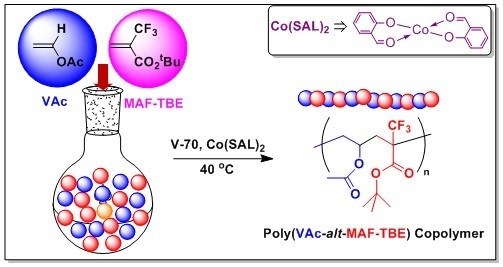
Bis(formylphenolato)cobalt(II)-Mediated Alternating Radical Copolymerization of tert-Butyl 2-Trifluoromethylacrylate with Vinyl Acetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.2.1. OMRP of VAc Initiated by V-70 in the Presence of 3
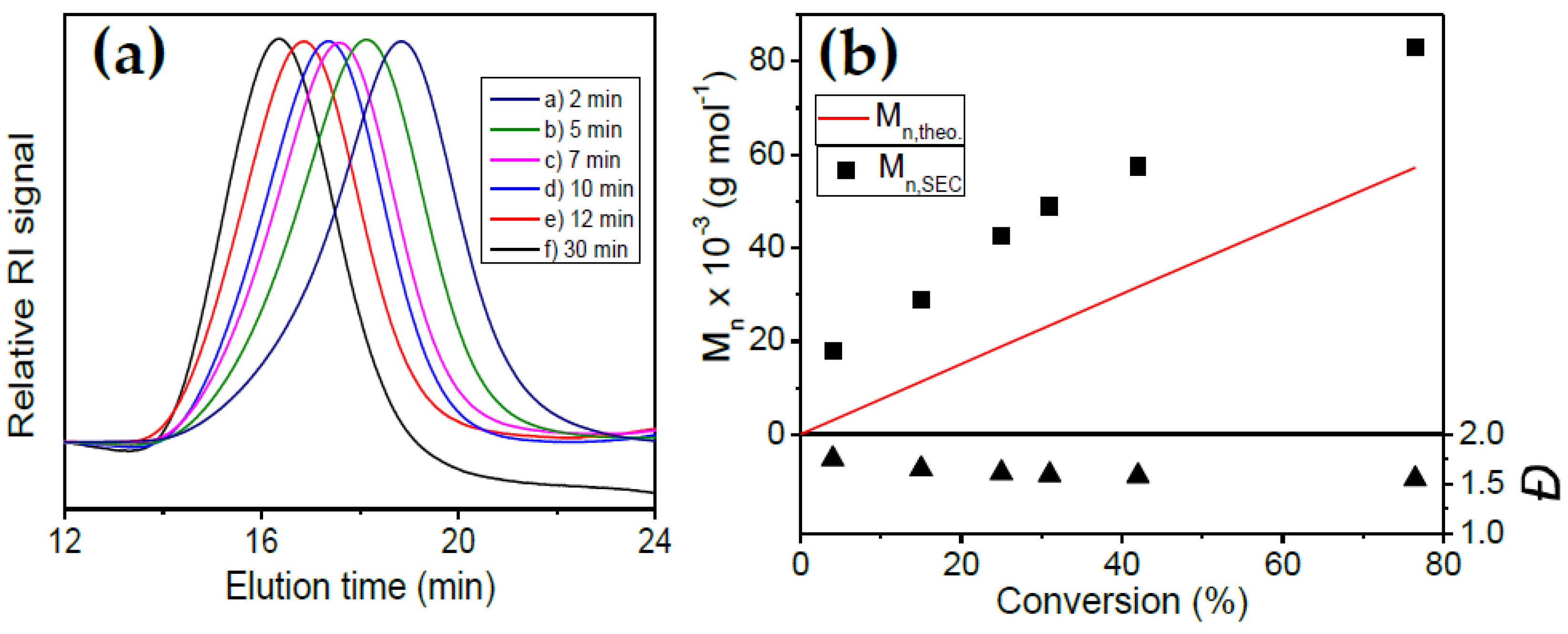
2.2.2. Co(SAL)2-Mediated Radical Copolymerization of VAc and MAF-TBE
2.3. Characterizations
2.3.1. Size Exclusion Chromatography (SEC)
2.3.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
3. Results and Discussion
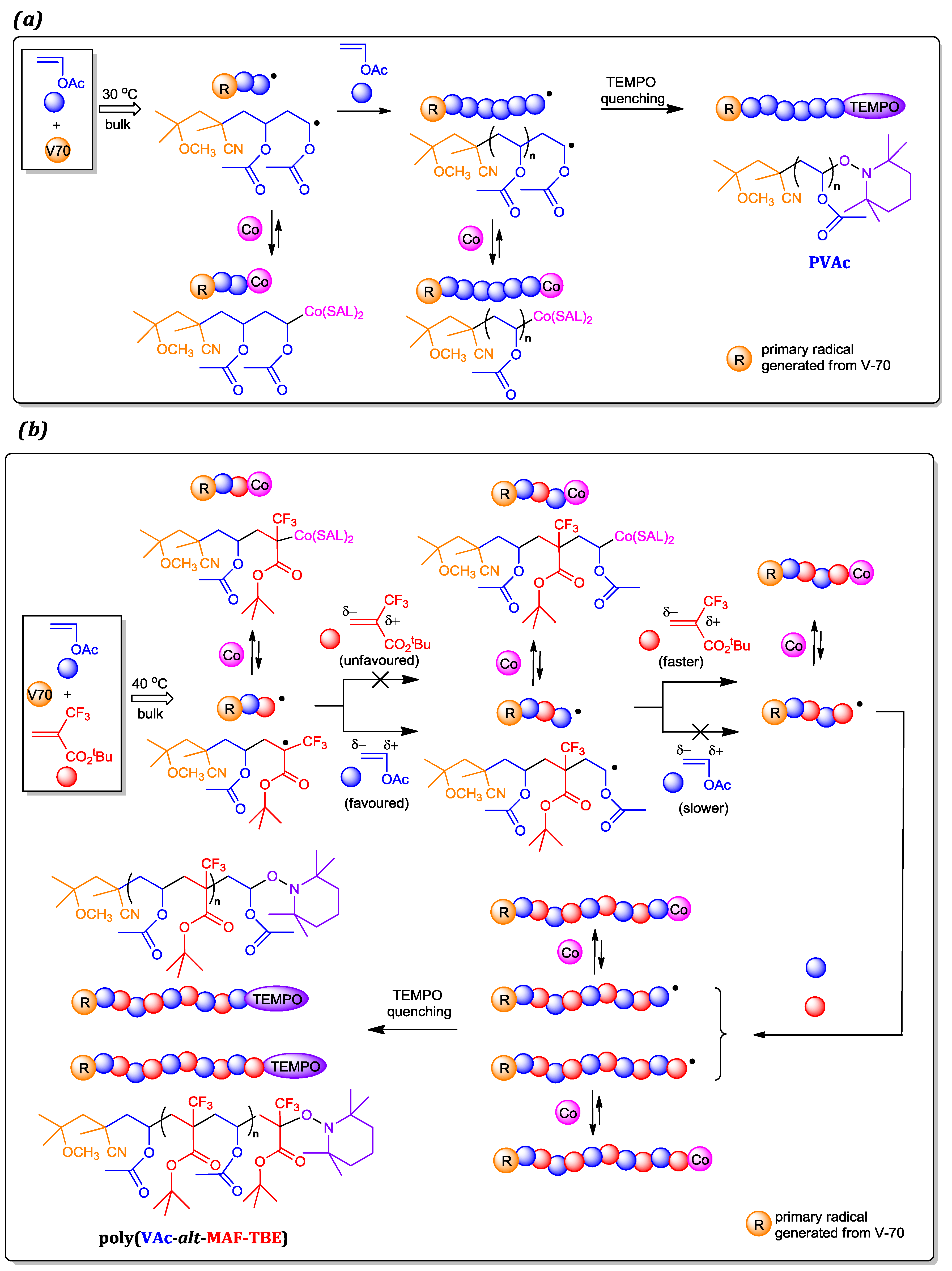
3.1. Homopolymerizations of VAc and MAF-TBE Mediated by 3
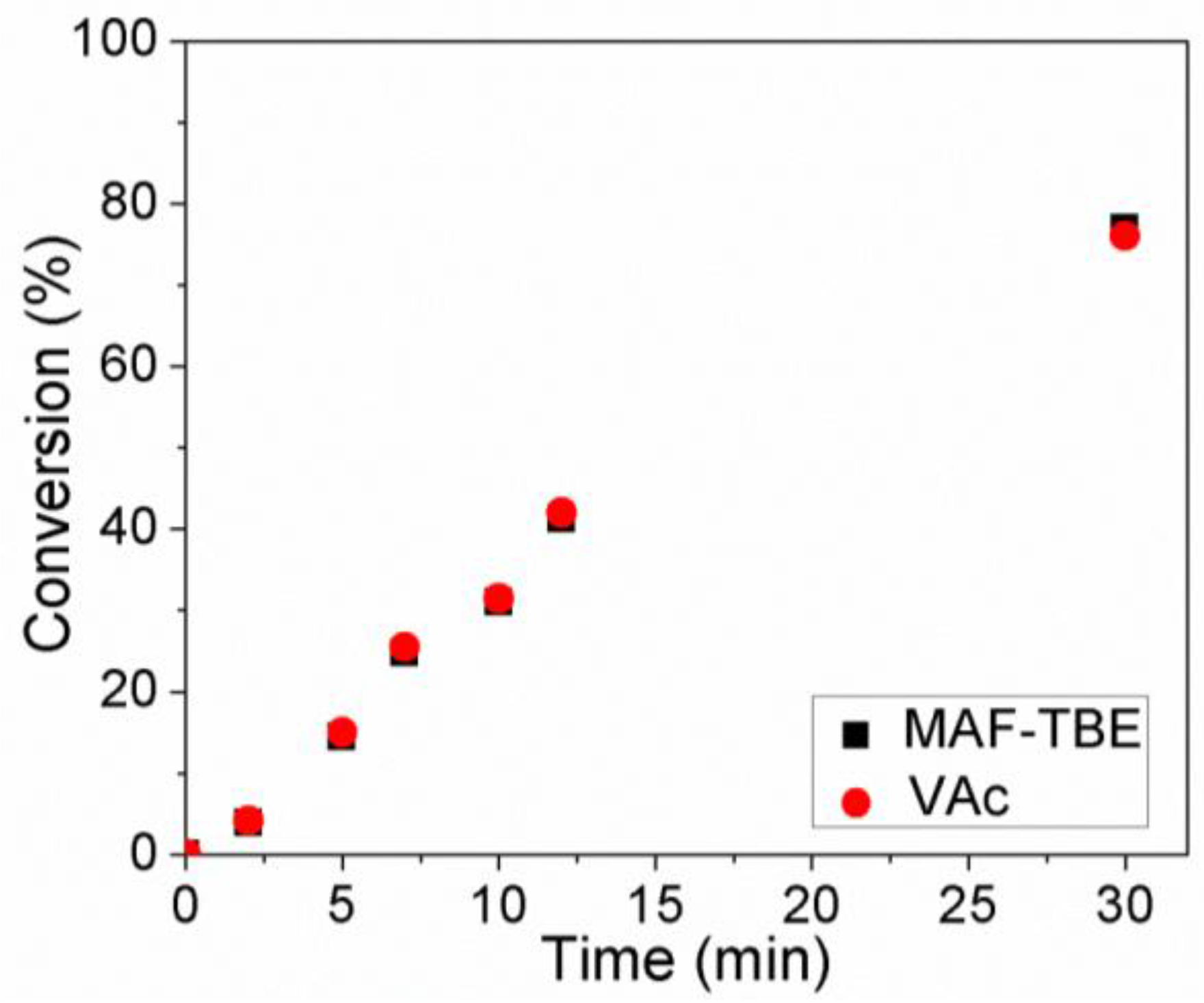
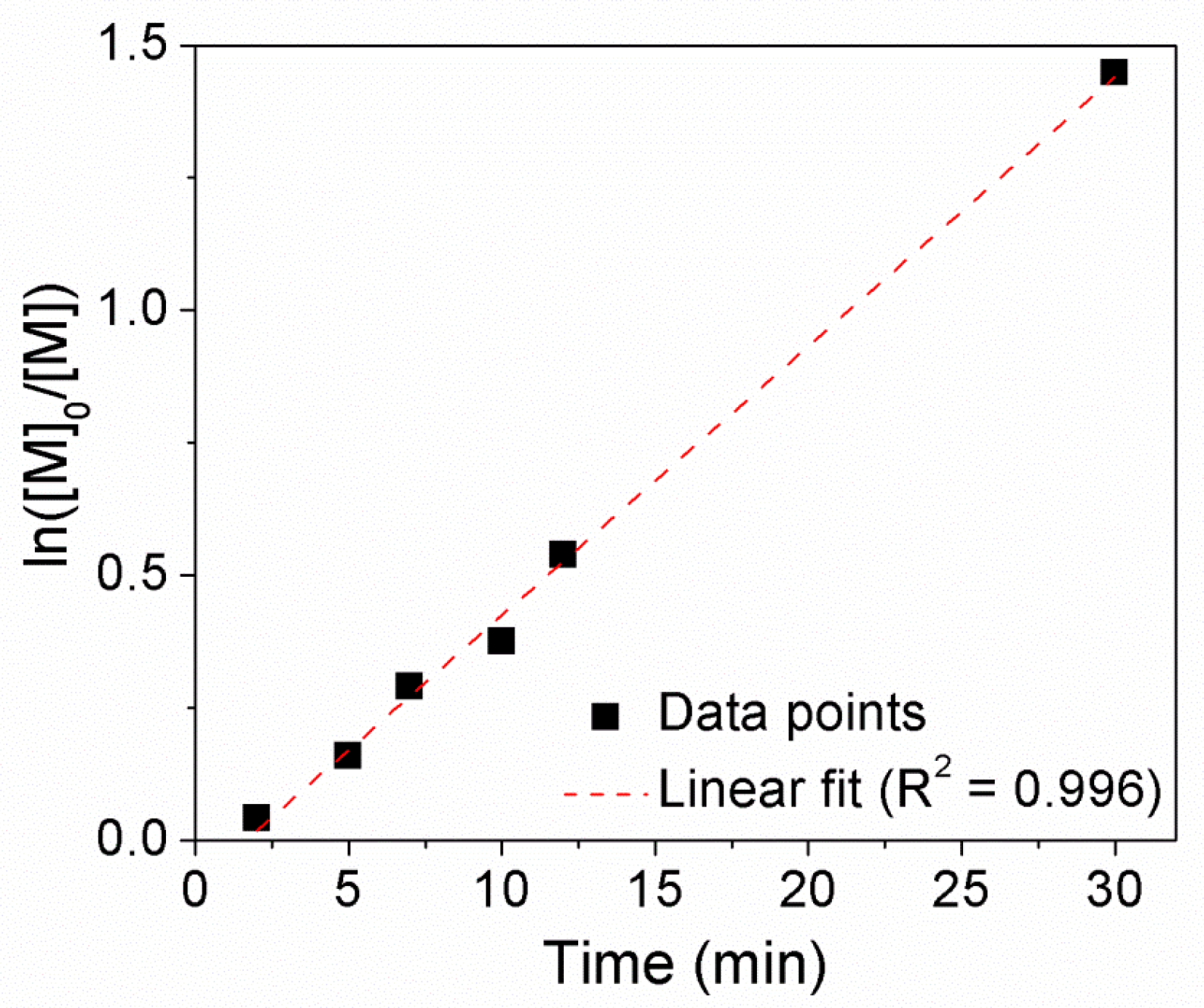
3.2. Copolymerization of VAc and MAF-TBE Mediated by 3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: From Fundamental to Practical Synthesis and Applications; Royal Society of Chemistry: Oxford, UK, 2016. [Google Scholar]
- Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sumerlin, B.S. Thermally-labile segmented hyperbranched copolymers: Using reversible-covalent chemistry to investigate the mechanism of self-condensing vinyl copolymerization. Chem. Sci. 2014, 5, 4646–4655. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Dai, Y.; Hill, M.R.; Ghiviriga, I.; Bapat, A.P.; Sumerlin, B.S. