Seawater Effects on Thermally Aged Ambient Cured Carbon/Epoxy Composites: Moisture Kinetics and Uptake Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processes
2.2. Procedure for Conditioning and Moisture Uptake Measurement
3. Results and Discussion
4. Summary and Conclusions
- (1)
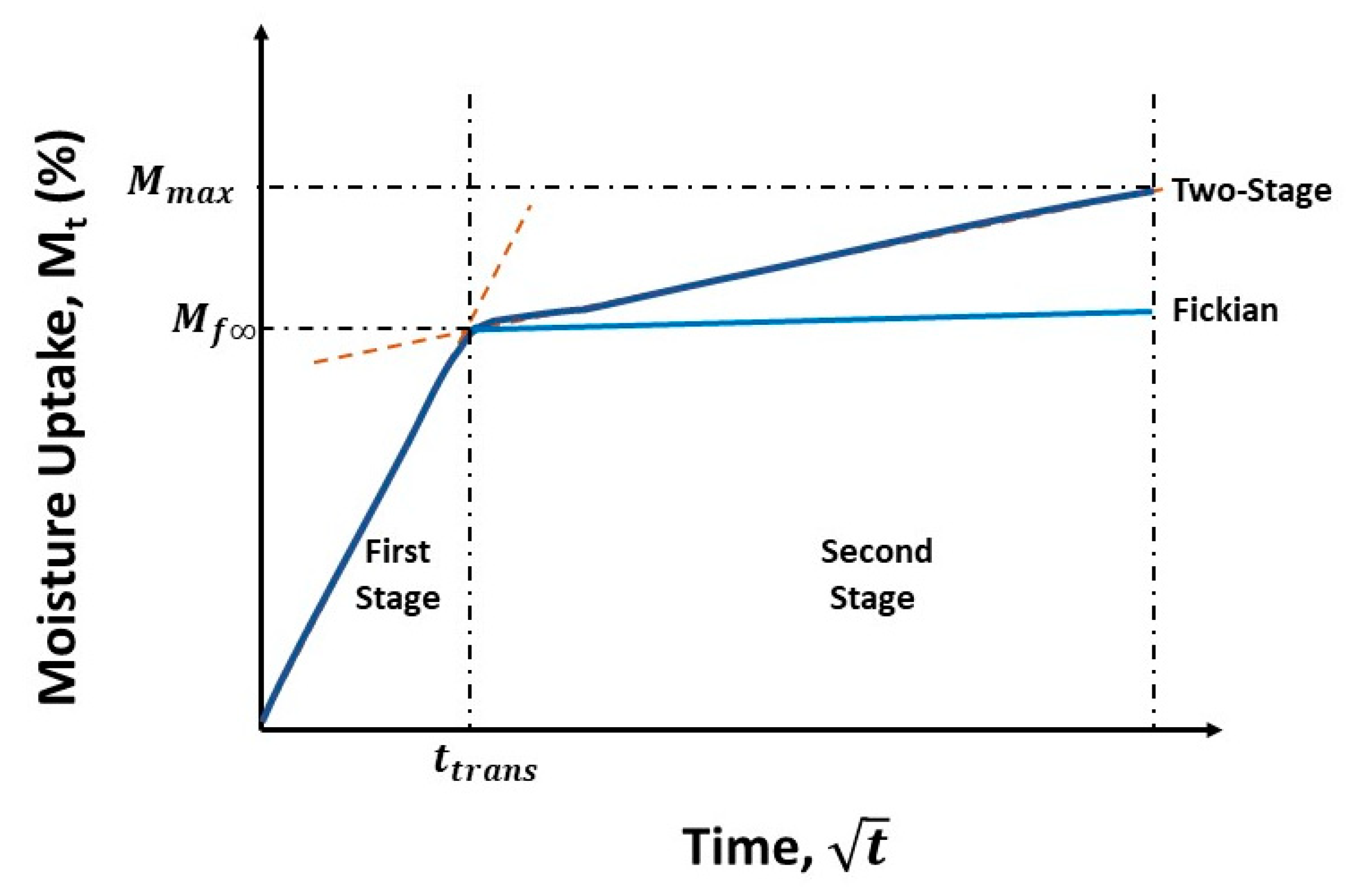
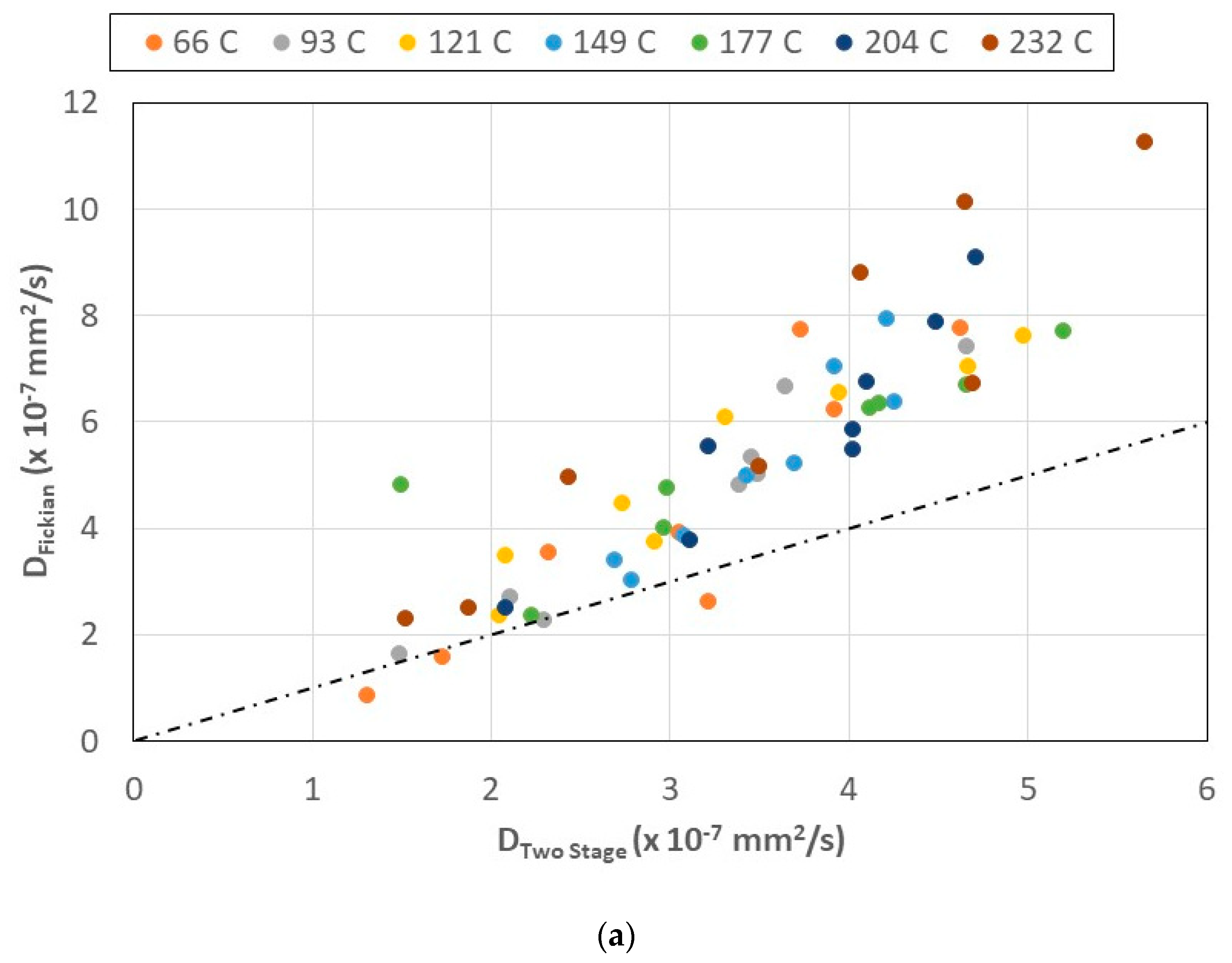
- The two-stage model better describes the moisture uptake and provides a wealth of moisture kinetics information, not only as related to stages and rates of uptake but also in terms of diffusion and relaxation/deterioration coefficients, representing the complex interactions between mechanisms initiated by the extent of prior thermal aging and those subsequently initiated through immersion in seawater.
