Epoxy-Based Shape-Memory Actuators Obtained via Dual-Curing of Off-Stoichiometric “Thiol–Epoxy” Mixtures
Abstract
:1. Introduction
2. Experimental Section
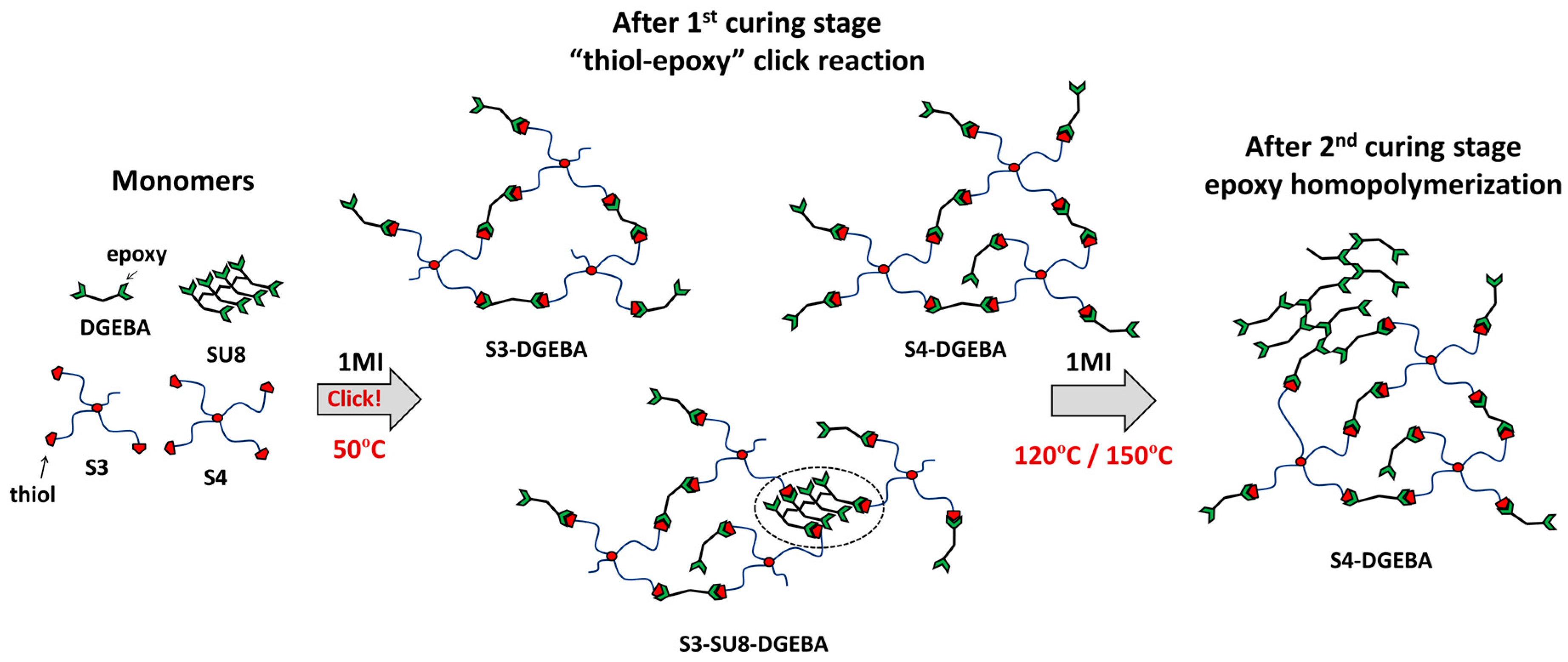
2.1. Materials and Methodology
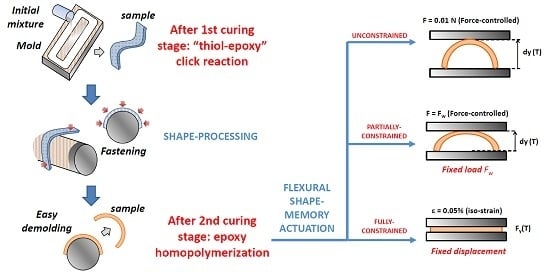
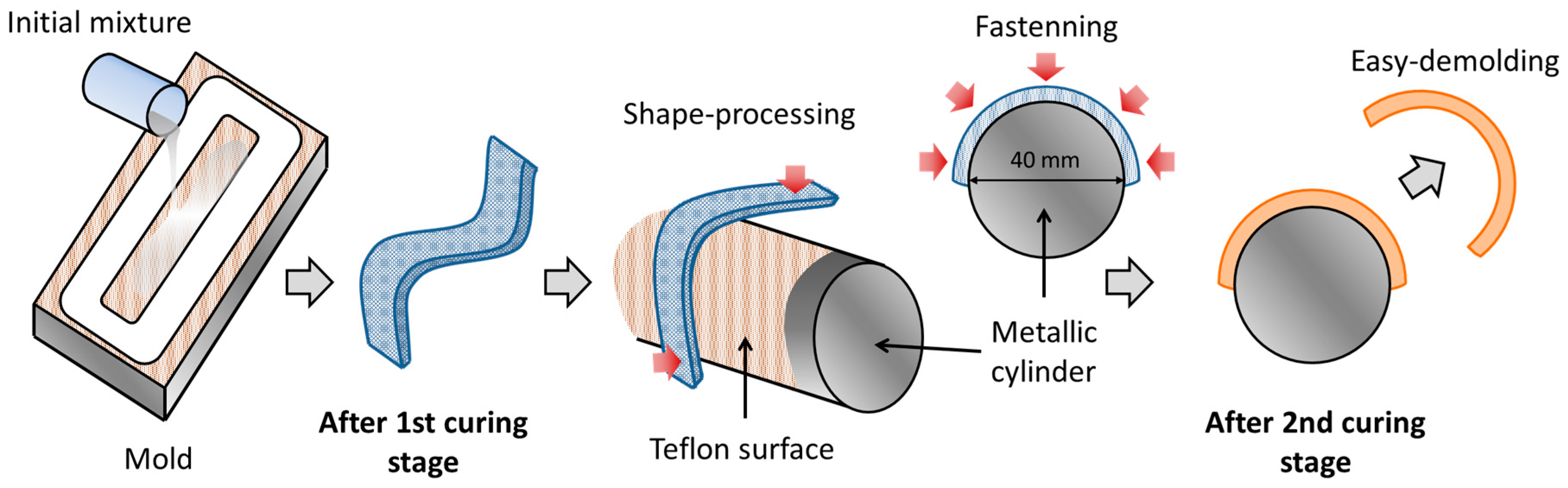
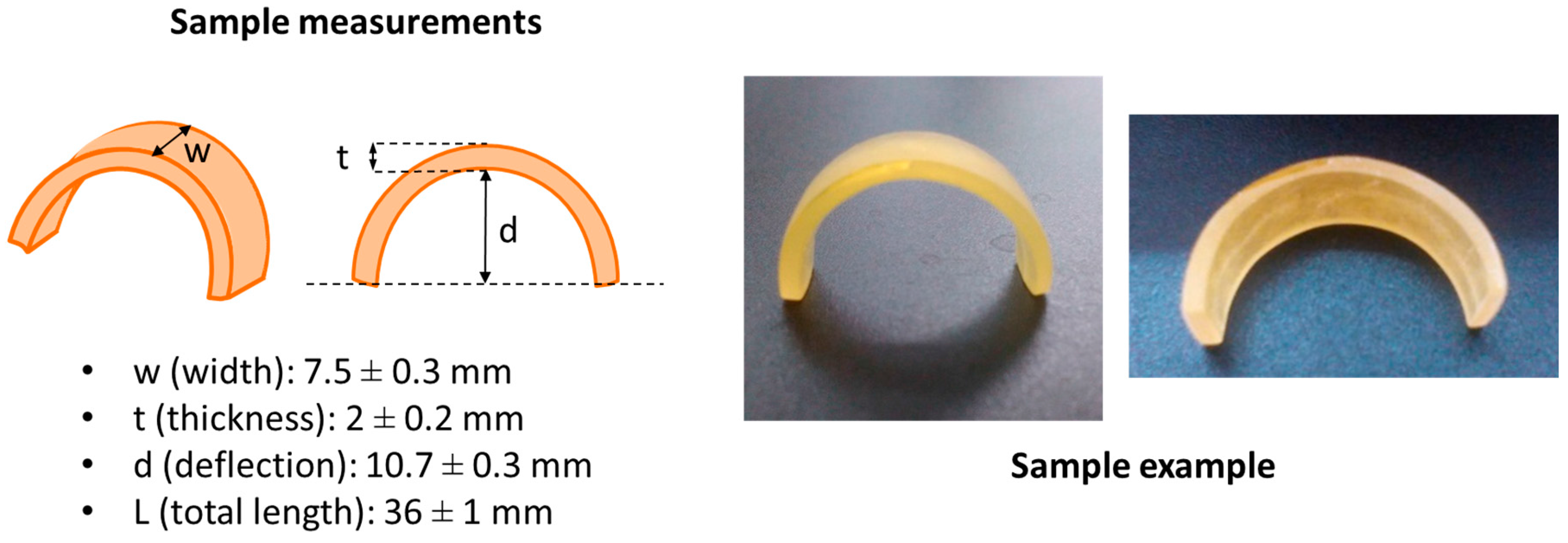
2.2. Sample Processing
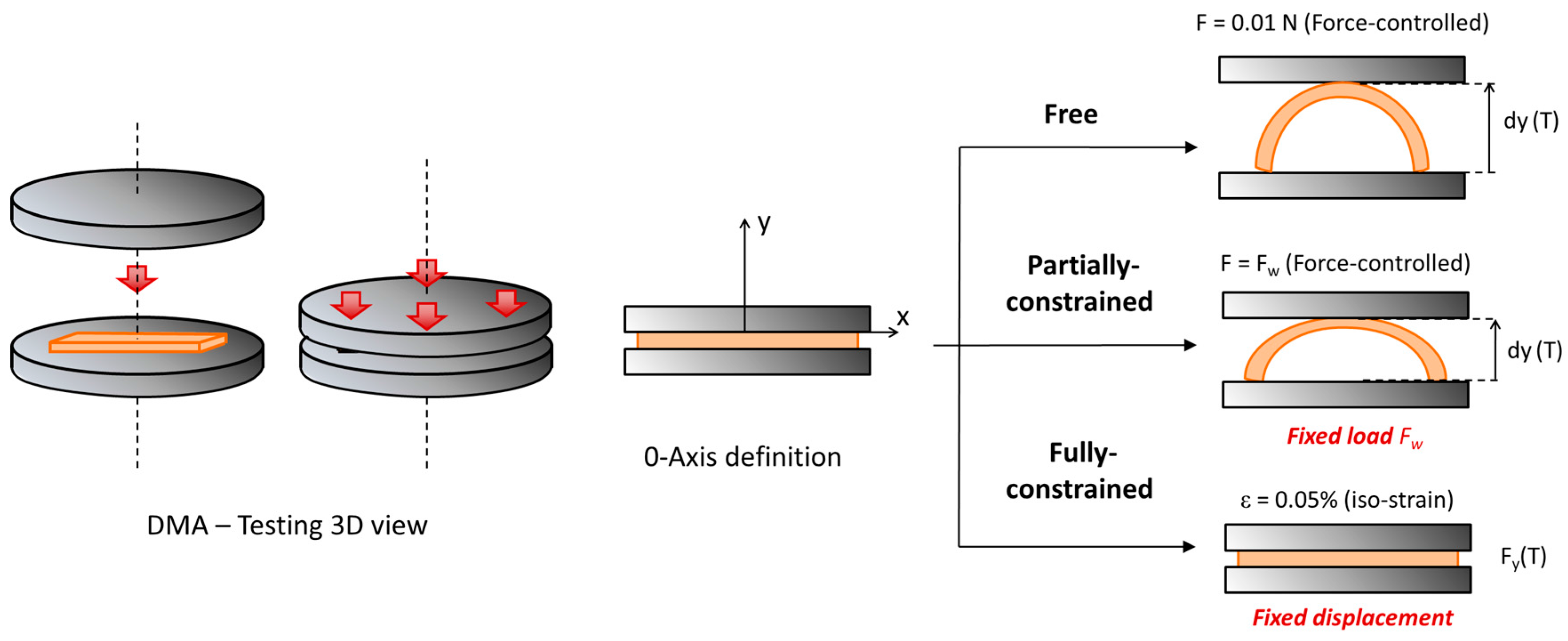
2.3. Thermomechanical Characterization