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat. Chem. 2017, 9, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tonnar, J.; Améduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of iodocompounds in radical polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Thenappan, A.; Ameduri, B. Synthesis of Chlorotrifluoroethylene-Based Block Copolymers by Iodine Transfer Polymerization. ACS Macro Lett. 2015, 4, 16–20. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef] [PubMed]
- Taton, D.; Destarac, M.; Zard, S.Z. Macromolecular Design by Interchange of Xanthates (MADIX): Background, Design, Scope, and Applications. In Handbook of RAFT Polymerization; Barner-Kowollik, C., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 373–421. [Google Scholar]
- Barner-Kowollik, C. Handbook of RAFT Polymerization; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S. Bioapplications of RAFT Polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; Carmean, R.N.; Sumerlin, B.S. Expanding the Scope of RAFT Polymerization: Recent Advances and New Horizons. Macromolecules 2015, 48, 5459–5469. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerization in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef] [PubMed]
- Poli, R. Relationship between one-electron transition metal reactivity and radical polymerization processes. Angew. Chem. Int. Ed. Engl. 2006, 45, 5058–5070. [Google Scholar] [CrossRef] [PubMed]
- Hurtgen, M.; Detrembleur, C.; Jerome, C.; Debuigne, A. Insight into Organometallic-Mediated Radical Polymerization. Polym. Rev. 2011, 51, 188–213. [Google Scholar] [CrossRef]
- Poli, R. Organometallic Mediated Radical Polymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 3, pp. 351–375. [Google Scholar]
