrossRef]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Reisner, E. Solar hydrogen production using carbon quantum dots and a molecular nickel catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef]
- Sahu, S.; Liu, Y.; Wang, P.; Bunker, C.E.; Fernando, K.A.S.; Lewis, W.K.; Guliants, E.A.; Yang, F.; Wang, J.; Sun, Y.P. Visible-light photoconversion of carbon dioxide into organic acids in an aqueous solution of carbon dots. Langmuir 2014, 30, 8631–8636. [Google Scholar] [CrossRef]
- Wu, W.; Zhan, L.; Fan, W.; Song, J.; Li, X.; Li, Z.; Wang, R.; Zhang, J.; Zheng, J.; Wu, M.; et al. Cu-N dopants boost electron transfer and photooxidation reactions of carbon dots. Angew. Chem. Int. Ed. 2015, 54, 6540–6544. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Li, H.; Wu, Y.; Wang, K.; Fan, H.; Feng, H.; Zhang, D. Transition metal-carbon quantum dots composites and their antibacterial properties. J. Biomater. Tissue Eng. 2018, 8, 309–316. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.X.; Zhang, W.D. Carbon quantum dots-doped CdS microspheres with enhanced photocatalytic performance. J. Alloy. Compd. 2013, 569, 102–110. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Kansal, S.K. Synthesis of ZnS/CQDs nanocomposite and its application as a photocatalyst for the degradation of an anionic dye, ARS. Superlattices Microstruct. 2016, 98, 86–95. [Google Scholar] [CrossRef]
- Zhao, S.; Li, C.; Wang, L.; Liu, N.; Qiao, S.; Liu, B.; Huang, H.; Liu, Y.; Kang, Z. Carbon quantum dots modified MoS2 with visible-light-induced high hydrogen evolution catalytic ability. Carbon 2016, 99, 599–606. [Google Scholar] [CrossRef]
- Atkin, P.; Daeneke, T.; Wang, Y.; Carey, B.J.; Berean, K.J.; Clark, R.M.; Ou, J.Z.; Trinchi, A.; Cole, I.S.; Kalantar-Zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13563–13571. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Zhu, C.; Sheng, Y.; Yan, Z.; Wang, Y.; Feng, B. The visible light catalytic properties of carbon quantum dots/ZnO nanoflowers composites. J. Mater. Sci. Mater. Electron. 2015, 26, 2861–2866. [Google Scholar] [CrossRef]
- Muthulingam, S.; Lee, I.H.; Uthirakumar, P. Highly efficient degradation of dyes by carbon quantum dots/N-doped zinc oxide (CQD/N-ZnO) photocatalyst and its compatibility on three different commercial dyes under daylight. J. Colloid Interface Sci. 2015, 455, 101–109. [Google Scholar] [CrossRef]
- Ding, D.; Lan, W.; Yang, Z.; Zhao, X.; Chen, Y.; Wang, J.; Zhang, X.; Zhang, Y.; Su, Q.; Xie, E. A simple method for preparing ZnO foam/carbon quantum dots nanocomposite and their photocatalytic applications. Mater. Sci. Semicond. Process. 2016, 47, 25–31. [Google Scholar] [CrossRef]
- Feng, C.; Deng, X.Y.; Ni, X.X.; Li, W.B. Fabrication of carbon dots modified porous ZnO nanorods with enhanced photocatalytic activity. Wuli Huaxue Xuebao/Acta Phys. Chim. Sin. 2015, 31, 2349–2357. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.P.; Zhao, J.X.; Ge, Z.H.; Zhao, X.K.; Zou, L. ZnO/carbon quantum dots heterostructure with enhanced photocatalytic properties. Appl. Surf. Sci. 2013, 279, 367–373. [Google Scholar] [CrossRef]
- Elkodous, M.A.; Hassaan, A.; Pal, K.; Ghoneim, A.I.; Abdeen, Z. C-dots dispersed macro-mesoporous TiO2 photocatalyst for effective waste water treatment. Charact. Appl. Nanomater. 2018, 1. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Appl. Catal. B Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Ângelo, J.; Girão, A.V.; Trindade, T.; Andrade, L.; Mendes, A. N-doped carbon quantum dots/TiO2 composite with improved photocatalytic activity. Appl. Catal. B Environ. 2016, 193, 67–74. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Saud, P.S.; Pant, B.; Alam, A.M.; Ghouri, Z.K.; Park, M.; Kim, H.Y. Carbon quantum dots anchored TiO2 nanofibers: Effective photocatalyst for waste water treatment. Ceram. Int. 2015, 41, 11953–11959. [Google Scholar] [CrossRef]
- Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P.; Zhan, J.; Li, M.; Liu, H. Carbon quantum dots/hydrogenated TiO2 nanobelt heterostructures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Dong, H.; Liu, Y.; Wang, L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D interface engineering of carbon quantum dots modified Bi2WO6 ultrathin nanosheets with enhanced photoactivity for full spectrum light utilization and mechanism insight. Appl. Catal. B Environ. 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Wang, H.; Zeng, G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 511, 296–306. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, T.; Xu, J.; Zeng, H.; Zhang, N. Carbon quantum dots/Bi2WO6 composites for efficient photocatalytic pollutant degradation and hydrogen evolution. NANO 2017, 12, 1750082. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ji, M.; Li, H.; Xu, H.; Li, H.; Chen, R. The synergistic role of carbon quantum dots for the improved photocatalytic performances of Bi2MoO6. Nanoscale 2015, 7, 11433–11443. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, P.; Zhuo, M.; Wang, F.; Su, Y.; Chen, T.; Yao, K.; Cai, Z.; Lv, W.; Liu, G. Degradation of indometacin by simulated sunlight activated CDs-loaded BiPO4 photocatalyst: Roles of oxidative species. Appl. Catal. B Environ. 2018, 221, 129–139. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Chen, X.; Ji, M.; Yin, S.; Zhang, Q.; Li, H. Tunable oxygen activation induced by oxygen defects in nitrogen doped carbon quantum dots for sustainable boosting photocatalysis. Carbon 2017, 114, 601–607. [Google Scholar] [CrossRef]
- Zhao, C.; Li, W.; Liang, Y.; Tian, Y.; Zhang, Q. Synthesis of BiOBr/carbon quantum dots microspheres with enhanced photoactivity and photostability under visible light irradiation. Appl. Catal. A Gen. 2016, 527, 127–136. [Google Scholar] [CrossRef]
- Xia, J.; Di, J.; Li, H.; Xu, H.; Li, H.; Guo, S. Ionic liquid-induced strategy for carbon quantum dots/BiOX (X=Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl. Catal. B Environ. 2016, 181, 260–269. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Q.; Yan, X.; Mo, Q.; Chen, Y.; Liu, B.; Teng, L.; Xiao, W.; Ge, L.; Wang, Q. Enhanced photocatalytic activity of the carbon quantum dot-modified BiOI microsphere. Nanoscale Res. Lett. 2016, 11, 60. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, S.; Li, X.; Li, H.; Zhang, T.; Cui, W. Enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight of novel CQDs/Bi2O2CO3 composite. Nanomaterials 2018, 8, 330. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.M.; Li, J.G.; Song, X.L.; Dai, H.Y.; Liang, Z.H. Construction of carbon quantum dots/proton-functionalized graphitic carbon nitride nanocomposite via electrostatic self-assembly strategy and its application. Appl. Surf. Sci. 2016, 370, 514–521. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, Y.; Zhang, G.; Yin, B.; Zhao, Y.; Shi, W.; Li, C. Facile fabrication of stable metal-free CQDs/g-C3N4 heterojunctions with efficiently enhanced visible-light photocatalytic activity. Sep. Purif. Technol. 2016, 171, 229–237. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Geng, F.; Guo, L.H.; Wan, B.; Yang, Y. Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ. 2016, 180, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yao, P.; Zhu, D.; Gu, C. A novel method for the development of a carbon quantum dot/carbon nitride hybrid photocatalyst that responds to infrared light irradiation. J. Mater. Chem. A 2015, 3, 13189–13192. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Zhang, Q.; Zhang, Y.; Cao, J.J.; Ho, W.; Lee, S.C. Biocompatible FeOOH-Carbon quantum dots nanocomposites for gaseous NOx removal under visible light: Improved charge separation and high selectivity. J. Hazard. Mater. 2018, 354, 54–62. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, M.; Yuan, X.; Si, M.; Jiang, L.; Wu, Z.; Wang, H.; Zeng, G. Nitrogen doped carbon quantum dots mediated silver phosphate/bismuth vanadate Z-scheme photocatalyst for enhanced antibiotic degradation. J. Colloid Interface Sci. 2018, 529, 11–22. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]







| Carbon Source | Nitrogen Source | QY | Application | Reference |
|---|---|---|---|---|
| Citric acid | Ethylenediamine | 80.6% | Ink, Fe3+ detection, CD/polymer composites | [51] |
| Ethylamine | 8.4% | -- | ||
| n-heptylamine | 7.7% | -- | ||
| Urea | 19.4% | -- | ||
| Sodium citrate | Ethylenediamine | 21.6% | -- | [51] |
| Citric acid | Ethylenediamine | 53% | -- | [52] |
| Hexamethylenetetramine | 17% | -- | ||
| Triethanolamine | 7% | -- | ||
| Citric acid | Ethylenediamine | 69.3% | Bioimaging | [53] |
| Diethylenetriamine | 68% | Bioimaging | ||
| Triethylenetetraamine | 33.4% | Bioimaging | ||
| Citric acid | Urea | 36% | Drug delivery | [4] |
| Acrylic acid | Ethylenediamine | 30.5% | Fluorescent polymers | [54] |
| p-phenylenediamine | Urea | up to 35% | Bioimaging | [49] |
| Calcium citrate | Urea | 10.1% | Ink | [34] |
| Citric acid | Ethylenediamine | 1.7% | -- | [31] |
| Precursor (Method 1) | QY | Application | Reference |
|---|---|---|---|
| Histidine (HT) | 10.7% | Melamine sensing | [6] |
| Cysteine (TD) | -- | Solar cells, optoelectronics | [36] |
| Serine (PT) | Blue fluorescence | -- | [55] |
| Glucose (US) | 7% | -- | [38] |
| Glucose (PT) | Blue fluorescence | -- | [55] |
| o-phenylenediamine (ST) | 10.4%, green | Multi-color bioimaging Flexible full-color emissive film | [8] |
| m-phenylenediamine (ST) | 4.8%, blue | ||
| p-phenylenediamine (ST) | 20.6%, red | ||
| Citric acid (TD) | 11% | Fe3+ detection | [35] |
| Citric acid (HT) | 7.2% | -- | [51] |
| Ascorbic acid (HT) | -- | Photocatalysis | [7] |
| Ethylenediamine (HT) | 3.8% | -- | [51] |
| Acrylamide (PT) | Blue fluorescence | -- | [55] |
| EDTA disodium salt (PT) | Blue fluorescence | -- | [55] |
| Starting Material | Synthesis Method | Application | Reference |
|---|---|---|---|
| Lignin + H2O2 | Hydrothermal, 180 °C, 40 min | Bioimaging | [56] |
| Chitosan | Hydrothermal, 180 °C, 12 h | Bioimaging | [47] |
| Xylan + NH4OH | Hydrothermal, 200 °C, 12 h | Bioimaging | [11] |
| Citrus pectin + NaOH | Hydrothermal, 100–180 °C, 2 h | Bioimaging | [45] |
| Silk fibroin | Microwave (300 W), 20 min | Biomedical | [57] |
| Gelatin | Hydrothermal, 200 °C, 3 h | Bioimaging, optical devices | [12] |
| Peach gum polysaccharide | Hydrothermal, 180 °C, 12 h | Optical devices | [44] |
| Cashew gum | Microwave (800 W), 30–40 min | -- | [58] |
| Peanut shell | Pyrolysis, 400 °C, 4 h | Metal ion detection (Cu2+) | [43] |
| Sweet potato | Hydrothermal, 180 °C, 18 h | Bioimaging, metal ion detection (Fe3+) | [59] |