- (2)
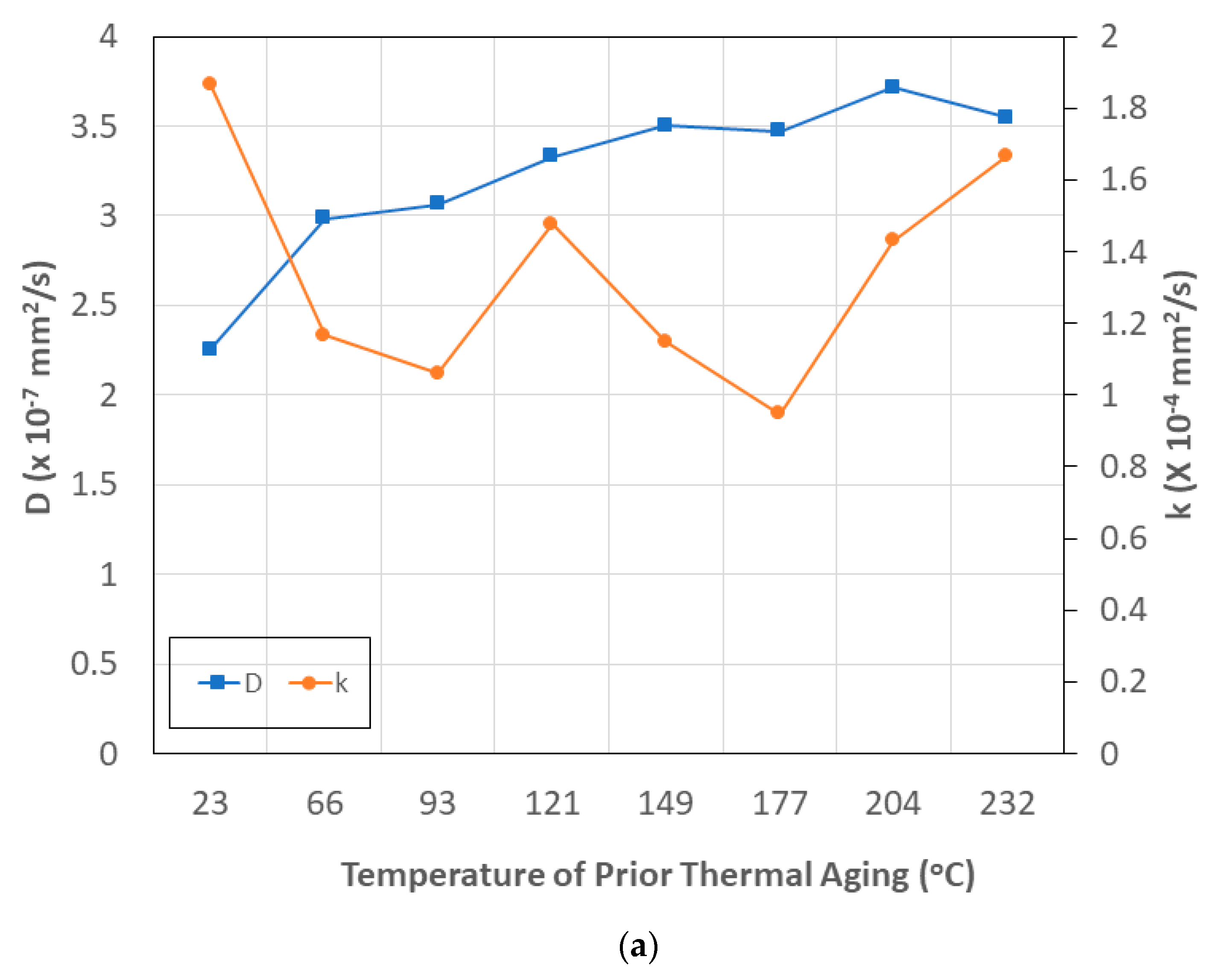
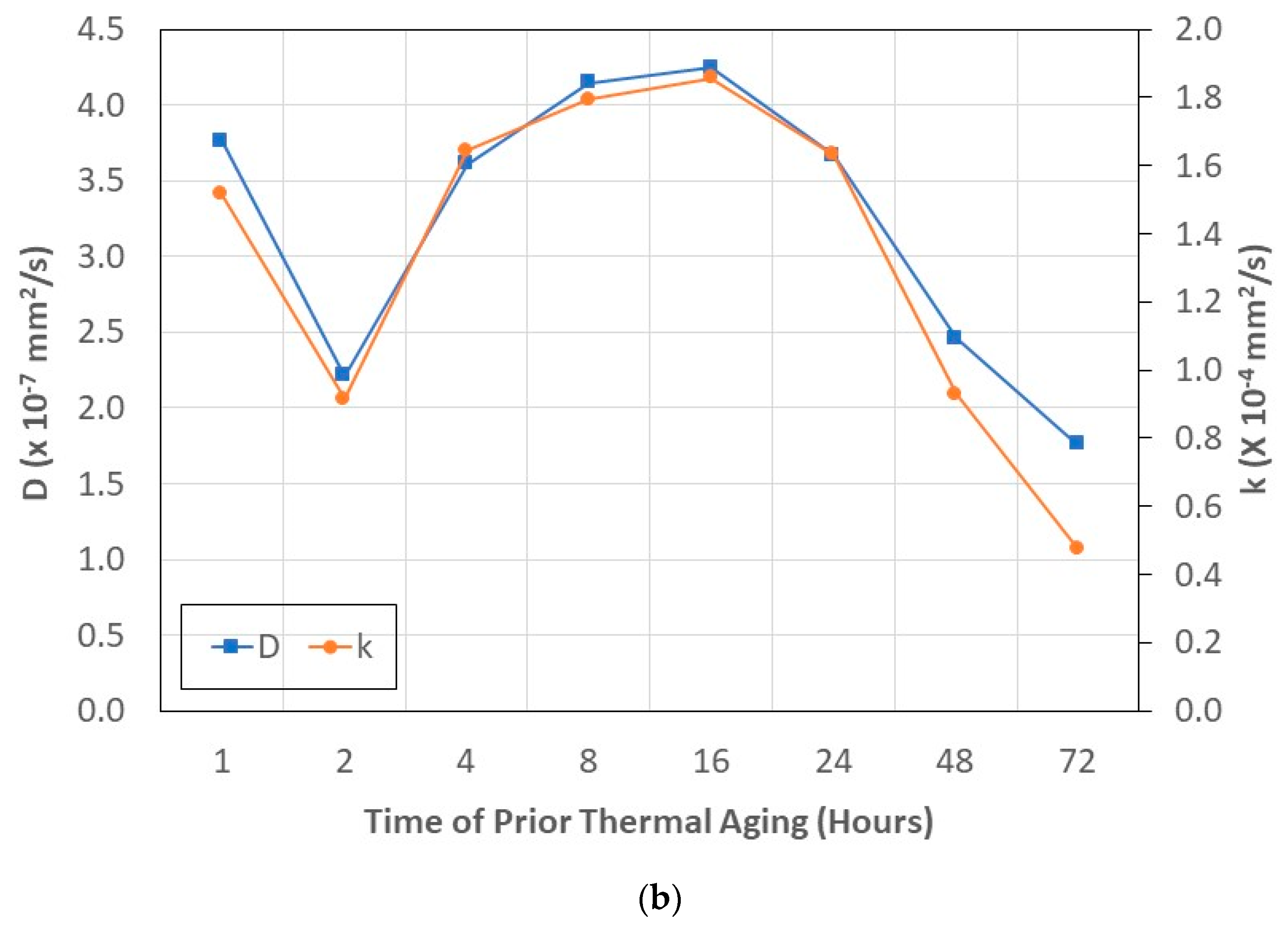
- The level of maximum uptake, Mmax, shows an increasing trend with temperature and time of aging, reaching near asymptote levels at the highest temperatures and longest time periods of prior thermal aging. The trends are related to the level of curing and cross-link density, which results in lower free volume.