- Poli, R. New phenomena in organometallic-mediated radical polymerization (OMRP) and perspectives for control of less active monomers. Chem. Eur. J. 2015, 21, 6988–7001. [Google Scholar] [CrossRef] [PubMed]
- Debuigne, A.; Jerome, C.; Detrembleur, C. Organometallic-mediated radical polymerization of ‘less activated monomers’: Fundamentals, challenges and opportunities. Polymer 2017, 115, 285–307. [Google Scholar] [CrossRef]
- Detrembleur, C.; Debuigne, A.; Hurtgen, M.; Jerome, C.; Pinaud, J.; Fevre, M.; Coupillaud, P.; Vignolle, J.; Taton, D. Synthesis of 1-Vinyl-3-ethylimidazolium-Based Ionic Liquid (Co)polymers by Cobalt-Mediated Radical Polymerization. Macromolecules 2011, 44, 6397–6404. [Google Scholar] [CrossRef]
- Patil, N.; Cordella, D.; Aqil, A.; Debuigne, A.; Admassie, S.; Jerome, C.; Detrembleur, C. Surface- and Redox-Active Multifunctional Polyphenol-Derived Poly(ionic liquid)s: Controlled Synthesis and Characterization. Macromolecules 2016, 49, 7676–7691. [Google Scholar] [CrossRef]
- Debuigne, A.; Morin, A.N.; Kermagoret, A.; Piette, Y.; Detrembleur, C.; Jérôme, C.; Poli, R. Key Role of Intramolecular Metal Chelation and Hydrogen Bonding in the Cobalt-Mediated Radical Polymerization of N-Vinyl amides. Chem. Eur. J. 2012, 18, 12834–12844. [Google Scholar] [CrossRef] [PubMed]
- Debuigne, A.; Poli, R.; Jérôme, C.; Jérome, R.; Detrembleur, C. Overview of cobalt-mediated radical polymerization: Roots, state of the art and future prospects. Prog. Polym. Sci. 2009, 34, 211–239. [Google Scholar] [CrossRef]