| Pomelo peel | Hydrothermal, 200 °C, 3 h | Metal ion detection (Hg2+) | [42] |
| Grass | Hydrothermal, 150–200 °C, 3 h | Metal ion detection (Cu2+) | [41] |
| Cow milk | Hydrothermal, 180 °C, 12 h | Antimicrobial | [60] |
| Egg white | Hydrothermal, 220 °C, 48 h | Metal ion detection, bioimaging, optical devices | [61] |
| Egg white or egg yolk | Plasma treatment, 3 min | Printing ink | [55] |
| Polymer | Structure | Synthesis Method | Application | Reference |
|---|---|---|---|---|
| Branched polyethyleneimine |  | Hydrothermal | Bioimaging, gene delivery | [3] |
| Polyethyleneimine |  | Hydrothermal | -- | [62] |
| Polyethyleneimine (+glycerol) | Same above | Microwave | Gene delivery | [5] |
| Polyamindoamine dendrimer |  | Hydrothermal | Fe3+ detection, ink | [63] |
| Polyacrylic acid (+EDA) |  | Hydrothermal | Graphic security, information encryption | [39,40] |
| Polyvinyl alcohol |  | Hydrothermal | Bioimaging | [62] |
| Photocatalysis | [64] | |||
| Polyacrylamide |  | Hydrothermal | Bioimaging | [65] |
| Polyacrylamide | Same above | Plasma treatment | -- | [55] |
| Polyethylene glycol |  | Ultrasonic | Photocatalysis | [37] |
| Polypropylene |  | Thermal decomposition | -- | [66] |
| P(methyl acrylate-r-EDY) |  | Thermal decomposition | -- | [67] |
| PMPC |  | Microwave | Biomedical | [70] |
| PCB-1 |  | Microwave | Biomedical | [70] |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutant 1: Degradation Efficiency/Time | Ref (year) |
|---|---|---|---|---|---|
| CDs/ZnO foam | nanocomposite | Dispersion in CDs solution | 250-W Xe (vis) (λ ≥ 400 nm) | MB > RhB > MO | [91] (2016) |
| CDs/ZnO | Porous nanorods | Solvent thermal + deposition | 300-W Xe (vis) (λ ≥ 420 nm) | Phenol: 94.3%/60 min | [92] (2015) |
| CDs/ZnO | Heterostructure | Sol-gel + spin coating | 18-W UV lamp (vis) (λ = 365 nm) | RhB: 30%/120 min | [93] (2013) |
| CDs/ZnO | Nanocomposite (20–30 nm) | Hydrothermal | 3 of 8-W visible light lamp | Benzene gas: 86%/24 h Methanol gas: 82%/24 h | [79] (2012) |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutant 1 | Degradation Efficiency/Time | Ref (Year) |
|---|---|---|---|---|---|---|
| CDs/TiO2 | Macro-mesoporous nanospheres | Dispersion | 300-W halogen lamp (vis) | MB | - | [94] (2018) |
| CDs/TiO2 | Composite | Hydrothermal-calcination | 350-W arc Xe lamp (vis) (λ < 420 nm) | GEM | 89%/8 min | [95] (2017) |
| N-CDs/TiO2 | Composite | Hydrothermal | 6-W fluorescent lamp (vis) (λ > 400 nm) UV lamp (UV) (λ=365 nm) | NO | 27%/120 h (vis) 79.6%/85 h (UV) | [96] (2016) |
| N-CDs/TiO2 | Hierarchical microspheres/nanorods | Hydrothermal | 500-W Xe (vis) (λ = 420 nm) | RhB | > 95%/30 min | [97] (2013) |
| CDs/TiO2 | Nanofibers | Hydrothermal | Natural sunny day (11 a.m. and 3 p.m.) | MB | 71%/95 min | [98] (2015) |
| CDs/Hydrogenated TiO2 | Nanobelt heterostructure | Hydrothermal + bath reflux | 350-W Hg lamp (UV) (λ = 365 nm) 300-W Xe arc lamp (vis) | MO | > 86%/25 min (UV) 50%/25 min (vis) | [99] (2015) |
| CDs/TiO2 | Nanohybrid | Hydrothermal | 500-W halogen lamp | MO | 96.7%/8 h (UV-vis) | [64] (2018) |
| CDs/TiO2 | Nanoparticles/microsphere hybrid | Sol-gel method | 500-W Xe lamp (vis) (λ > 420 nm) | MB | 90%/2 h | [7] (2017) |
| CDs/rutile TiO2 | Nanocomposite | Mix + vacuum drying | 350-W Xe lamp (vis) (λ > 420 nm) | MB | 97%/1 h | [15] (2012) |
| CDs/TiO2 | Nanocomposite | Sol-gel method | 300-W halogen lamp (vis, λ not specified) | MB | ca. 100%/25 min | [16] (2010) |