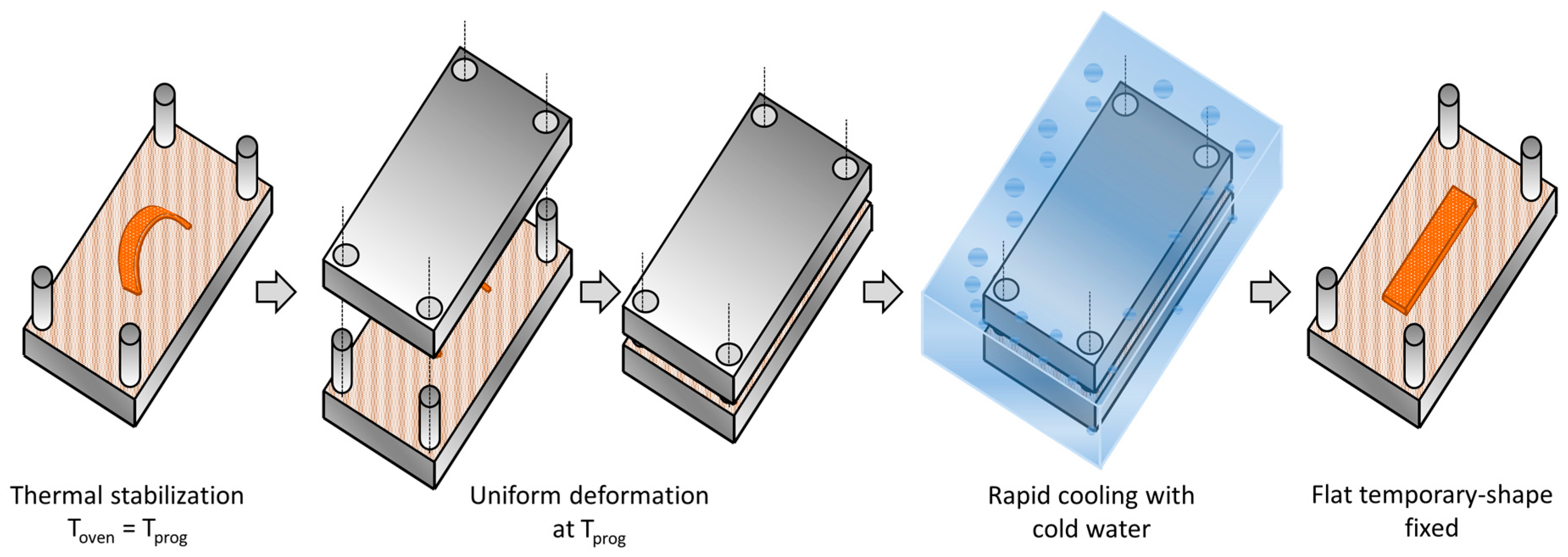
2.4. Shape-Memory Characterization
3. Results and Discussion
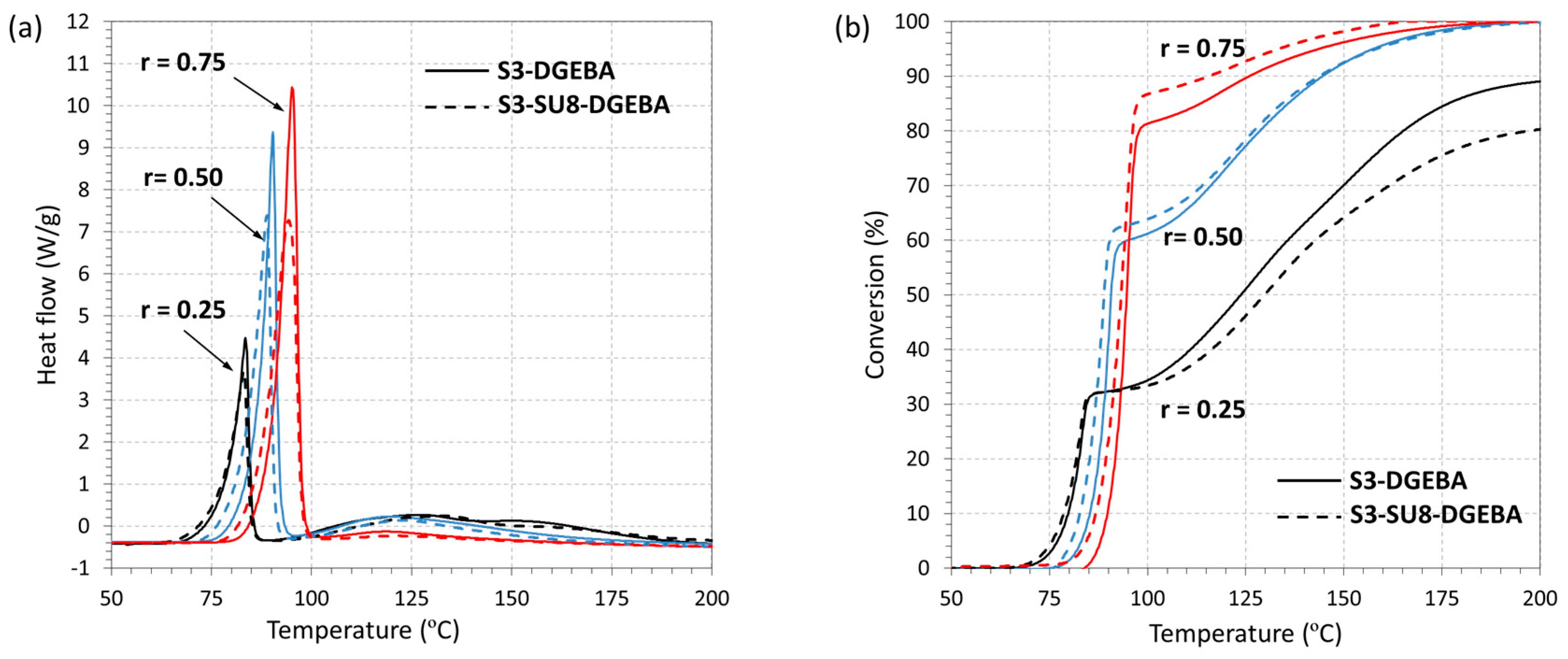
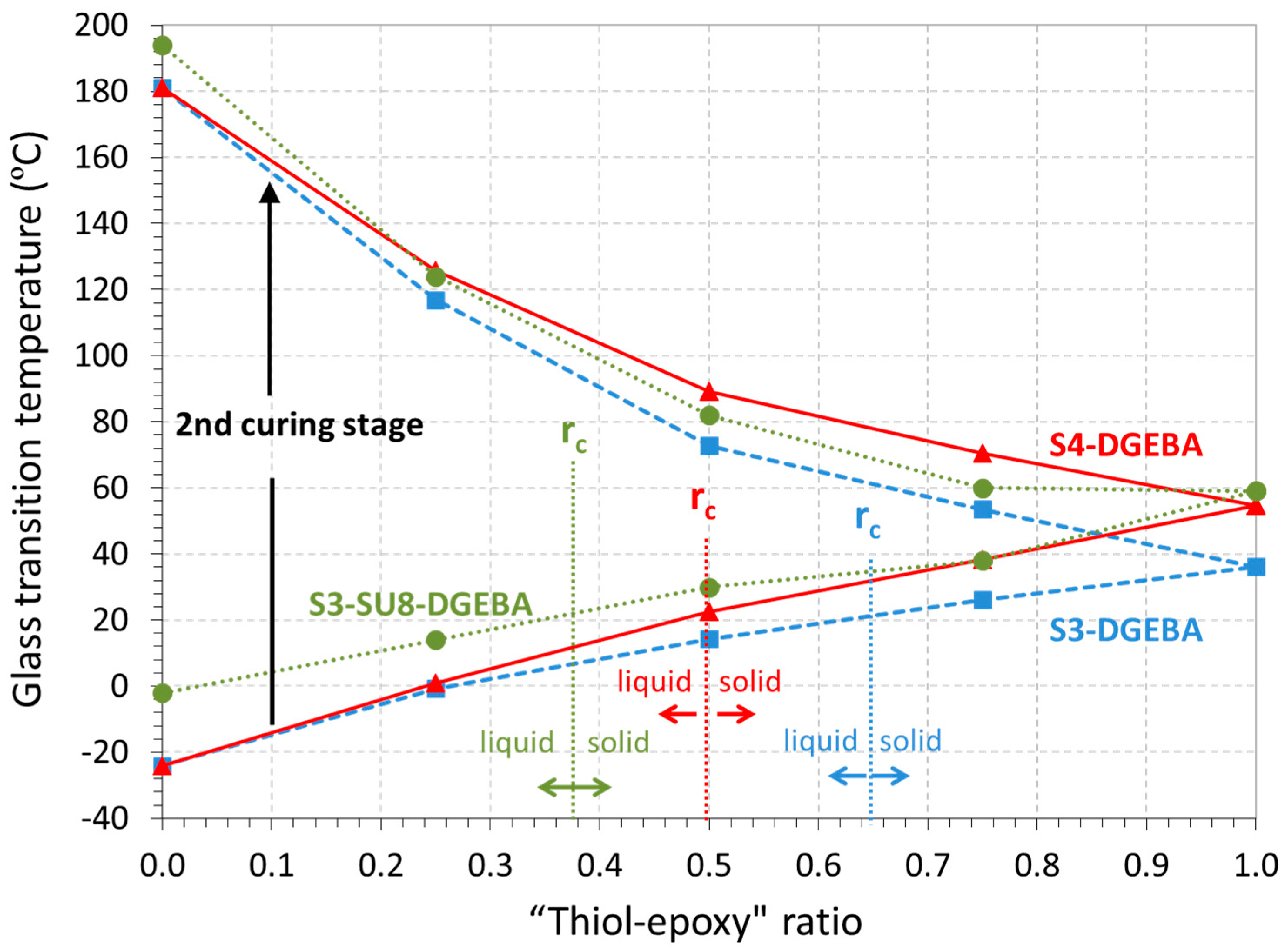
3.1. DSC Analysis of the Dual-Curing Process
3.2. Thermomechanical Results
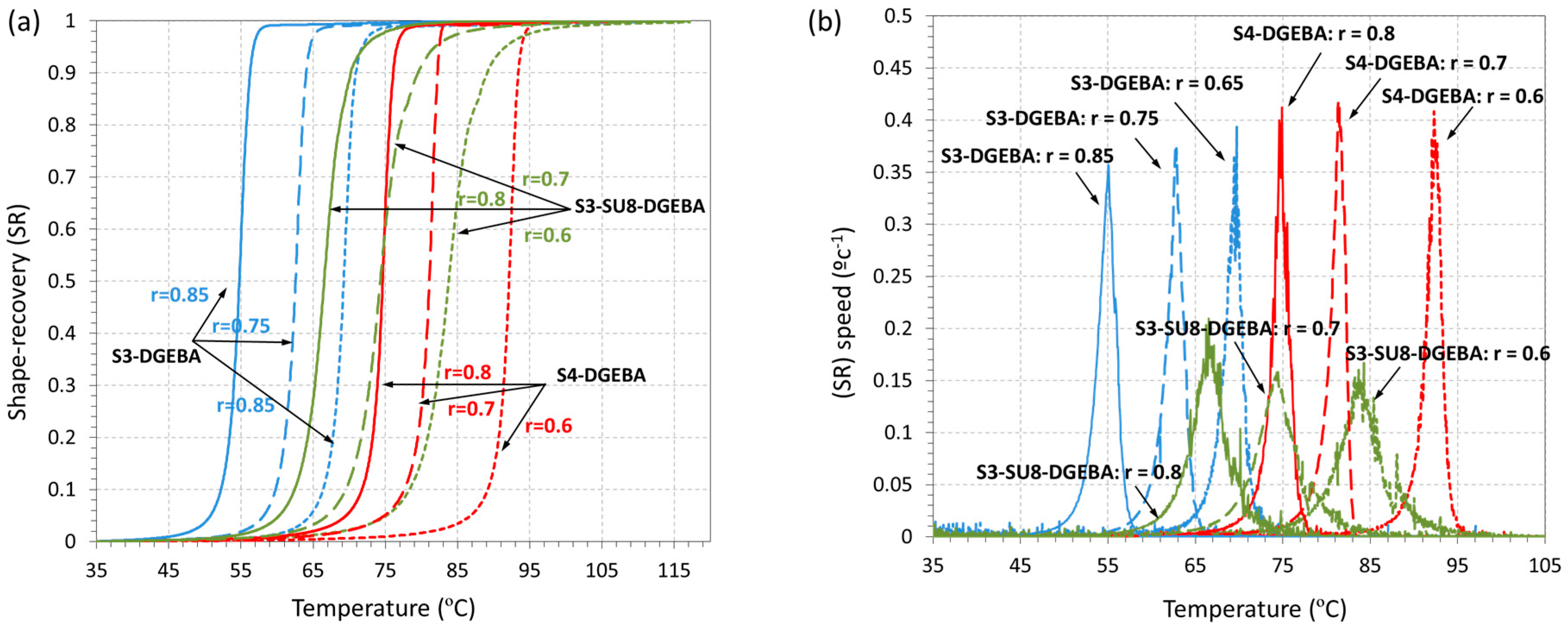
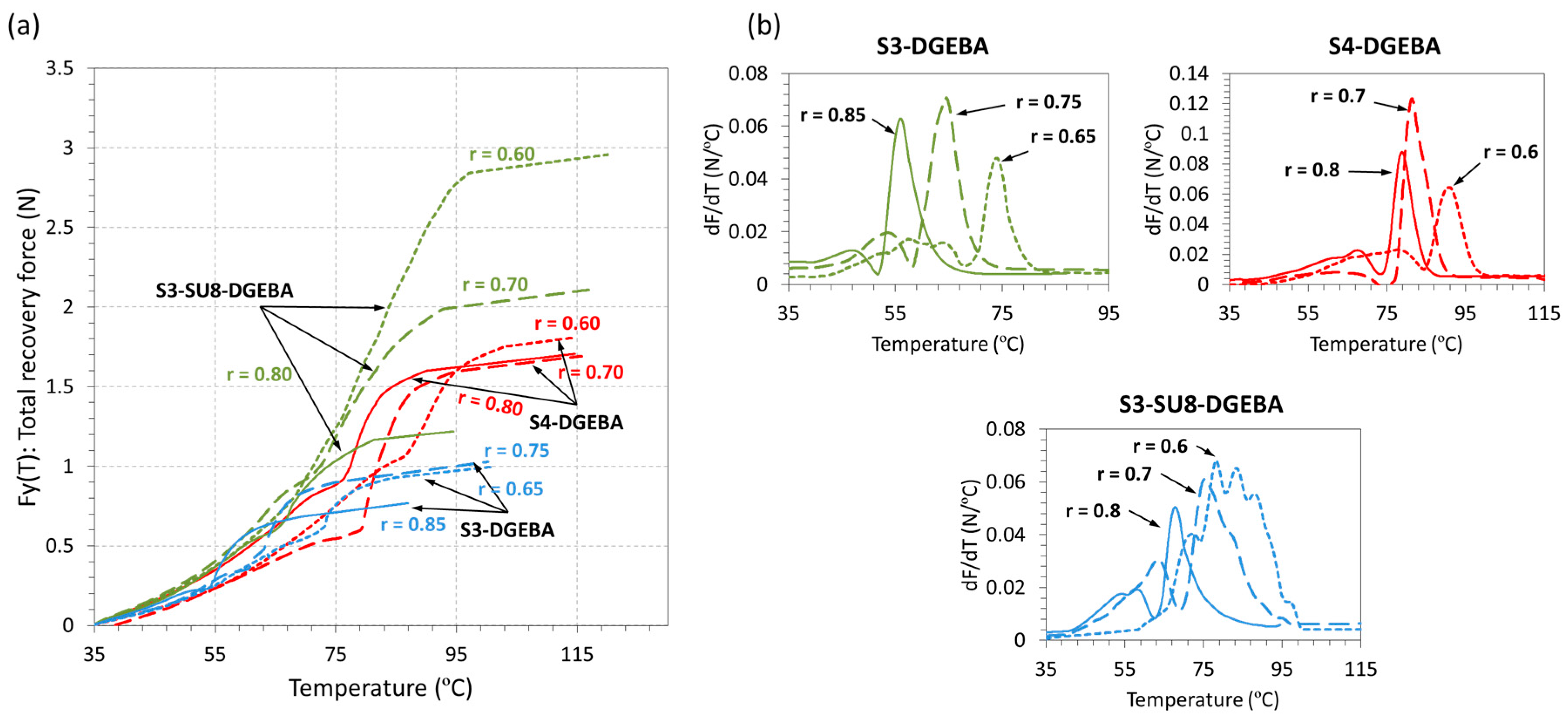
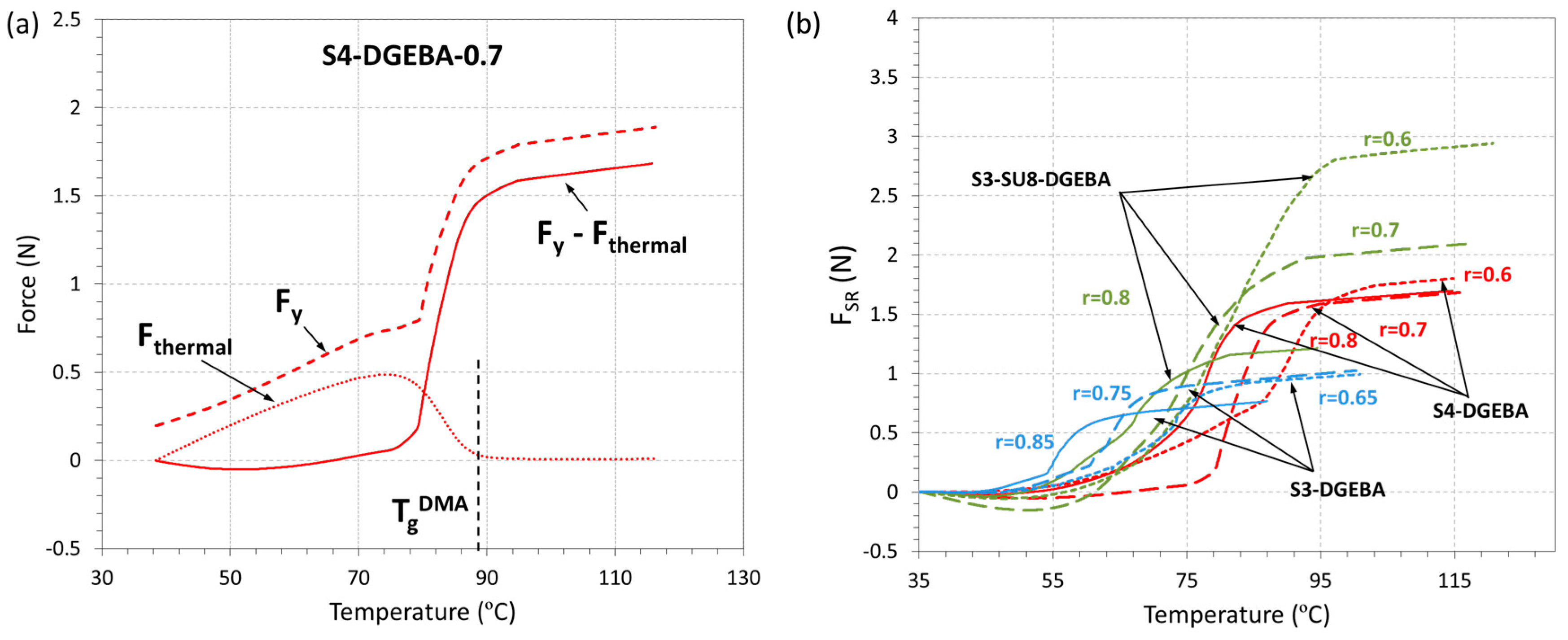
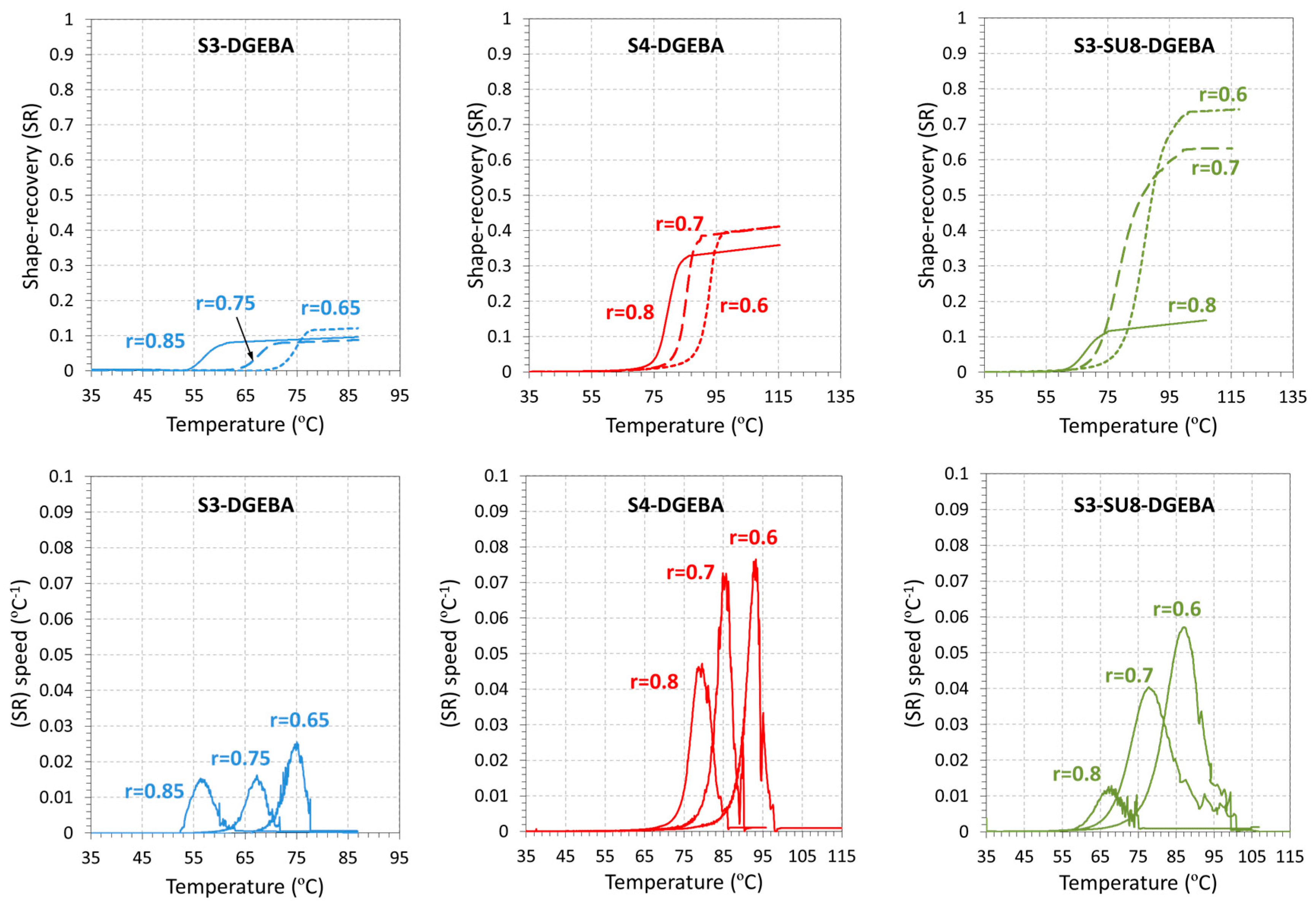