- Wayland, B.B.; Poszmik, G.; Mukerjee, S. Living radical polymerization of acrylates by organocobalt porphyrin complexes. J. Am. Chem. Soc. 1994, 116, 7943–7944. [Google Scholar] [CrossRef]
- Debuigne, A.; Caille, J.R.; Jérôme, R. Highly efficient cobalt-mediated radical polymerization of vinyl acetate. Angew. Chem. Int. Ed. Engl. 2005, 44, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Kaneyoshi, H.; Matyjaszewski, K. Radical (Co)polymerization of vinyl chloroacetate and N-vinylpyrrolidone mediated by bis(acetylacetonate)cobalt derivatives. Macromolecules 2006, 39, 2757–2763. [Google Scholar] [CrossRef]
- Santhosh Kumar, K.S.; Gnanou, Y.; Champouret, Y.; Daran, J.-C.; Poli, R. Radical Polymerization of Vinyl Acetate with Bis(tetramethylheptadionato)cobalt(II): The Cohabitation of Three Different Mechanisms. Chem. Eur. J. 2009, 15, 4874–4885. [Google Scholar] [CrossRef] [PubMed]
- Bellan, E.V.; Thevenin, L.; Gayet, F.; Fliedel, C.; Poli, R. Catalyzed Chain Transfer in Vinyl Acetate Polymerization mediated by 9-Oxyphenalenone Cobalt(II) complexes. ACS Macro Lett. 2017, 6, 959–962. [Google Scholar] [CrossRef]
- Améduri, B. Controlled Radical (Co)polymerization of Fluoromonomers. Macromolecules 2010, 43, 10163–10184. [Google Scholar] [CrossRef]
- Demarteau, J.; Ameduri, B.; Ladmiral, V.; Mees, M.A.; Hoogenboom, R.; Debuigne, A.; Detrembleur, C. Controlled Synthesis of Fluorinated Copolymers via Cobalt-Mediated Radical Copolymerization of Perfluorohexylethylene and Vinyl Acetate. Macromolecules 2017, 50, 3750–3760. [Google Scholar] [CrossRef]