- (3)
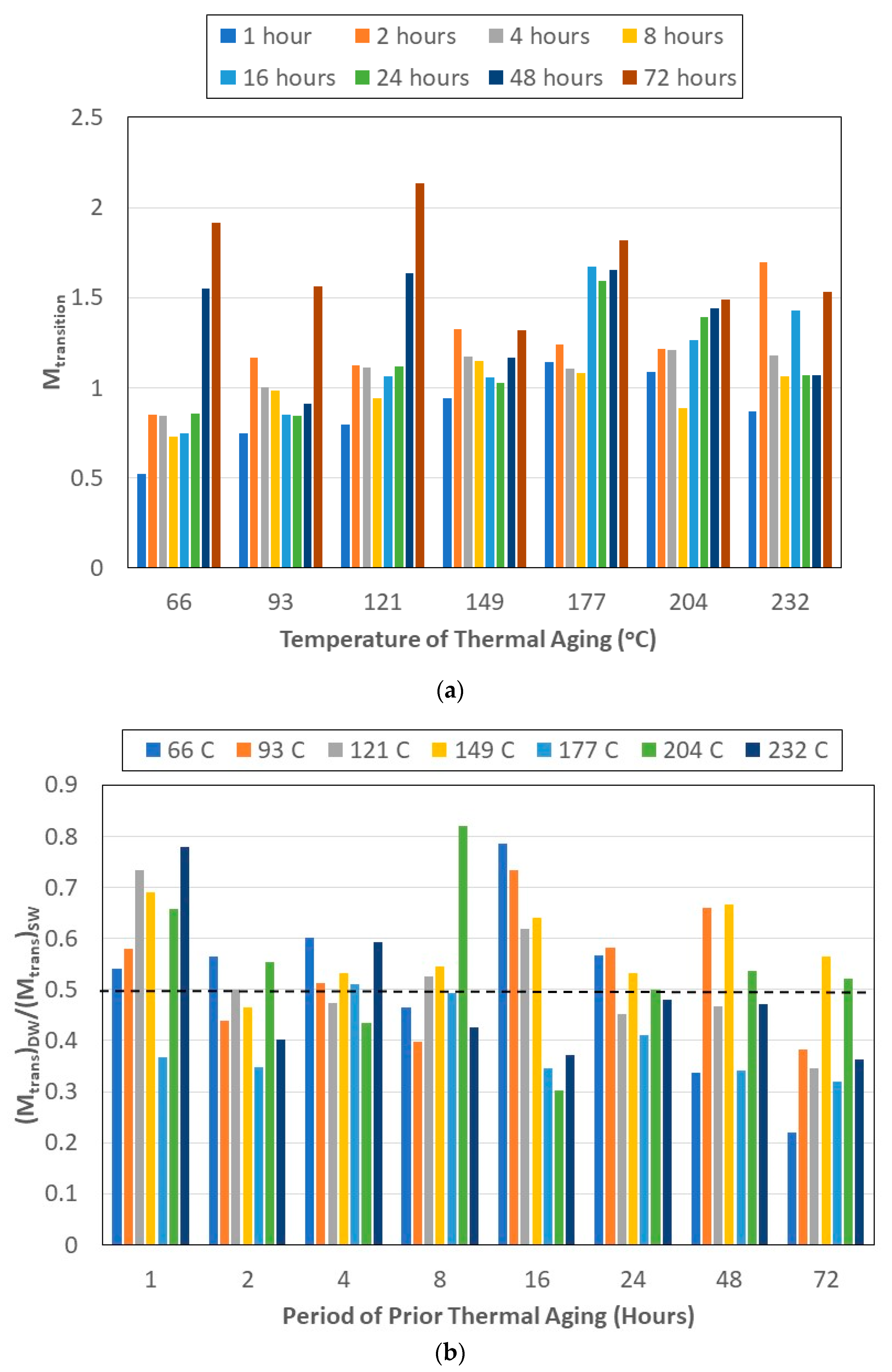
- The transition level of uptake, Mtrans, indicative of the shift from the faster diffusion-dominated initial regime to the slower second stage, shows an initial increase with the time of prior thermal aging, followed by a decrease.