| CDs/TiO2 | Nanodots/microcolumn composite | One-pot hydrothermal | 14 W UV lamp 500-W Xe lamp (vis) (λ > 420 nm) | RhB | ca. 100%/75 min (UV) 77%/150 min (vis) | [77] (2015) |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutant 1: Degradation Efficiency/Time | Ref (Year) |
|---|---|---|---|---|---|
| CDs/Bi2WO6 | 0D/2D ultrathin nanosheets | Hydrothermal | 300-W Xe (vis) | MO: 94.1%/120 min BPA: 99.5%/60 min | [100] (2018) |
| N-CDs/Bi2WO6 | Hybrid material | Hydrothermal | 300-W Xe (vis) (λ = 420 nm) | TC: 97%/25 min | [101] (2018) |
| CDs/Bi2WO6 | Nanocomposite | Hydrothermal | 500-W Xe (solar light) | RhB: 97%/10 min Phenol: 33.4%/120 min | [102] (2017) |
| CDs/Bi2WO6 | Hybrid material | Hydrothermal | 300-W Xe (vis) (λ = 400 nm) | RhB: ~98%/120 min CIP: 87%/120 min BPA: ~45%/120 min TC-HCl: ~78%/120 min | [103] (2015) |
| CDs/Bi2MoO6 | Irregular nanosheets | Hydrothermal | 300-W Xe (vis) (λ = 400 nm) | CIP: 88%/120 min BPA: 54%/120 min | [104] (2015) |
| CDs/BiPO4 | Nanorods | Hydrothermal- calcination | 350-W Xe (λ ≥ 290 nm) | IDM: ~ 90%/120 min | [105] (2018) |
| N-CDs/BiPO4 | Nanoparticles /nanorods | Ionic liquid assisted solvothermal | 250-W high pressure Hg (UV) | CIP: 87.5%/120 min | [106] (2017) |
| CDs/BiOBr | Microspheres | Solvothermal and hydrothermal | 300-W Xe (vis) (λ = 400 nm) | RhB: ~100%/145 min PNP: 26%/320 min | [107] (2016) |
| CDs/BiOX (X=Br, Cl) | Hybrid nanosheets | Ionic liquid induced | 300-W Xe (vis) (λ = 400 nm) | BiOBr: RhB: ~100%/30 min CIP: 44.3%/240 min | [108] (2016) |
| CDs/BiOI | Uniform layered structure nanoplates | Hydrothermal | 150-W Xe (vis) (λ = 420 nm) | MO: 98%/50 min | [109] (2016) |
| CDs/Bi2O2CO3 | Nanoparticles /flower-like nanosheets | Dynamic- adsorption precipitation | 400-W metal halide (vis) (λ > 400 nm) | MB: 94.45%/120 min Phenol: 61.46%/120 min | [110] (2018) |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutant 1 | Degradation Efficiency/Time | Ref (Year) |
|---|---|---|---|---|---|---|
| NCDs/g-C3N4 | Composite | Polymerization | 350-W Xe (vis) (λ = 420 nm) | IDM | 91.5%/90 min | [111] (2017) |
| CDs/g-C3N4 | Nanocomposite | Electrostatic adsorption | (vis) | MB | > 90%/90 min | [112] (2016) |
| CDs/g-C3N4 | Heterojunction | Low temperature method | 250-W Xe (vis) (λ = 420 nm) | RhB and TC-HCl | RhB: 95.2%/210 min TC-HCl: 78.6%/240 min | [113] (2016) |
| CDs/g-C3N4 | Heterojunction | Impregnation- thermal | 300-W Xe (vis) (λ < 400 nm) | Phenol | 100%/within 200 min | [114] (2016) |
| CDs/carbon nitride | Hybrid composite | High temperature treatment | Infrared light (λ > 800 nm) | MO | 90%/4 h | [115] (2015) |
| CDs/FeOOH | Nanocomposite | Hyrothermal | 300-W Xe (vis) (λ > 420 nm) | NO | 22%/30 min | [116] (2018) |
| N-CDs/Ag3PO4/BiVO4 | Z-scheme hybrid material | Solvothermal- precipitation | 300-W Xe (vis) (λ = 420 nm) | TC-HCl | 88.9%/30 min | [117] (2018) |
| CDs/MoO3 /g-C3N4 | Z-scheme microstructure | Calcination | 350-W Xe (vis) (λ = 420 nm) | TC | 88.4%/90 min | [118] (2018) |
| CDs/CdSe/rGO | Hybrid nanomaterial | Hydrothermal | 350-W Xe (vis) | TC-HCl | 90%/60 min | [32] (2017) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. https://doi.org/10.3390/polym11040689
Chu K-W, Lee SL, Chang C-J, Liu L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers. 2019; 11(4):689. https://doi.org/10.3390/polym11040689
Chicago/Turabian StyleChu, Kuan-Wu, Sher Ling Lee, Chi-Jung Chang, and Lingyun Liu. 2019. "Recent Progress of Carbon Dot Precursors and Photocatalysis Applications" Polymers 11, no. 4: 689. https://doi.org/10.3390/polym11040689