- Tyson, G.N.; Adams, S.C. The configuration of some cupric, nickelous and cobaltous complexes by means of magnetic measurements. J. Am. Chem. Soc. 1940, 62, 1228–1229. [Google Scholar] [CrossRef]
- Cotton, F.A.; Holm, R.H. Magnetic investigations of spin-free cobaltous complexes 3. On the existence of planar complexes. J. Am. Chem. Soc. 1960, 82, 2979–2983. [Google Scholar] [CrossRef]
- Banerjee, S.; Domenichelli, I.; Ameduri, B. Nitroxide-Mediated Alternating Copolymerization of Vinyl Acetate with tert-Butyl-2-trifluoromethacrylate Using a SG1-Based Alkoxyamine. ACS Macro Lett. 2016, 5, 1232–1236. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Rahaman, S.M.W.; Poli, R.; Améduri, B. Organometallic-Mediated Alternating Radical Copolymerization of tert-Butyl-2-trifluoromethacrylate with Vinyl Acetate and Synthesis of Block Copolymers Thereof. Macromol. Rapid Commun. 2017, 38, 1700203. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Ameduri, B. Advances in the (Co)polymerization of Alkyl 2-Trifluoromethacrylates and 2-(Trifluoromethyl)acrylic Acid. Prog. Polym. Sci. 2013, 38, 703–739. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer crosslinking and post-crosslinking at room and medium temperature. Prog. Polym. Sci. 2010, 36, 191–217. [Google Scholar] [CrossRef]