- (4)
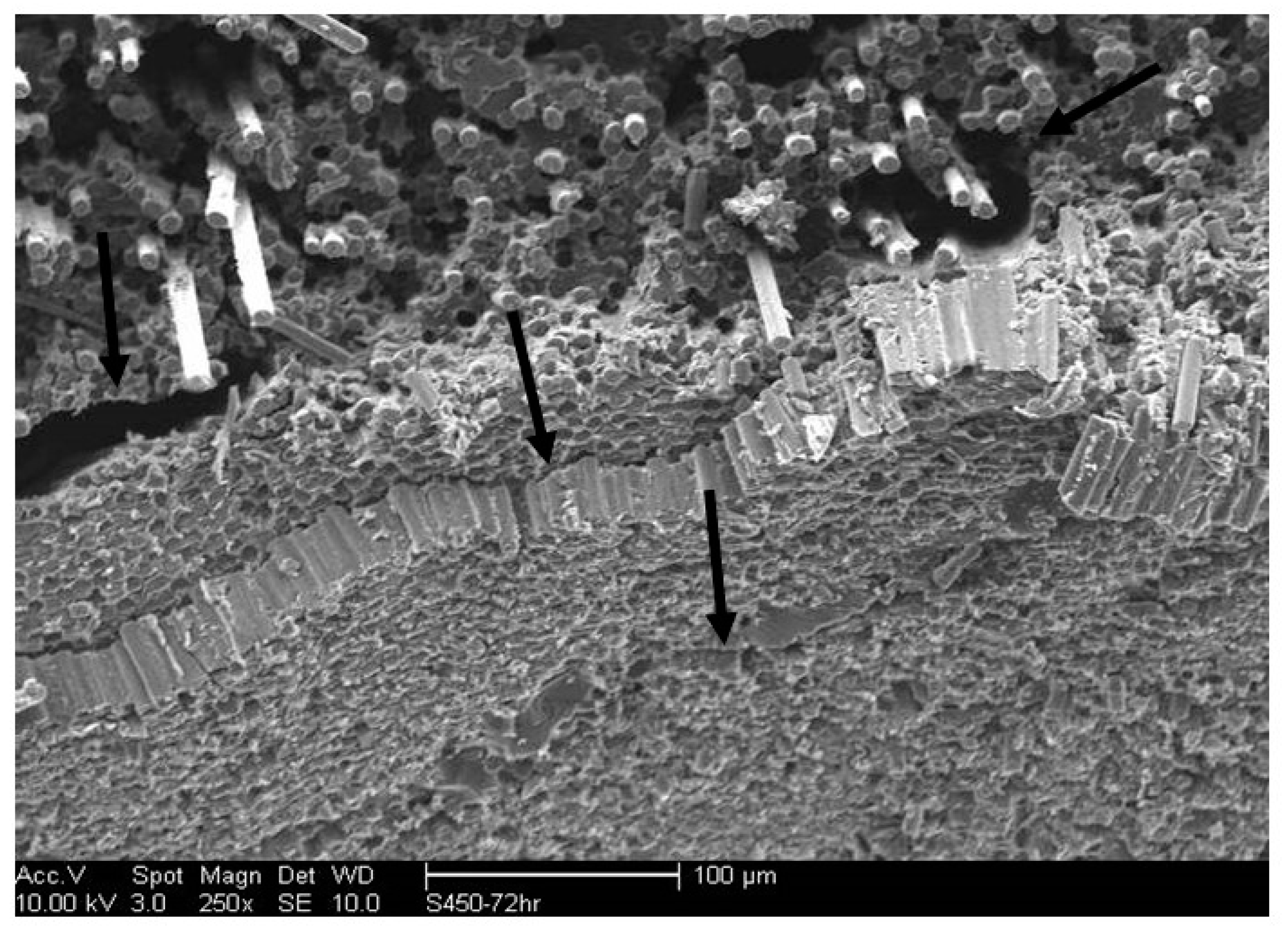
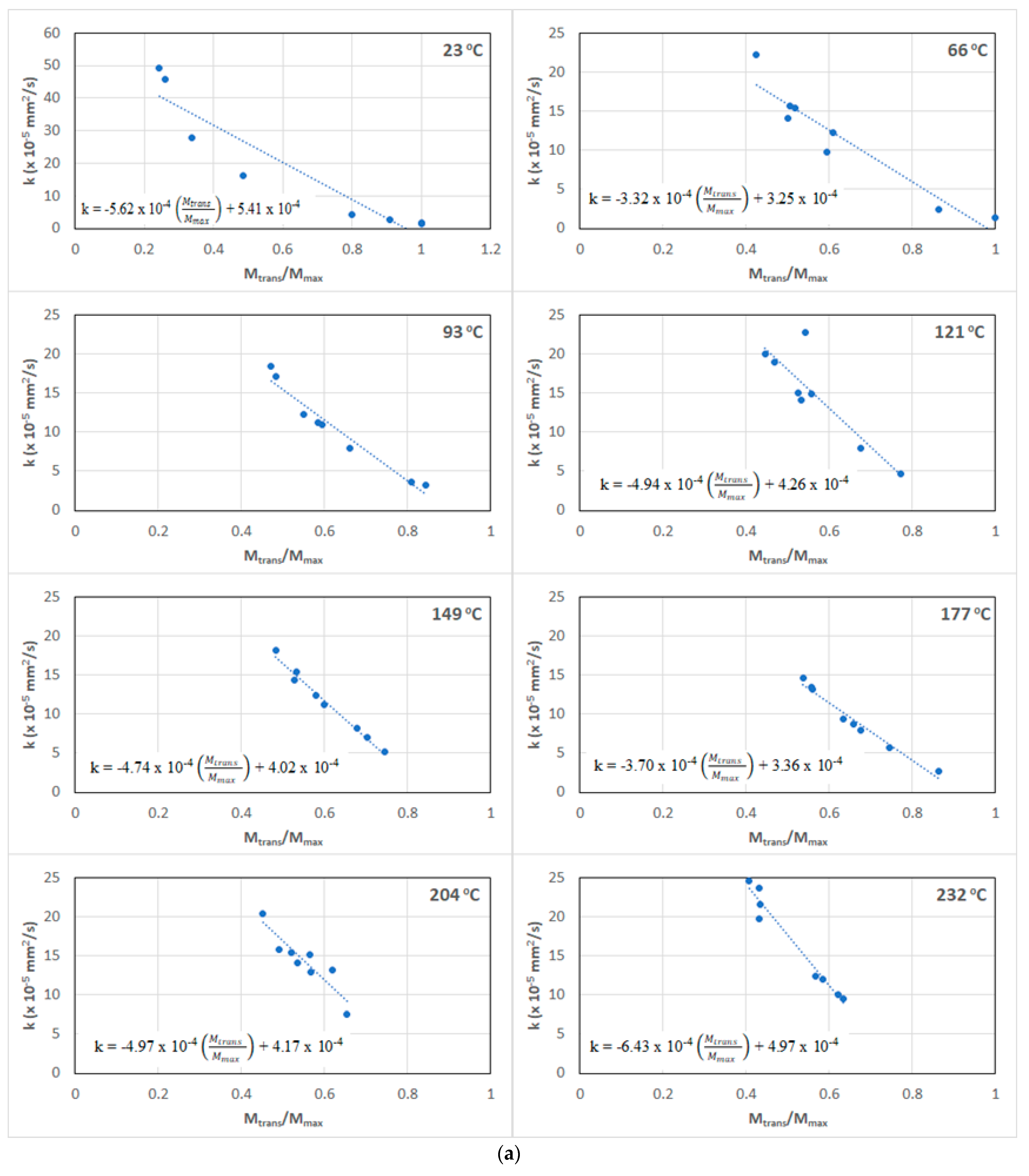
- The ratio Mtrans/Mmax provides further insight into the relative influence and length of the two primary mechanisms involved—an initial diffusion-dominated regime and a subsequent relaxation-/deterioration-based regime—the latter of which has a far more complex set of competing phenomena, including that of longer-term hydrolysis of the resin, irreversible changes in the network including those of postcuring, and of deterioration through the growth of microcavities and increased fiber–matrix debonding. The trends are in accordance with the phenomena reported in earlier studies related to moisture absorption and temperature, indicating that there are some similarities between sequential exposure and those of simultaneous hydrothermal loading.