3.3. Shape-Memory Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Lendlein, A.; Sauter, T. Shape-memory effect in polymers. Macromol. Chem. Phys. 2013, 214, 1175–1177. [Google Scholar] [CrossRef]
- Wu, X.; Huang, W.M.; Zhao, Y.; Ding, Z.; Tang, C.; Zhang, J. Mechanisms of the shape memory effect in polymeric materials. Polymers 2013, 5, 1169–1202. [Google Scholar] [CrossRef]
- Li, G.; Wang, A. Cold, warm, and hot programming of shape memory polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1319–1339. [Google Scholar] [CrossRef]
- Arrieta, S.; Diani, J.; Gilormini, P. Experimental characterization and thermoviscoelastic modeling of strain and stress recoveries of an amorphous polymer network. Mech. Mater. 2014, 68, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Guo, J.; Nguyen, T.D. Modeling the multiple shape memory effect and temperature memory effect in amorphous polymers. RSC Adv. 2015, 5, 416–423. [Google Scholar] [CrossRef]
- Lewis, C.L.; Meng, Y.; Anthamatten, M. Well-Defined Shape-Memory Networks with High Elastic Energy Capacity. Macromolecules 2015, 48, 4918–4926. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49–50, 1–42. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, D.; Qiu, X.; Zhang, W.; Zhang, F.; Liu, L.; Liu, Y.; Zhang, S.; Hu, Y.; Leng, J. Shape-Memory Polymers with Adjustable High Glass Transition Temperatures. Macromolecules 2015, 48, 3582–3589. [Google Scholar] [CrossRef]