- Banerjee, S.; Wehbi, M.; Manseri, A.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Poly(vinylidene fluoride) Containing Phosphonic Acid as Anticorrosion Coating for Steel. ACS Appl. Mater. Interfaces 2017, 9, 6433–6443. [Google Scholar] [CrossRef] [PubMed]
- Soulestin, T.; Dos Santos Filho, P.M.; Ladmiral, V.; Lannuzel, T.; Domingues Dos Santos, F.; Ameduri, B. Ferroelectric fluorinated copolymers with improved adhesion properties. Polym. Chem. 2017, 8, 1017–1027. [Google Scholar] [CrossRef]
- Maria, S.; Kaneyoshi, H.; Matyjaszewski, K.; Poli, R. Effect of Electron Donors on the Radical Polymerization of Vinyl Acetate Mediated by Co(acac)2: Degenerative transfer versus reversible homolytic cleavage of an organocobalt(III) complex. Chem. Eur. J. 2007, 13, 2480–2492. [Google Scholar] [CrossRef] [PubMed]
- Debuigne, A.; Champouret, Y.; Jérôme, R.; Poli, R.; Detrembleur, C. Mechanistic Insights into Cobalt Mediated Radical Polymerization (CMRP) of Vinyl Acetate via Cobalt(III) Adducts as Initiators. Chem. Eur. J. 2008, 14, 4046–4059. [Google Scholar] [CrossRef] [PubMed]
- Kharas, G.; Kohn, D.H. Characterization of copolymers of vinyl acetate with ethyl α-cyanocinnamate. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 577–582. [Google Scholar] [CrossRef]
- Kharas, G.; Kohn, D.H. Copolymerization of vinyl acetate with benzylidenemalononitrile. J. Polym. Sci. Part A Polym. Chem. 1984, 22, 583–588. [Google Scholar] [CrossRef]
- Kharas, G.B.; Barbarawi, H.A.; Crawford, A.L.; Dotson-Newman, O.I.; Dukarevich, I.J.; Ekram, A.; Galzin, N.A.; Ghattas, R.M.; Hoang, J.M.; Hyland, L.A.; et al. Novel co-polymers of vinyl acetate and halogen ring-substituted methyl 2-cyano-3-phenyl-2-propenoates. Des. Monomers Polym. 2005, 8, 309–317. [Google Scholar] [CrossRef]