- (5)
- The levels of maximum uptake were generally higher in deionized water than in seawater. The opposite trend is noted for uptake levels at the transition point at which the uptake in seawater is about twice that in deionized water, with the difference increasing with the time of prior thermal pre-aging. The highest ratios are seen from 24 h of prior aging onwards, irrespective of the temperature of thermal aging.
- (6)
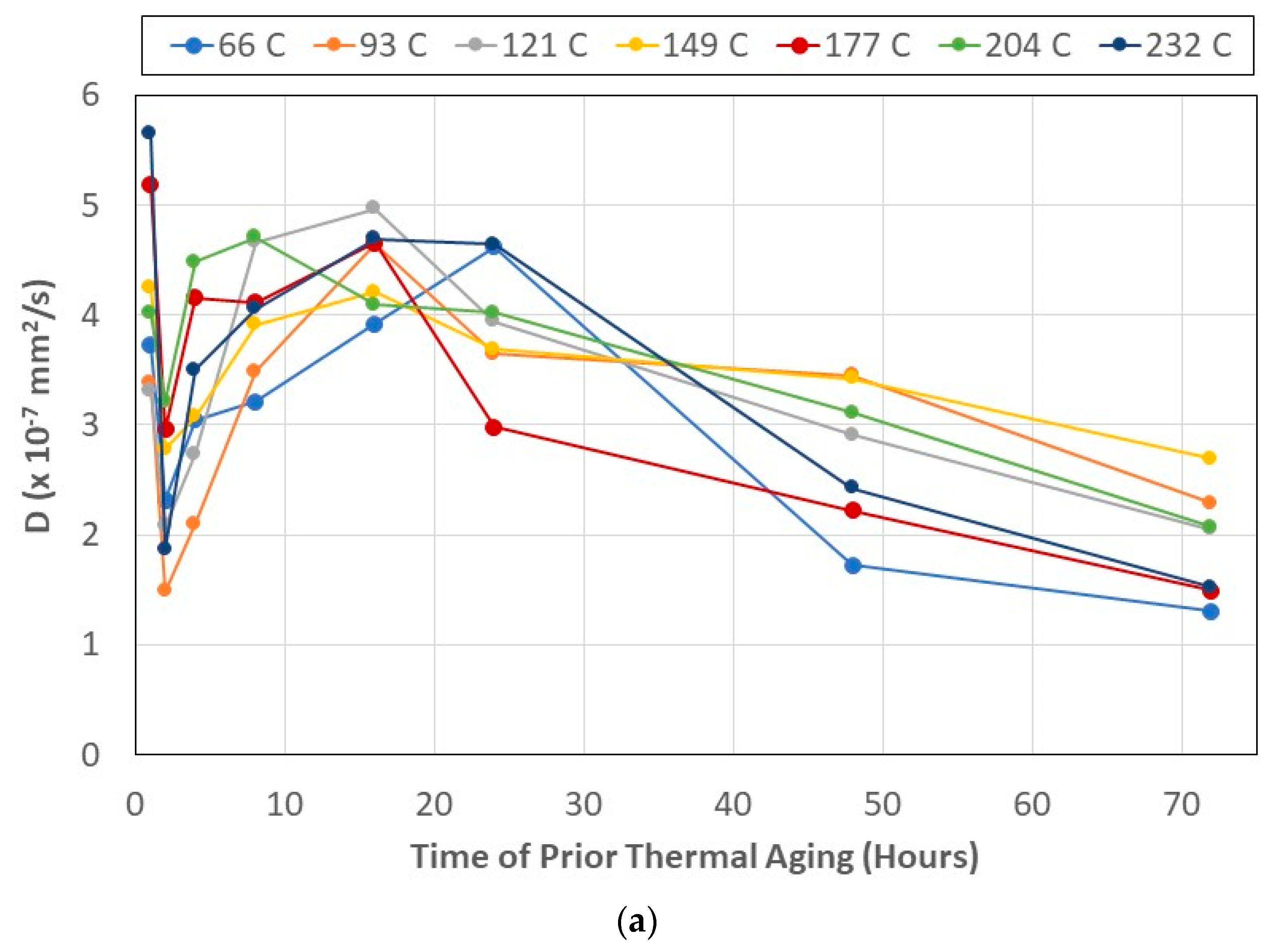
- Diffusivity, D, is directly related to both the temperature and time of prior thermal aging, and the relaxation/deterioration coefficient, k, is related through the ratio of mass uptakes, Mtrans/Mmax, since relaxation is not just a function of moisture uptake-driven mechanisms but rather depends on the evolving network structure of the bulk polymer and the composite and the integrity of the fiber–matrix interface, which is intrinsically dependent on the prior temperature and time of thermal aging. The relaxation/deterioration coefficient, k, increases as the ratio Mtrans/Mmax decreases, i.e., as the uptake in Stage II becomes larger, i.e., the diffusion-dominated first stage ends earlier in the uptake process. This can be related to the moisture ratio through a single master cure of exponential form.
- (7)
- The investigation shows similarities in the effects of the sequential exposure of thermal aging and subsequent immersion in seawater to that of immersion at elevated temperatures reported more extensively in the literature. It emphasizes the complex interactions between thermal- and moisture-driven phenomena that need to be more comprehensively understood in order to develop accelerated test processes and reliable estimates of long-term response and durability after extreme thermal events. At a first approximation for low levels of prior thermal aging, results from existing elevated temperature immersion can be used as reference levels.
- (8)
- Based on characteristic relationships, the use of moisture uptake levels and especially the ratio Mtrans/Mmax may provide better metrics for the modeling of long-term performance than that provided by the time of immersion.
- (9)
- The investigation indicates that in general, at temperatures lower than 232 °C, and for the time periods considered, the effects may not be serious enough to warrant removing components from service after exposure as tested, which is of significant practical value in post-extreme event integrity assessment and continued operation.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubino, F.; Nistico, A.; Tucci, F.; Carlone, P. Marine application of fiber reinforced composites: A review. J. Mar. Sci. Eng. 2020, 8, 26, 8010026, 28 pp. [Google Scholar] [CrossRef] [Green Version]
- Davies, P. Behavior of marine composite materials under deep submergence. In Chapter 6 in Marine Applications of Advanced Fibre-Reinforced Composites; Graham-Jones, J., Summerscales, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; Chapter 6; pp. 125–145. [Google Scholar]
- Sloan, J. Composites Enable Novel Flying Speedboat. Composites World. 1 January 2020. Available online: https://www.compositesworld.com/articles/composites-enable-novel-flying-speedboat (accessed on 26 March 2023).
- Gardiner, G. Composites End Markets: Boat Building and Marine 2022. Composites World. 1 December 2021. Available online: https://www.compositesworld.com/articles/composites-end-markets-boatbuilding-and-marine-2022 (accessed on 26 March 2023).
- Mouritz, A.P.; Gellert, E.; Burchill, P.; Challis, K. Review of advanced composite structures for naval ships and vessels. Compos. Struct. 2001, 53, 21–41. [Google Scholar] [CrossRef]
- Barsotti, B.; Gaiotti, M.; Rizzo, C.M. Recent industrial developments of marine composites limit states and design approaches on strength. J. Mar. Sci. Appl. 2020, 19, 553–566. [Google Scholar] [CrossRef]
- Lowde, M.J.; Peters, H.G.A.; Geraghty, R.; Graham-Jones, J.; Pemberton, R.; Summerscales, J. The 100-m composite ship. J. Mar. Sci. Eng. 2022, 10, 408, 10030408, 18 pp. [Google Scholar] [CrossRef]
- Pemberton, R.; Summerscales, J.; Graham-Jones, J. (Eds.) Marine Composites Design and Performance; Elsevier: Cambridge, UK, 2019. [Google Scholar]
- Graham-Jones, J.; Summerscales, J. (Eds.) Marine Applications of Advanced Fibre-Reinforced Composites; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Roseman, M.; Marton, R.; Morgan, G. Composites in offshore oil and gas applications. In Marine Applications of Advanced Fibre-Reinforced Composites; Graham-Jones, J., Summerscales, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; Chapter 10; pp. 233–257. [Google Scholar]
- Amaechi, C.V.; Chesterton, C.; Butler, H.D.; Gillet, N.; Wang, C.; Jae, I.A.; Reda, A.; Odijie, A.C. Review of composite marine risers for deep-water applications; Design development and mechanics. J. Compos. Sci. 2022, 6, 96, 50 pp. [Google Scholar] [CrossRef]
- Harper, P.; Hallett, S.; Fleming, A.; Dawson, M. Advanced fibre-reinforced composites for marine renewable energy devices. In Chapter 9 in Marine Applications of Advanced Fibre-Reinforced Composites; Graham-Jones, J., Summerscales, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 217–232. [Google Scholar]
- Tual, N.; Carrere, N.; Davies, P.; Bonnemains, T.; Lolive, E. Characterization of seawater aging effects on mechanical properties of carbon/epoxy composites for tidal turbine blades. Compos. A 2015, 78, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Bone, J.E.; Sims, G.D.; Maxwell, A.S.; Frenz, S.; Ogin, S.L.; Foreman, C.; Dorey, R.A. On the relationship between moisture uptake and mechanical property changes in a carbon fibre/epoxy composite. J. Compos. Mater. 2022, 56, 2189–2199. [Google Scholar] [CrossRef]
- Davies, P.; Rajapakse, Y.D.S. Durability of Composites in a Marine Environment—I; Springer Berlin: Berlin, Germany, 2014. [Google Scholar]
- Davies, P.; Rajapakse, Y.D.S. Durability of Composites in a Marine Environment—II; Springer Berlin: Berlin, Germany, 2018. [Google Scholar]
- Apicella, A.; Nicolais, L.; de Cataldis, C. Characterization of the morphological fine structure of commercial thermosetting resins through hygrothermal exposure. Adv. Polym. Sci. 1985, 66, 189–207. [Google Scholar]
- Apicella, A.; Tessieri, R.; de Cataldis, C. Sorption modes of water in glassy epoxies. J. Membr. Sci. 1984, 18, 211–225. [Google Scholar] [CrossRef]
- Moy, P.; Karasz, F.E. Epoxy-water interactions. Polym. Eng. Sci. 1980, 20, 315–319. [Google Scholar] [CrossRef]
- Pethrick, R.A.; Hollins, E.A.; McEwan, L.; Pollock, A.; Hayward, D.; Johncock, P. Effect of cure temperature on the structure and water absorption of epoxy/amine thermosets. Polym. Int. 1996, 39, 275–288. [Google Scholar] [CrossRef]
- Zhao, J.M.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part I: The nature of water in epoxy. Polymer 1999, 40, 5505–5512. [Google Scholar] [CrossRef]
- Guo, R.; Xian, G.; Li, F.; Li, C.; Hong, B. Hygrothermal resistance of pultruded carbon, glass and carbon/glass hybrid reinforced epoxy composites. Constr. Build. Mater. 2022, 315, 125710, 21 pp. [Google Scholar] [CrossRef]
- Guo, R.; Li, C.; Xian, G. Water absorption and long-term thermal and mechanical properties of carbon/glass hybrid rod for bridge cable. Eng. Struct. 2023, 274, 115176, 17 pp. [Google Scholar] [CrossRef]
- Hahn, H.T. Residual stresses in polymer matrix composite laminates. J. Compos. Mater. 1976, 10, 266–278. [Google Scholar] [CrossRef]
- Xiao, G.Z.; Delmar, M.; Shanahan, M.E.R. Irreversible interactions between water and DGEBA/DDA epoxy resin during hygrothermal aging. J. Appl. Polym. Sci. 1977, 65, 449–458. [Google Scholar] [CrossRef]
- Chateauminois, A.; Chabert, B.; Soulier, J.P.; Vincent, L. Dynamic-mechanical analysis of epoxy composites plasticized by water—Artifact and reality. Polym. Compos. 1995, 16, 288–296. [Google Scholar] [CrossRef]
- Adamson, M.J. Thermal expansion and swelling of cured epoxy-resin used in graphite-epoxy composite materials. J. Mater. Sci. 1980, 15, 1736–1745. [Google Scholar] [CrossRef]
- Nogueira, P.; Ramirez, C.; Torres, A.; Abad, M.J.; Cano, J.; Lopez, J.; Lopez-Bueno, I.; Barral, L. Effect of water sorption on the structure and mechanical properties of an epoxy system. J. Appl. Polym. Sci. 2001, 80, 71–80. [Google Scholar] [CrossRef]
- La Plante, G.; Lee-Sullivan, P. Moisture effects on FM300 structural film adhesive: Stress relaxation, fracture toughness, dynamic mechanical analysis. J. Appl. Polym. Sci. 2005, 95, 1285–1294. [Google Scholar] [CrossRef]
- Abanilla, M.A.; Li, Y.; Karbhari, V.M. Interlaminar and intralaminar durability characterization of wet layup carbon/epoxy used in external strengthening. Compos. B 2006, 37, 650–661. [Google Scholar] [CrossRef]
- Mazor, A.; Broutman, L.J.; Eckstein, B.H. Effect of long-term water exposure on the properties of carbon and graphite fiber reinforced epoxies. Polym. Eng. Sci. 1978, 18, 341–349. [Google Scholar] [CrossRef]
- Le Guen-Geffroy, A.; Le Gac, P.-Y.; Habert, B.; Davies, P. Physical aging of epoxy in a wet environment; Coupling between plasticization and physical aging. Polym. Degrad. Stab. 2019, 168, 108947, 8 pp. [Google Scholar] [CrossRef]