- Kochi, T.; Nakamura, A.; Ida, H.; Nozaki, K. Alternating Copolymerization of Vinyl Acetate with Carbon Monoxide. J. Am. Chem. Soc. 2007, 129, 7770–7771. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Améduri, B. First RAFT/MADIX radical copolymerization of tert-butyl 2-trifluoromethacrylate with vinylidene fluoride controlled by xanthate. Polym. Chem. 2013, 4, 2783–2799. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Bellan, E.V.; Gayet, F.; Debuigne, A.; Detrembleur, C.; Poli, R.; Améduri, B.; Ladmiral, V. Bis(formylphenolato)cobalt(II)-Mediated Alternating Radical Copolymerization of tert-Butyl 2-Trifluoromethylacrylate with Vinyl Acetate. Polymers 2017, 9, 702. https://doi.org/10.3390/polym9120702
Banerjee S, Bellan EV, Gayet F, Debuigne A, Detrembleur C, Poli R, Améduri B, Ladmiral V. Bis(formylphenolato)cobalt(II)-Mediated Alternating Radical Copolymerization of tert-Butyl 2-Trifluoromethylacrylate with Vinyl Acetate. Polymers. 2017; 9(12):702. https://doi.org/10.3390/polym9120702
Chicago/Turabian StyleBanerjee, Sanjib, Ekaterina V. Bellan, Florence Gayet, Antoine Debuigne, Christophe Detrembleur, Rinaldo Poli, Bruno Améduri, and Vincent Ladmiral. 2017. "Bis(formylphenolato)cobalt(II)-Mediated Alternating Radical Copolymerization of tert-Butyl 2-Trifluoromethylacrylate with Vinyl Acetate" Polymers 9, no. 12: 702. https://doi.org/10.3390/polym9120702