- Weitsman, Y.J. Fluid Effects in Polymers and Polymer Composites; Springer Science and Business Media: Boston, MA, USA, 2012. [Google Scholar]
- McKague, E.L.; Reynolds, J.D.; Halkias, J.E. Swelling and glass transition relations for epoxy material in humid environments. J. Appl. Polym. Sci. 1978, 22, 1643–1654. [Google Scholar] [CrossRef]
- Gaur, U.; Miller, B. Effects of environmental exposure on fiber/epoxy interfacial shear strength. Polym. Compos. 1990, 11, 217–222. [Google Scholar] [CrossRef]
- Nairn, J.A.; Hu, S. The initiation and growth of delaminations induced by matrix microcracks in laminated composites. Int. J. Fract. 1992, 57, 1–24. [Google Scholar] [CrossRef]
- Gillat, O.; Broutman, L.J. Effect of an external stress on moisture diffusion and degradation in a graphite epoxy laminate. In Advanced Composite Materials—Environmental Effects; ASTM STP, 658, Vinson, J.R., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1978; pp. 61–83. [Google Scholar]
- Roy, S.; Xu, W. Modelling of diffusion in the presence of damage in polymer matrix composites. Int. J. Solids Struct. 2001, 38, 115–126. [Google Scholar] [CrossRef]
- Tsenoglou, C.J.; Pavlidou, S.; Papaspyrides, C.D. Evaluation of interfacial relaxation due to water absorption in fiber polymer composites. Compos. Sci. Technol. 2006, 66, 2855–2864. [Google Scholar] [CrossRef]
- Loos, A.C.; Springer, G.S. Environmental Effects on Composite Materials; GS Springer. Technomic: Westport, CT, USA, 1981; Volume 1. [Google Scholar]
- Scott, P.; Lees, J.M. Water, saltwater and alkaline solution uptake in epoxy thin films. J. Appl. Polym. Sci. 2013, 130, 1898–1908. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.W.; Nguyen, T.; Aouadi, K. Sorption and diffusion of water, saltwater, and concrete pore solution in composite matrices. J. Appl. Polym. Sci. 1999, 71, 483–492. [Google Scholar] [CrossRef]
- Soulier, J.P.; Berruet, R.; Chateauminois, A.; Chabert, B.; Gauthier, R. Interactions of fibre-reinforced epoxy composites with different salt water solutions including isotonic liquid. Polym. Commun. 1988, 29, 243–246. [Google Scholar]
- Karbhari, V.M.; Hassanpour, B. Water, saltwater, and concrete leachate solution effects on durability of ambient-temperature cure carbon-epoxy composites. J. Appl. Polym. Sci. 2022, 139, E52496. [Google Scholar] [CrossRef]
- Ghorbel, I.; Valentin, D. Hydrothermal effects on the physico-chemical properties of pure and glass fiber reinforced polyester and vinylester resins. Polym. Compos. 1993, 14, 324–334. [Google Scholar] [CrossRef]
- Marshall, J.M.; Marshall, G.P.; Pinzelli, R.F. The diffusion of liquids into resins and composites. Polym. Compos. 1982, 3, 131–137. [Google Scholar] [CrossRef]
- Guttierrez, J.; Le Lay, F.; Herau, P. A study of the aging of glass fibre-resin composites in a marine environment. In Nautical Construction with Composite Materials; Davies, P., Lemoine, L., Eds.; Ifremer-Centre de Brest: Paris, France, 1992; p. 338. [Google Scholar]
- Gellert, E.P.; Turley, D.M. Seawater immersion aging of glass-fibre reinforced polymer laminates for marine applications. Compos. A 1999, 30, 1259–1265. [Google Scholar] [CrossRef]
- Kahraman, R.; Al-Harthi, M. Moisture diffusion into aluminum powder-filled epoxy adhesive in sodium chloride solutions. Int. J. Adhes. Adhes. 2005, 25, 337–341. [Google Scholar] [CrossRef]
- Tai, R.C.L.; Szklarska-Smialowska, Z. Effect of fillers on the degradation of automotive epoxy adhesives in aqueous solutions. I: Absorption of water by different fillers- incorporated automotive epoxy adhesives. J. Mater. Sci. 1993, 28, 6199–6204. [Google Scholar] [CrossRef]
- Tai, R.C.L.; Szklarska-Smialowska, Z. Effect of fillers on the degradation of automotive epoxy adhesives in aqueous solutions. II: The micro hardness change and delamination of automotive epoxy adhesives in distilled water and NaCl solutions. J. Mater. Sci. 1993, 28, 6205–6210. [Google Scholar] [CrossRef]
- Zafar, A.; Bertocco, F.; Schjodt-Thomsen, J.; Ruhe, J.C. Investigation of long-term effects of moisture on carbon fibre and epoxy matrix composites. Compos. Sci. Technol. 2012, 72, 656–666. [Google Scholar] [CrossRef]
- da Silva, L.V.; de Menezes, E.A.W.; Tarpani, J.R.; Amico, S.C. Accelerated aging effects on short-beam strength behavior of pultruded Cfrp rods. Appl. Compos. Mater. 2022, 29, 855–869. [Google Scholar] [CrossRef]
- Zavatta, N.; Rondina, F.; Falaschatt, M.P.; Donati, L. Effect of thermal aging on the mechanical strength of carbon fibre reinforced epoxy composites. Polymers 2021, 13, 2006, 10 pp. [Google Scholar] [CrossRef] [PubMed]
- Brkic, D.; Praks, P. Probability analysis and prevention of offshore oil and gas accidents: Fire as a cause and consequence. Fire 2021, 4, 71, 23 pp. [Google Scholar] [CrossRef]
- Firmo, J.P.; Correia, J.R.; Bisby, L.A. Fire behavior of FRP-strengthened reinforced concrete structural elements: A state-of-the-art review. Compos. B 2015, 80, 198–216. [Google Scholar] [CrossRef]
- Maravenas, C.; Miamis, K.; Vrakas, A.A. Fiber reinforced polymer strengthened/reinforced concrete exposed to fire: A review. Struct. Eng. Int. 2012, 22, 500–513. [Google Scholar] [CrossRef]
- Hoffman, E.; Skidmore, T.E. Radiation effects on epoxy/carbon fiber composites. J. Nucl. Mater. 2001, 392, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yin, X.; Wang, Y.; Zhang, L.; Zhang, Z.; Liu, Y.; Xian, G. Mechanical property evolution and service life prediction of pultruded carbon/glass hybrid rod exposed in harsh oil-well condition. Compos. Struct. 2020, 246, 112418, 12 pp. [Google Scholar] [CrossRef]
- Yuan, Y.; Goodson, J.E. Advanced composite downhole applications and the HTHP environmental challenges, 2004, Corrosion 2004, New Orleans, LA, March, paper # NACE-04616.
- Holliday, R.J.; Gibson, A.G.; Humphrey, J.; DiModica, P.; Christko, S.; Kotsihas, G. Post-fire structural integrity of composite gratings for offshore platforms. In Proceedings of the SPE Offshore Oil and Gas Conference, Aberdeen, UK, 3–6 September 2013. paper # SPE- 166587- M6. [Google Scholar]
- Hong, S.K.; Karbhari, V.M. Effect of thermal exposure on residual properties of wet layup carbon fiber reinforced epoxy composites. Polymers 2022, 14, 2957, 22 pp. [Google Scholar] [CrossRef]
- Papanicolaou, G.; Fotou, P.; Zetos, A. Modeling the mechanical degradation due to moisture absorption and polymer matrix composites. UPB Sci Bull. Ser. D 2016, 78, 37–47. [Google Scholar]
- Jose-Trujillo, E.; Rubio-Gonzalez, C.; Rodriguez-Gonzalez, J.A. Seawater aging effect on the mechanical properties of composites with different fibers and matrix types. J. Compos. Mater. 2019, 53, 3229–3241. [Google Scholar] [CrossRef]
- Hamadi, Y.K.; Levent, A.; Altan, M.C. Thermal history effects on moisture absorption of fiber-reinforced polymer composites. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1914, p. 030012, 5 pp. [Google Scholar]
- Karbhari, V.M.; Hong, S.K. Effect of sequential thermal aging and water immersion on moisture kinetics and SBS strength of wet layup carbon epoxy composites. J. Compos. Sci. 2022, 6, 306, 23 pp. [Google Scholar] [CrossRef]
- Xian, G.; Karbhari, V.M. Segmental relaxation of water-aged ambient cured epoxy. Polym. Degrad. Stab. 2007, 92, 1650–1659. [Google Scholar] [CrossRef]
- Shen, C.-H.; Springer, G.S. Moisture absorption and desorption of composite materials. J. Compos. Mater. 1976, 10, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Berens, A.R.; Hopfenberg, H.B. Diffusion and relaxation in glassy polymer powders: 2 Separation of diffusion and relaxation parameters. Polymer 1978, 19, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Bagley, E.; Long, F.A. Two-stage sorption and desorption of organic vapors in cellulose acetate. J. Am. Chem. Soc. 1955, 77, 2172–2182. [Google Scholar] [CrossRef]
- Bao, L.-R.; Yee, A.F.; Lee, C.Y.-C. Moisture absorption and hygrothermal aging in a bismaleimide resin. Polymer 2001, 42, 7327–7333. [Google Scholar] [CrossRef]
- Xian, G.; Karbhari, V.M. DMTA based investigation of hygrothermal aging of an epoxy system used in rehabilitation. J. Appl. Polym. Sci. 2007, 104, 1084–1094. [Google Scholar] [CrossRef]
- Karbhari, V.M. Long-term hydrothermal aging of carbon-epoxy materials for rehabilitation of civil infrastructure. Compos. A 2022, 153, 106705, 12 pp. [Google Scholar] [CrossRef]
- Morgan, R.J.; O’neal, J.E.; Fenter, D.L. The effect of moisture on the physical and mechanical integrity of epoxies. J. Mater. Sci. 1980, 15, 751–764. [Google Scholar] [CrossRef]
- Tam, L.H.; He, L.; Wu, C. Molecular dynamics study on the effect of salt environment on interfacial structure, stress and adhesion of carbon fiber/epoxy interface. Compos. Interfaces 2019, 26, 431–447. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Chen, J.; Huang, L.; Lv, Y. Effect of marine environment on the mechanical properties, degradation and long-term creep failure of CFRP. Mater. Today Commun. 2022, 31, 103834, 15 pp. [Google Scholar] [CrossRef]
- Tang, J.; Springer, G.S. Effects of cure and moisture on the properties of Fiberite 976 resin. J. Compos. Mater. 1988, 22, 2–14. [Google Scholar] [CrossRef]
- de Parscau du Plessix, B.; Jacquemin, F.; Lefebure, P.; Le Corre, S. Characterization and modeling of the polymerization-dependent moisture absorption behavior of an epoxy-carbon fiber-reinforced composite material. J. Compos. Mater. 2016, 50, 2495–2505. [Google Scholar] [CrossRef]
- Baghad, A.; El Mabrouk, K.; Vandereuil, S.; Nouneh, K. Effects of high operating temperatures and holding times on the thermomechanical and mechanical properties of autoclaved epoxy/carbon composite laminates. Polym. Compos. 2022, 43, 862–873. [Google Scholar] [CrossRef]
- Karbhari, V.M.; Xian, G.; Hong, S.K. Effect of thermal exposure on carbon fiber reinforced composites used in civil infrastructure rehabilitation. Compos. A 2021, 149, 106570. [Google Scholar] [CrossRef]
- Zheng, Y.; Priestley, R.D.; McKenna, G.B. Physical aging of an epoxy subsequent to relative humidity jumps through the glass concentration. J. Polym. Sci. B Polym. Phys. 2004, 42, 2107–2121. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Jones, F.R. Thermal spike enhanced moisture absorption by polymer-matrix carbon-fibre composites. Compos. Sci. Technol. 1997, 57, 451–461. [Google Scholar] [CrossRef]
- Loos, A.C.; Springer, G.S. Effects of thermal spiking on graphite-epoxy composites. J. Compos. Mater. 1979, 13, 17–34. [Google Scholar] [CrossRef]
- Adamson, N.J. A Model of the Thermal Spike Mechanism in Graphite Epoxy Laminates; NASA TM-8429; NASA: Washington, DC, USA, 1982. [Google Scholar]
- Bao, L.-R.; Yee, A.F. Effect of temperature on moisture absorption in a bismaleimide resin and its carbon fiber composites. Polymer 2000, 43, 3987–3997. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Gopferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.S.; Karasz, F.E. Interaction of epoxy resins with water: The depression of glass transition temperature. Polymer 1984, 25, 664–669. [Google Scholar] [CrossRef]
- Ivanova, K.I.; Pethrick, A.A.; Affrossman, S. Hygrothermal aging of rubber-modified and mineral filled dicyandiamide-cured DGEBA epoxy resin II: Dynamic mechanical thermal analysis. J. Appl. Polym. Sci. 2001, 82, 3477–3485. [Google Scholar] [CrossRef]








| Temperature of Thermal Aging (°C) | Rate ×10−8 mm2/s/Week |
|---|---|
| 66 | 4.08 |
| 93 | 1.59 |
| 121 | 2.49 |
| 149 | 1.79 |
| 177 | 1.55 |
| 204 | 1.99 |
| 232 | 3.04 |
| Temperature of Pre-Immersion Thermal Aging (°C) | m | R2 | Time of Pre-Immersion Thermal Aging (hours) | m | R2 |
|---|---|---|---|---|---|
| 66 | 1.52 | 0.94 | 1 | 1.69 | 0.97 |
| 93 | 1.51 | 0.98 | 2 | 1.44 | 0.97 |
| 121 | 1.56 | 0.99 | 4 | 1.52 | 0.99 |
| 149 | 1.53 | 0.97 | 8 | 1.66 | 0.96 |
| 177 | 1.50 | 0.97 | 16 | 1.58 | 0.99 |
| 204 | 1.61 | 0.98 | 24 | 1.72 | 0.98 |
| 232 | 1.87 | 0.97 | 48 | 1.41 | 0.96 |
| 72 | 1.33 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbhari, V.M.; Acharya, R.; Hong, S. Seawater Effects on Thermally Aged Ambient Cured Carbon/Epoxy Composites: Moisture Kinetics and Uptake Characteristics. Polymers 2023, 15, 2138. https://doi.org/10.3390/polym15092138
Karbhari VM, Acharya R, Hong S. Seawater Effects on Thermally Aged Ambient Cured Carbon/Epoxy Composites: Moisture Kinetics and Uptake Characteristics. Polymers. 2023; 15(9):2138. https://doi.org/10.3390/polym15092138
Chicago/Turabian StyleKarbhari, Vistasp M., Rabina Acharya, and SoonKook Hong. 2023. "Seawater Effects on Thermally Aged Ambient Cured Carbon/Epoxy Composites: Moisture Kinetics and Uptake Characteristics" Polymers 15, no. 9: 2138. https://doi.org/10.3390/polym15092138