- Zhou, J.; Sheiko, S.S. Reversible shape-shifting in polymeric materials. J. Polym. Sci. Part B Polym. Phys. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Lewis, C.L.; Dell, E.M. A Review of Shape Memory Polymers Bearing Reversible Binding Groups. J. Polym. Sci. Part B Polym. Phys. 2016, 1340–1364. [Google Scholar] [CrossRef]
- Tandon, G.P.; McClung, A.J.; Baur, J.W. Shape-Memory Polymers for Aerospace Applications; DEStech Publications: Lancaster, PA, USA, 2015; p. 658. [Google Scholar]
- Leng, J.; Yu, K.; Sun, J.; Liu, Y. Deployable morphing structure based on shape memory polymer. Aircr. Eng. Aerosp. Technol. 2015, 87, 218–223. [Google Scholar] [CrossRef]
- Boyle, M.A.; Martin, C.J.; Neuner, J.D. Epoxy Resins. In ASM Handbook Volume 21, Composites (ASM International); Miracle, D.B., Donaldson, S.L., Eds.; ASM International: Novelty, OH, USA, 2001; pp. 78–89. [Google Scholar]
- Santhosh Kumar, K.S.; Biju, R.; Reghunadhan Nair, C.P. Progress in shape memory epoxy resins. React. Funct. Polym. 2013, 73, 421–430. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, G.; Cao, Z.; Zhao, Q.; Xie, T. High strain epoxy shape memory polymer. Polym. Chem. 2015, 6, 3046–3053. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Shape memory epoxy vitrimers based on DGEBA crosslinked with dicarboxylic acids and their blends with citric acid. RSC Adv. 2016, 6, 88647–88655. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Wei, Y.; Ji, Y. Regional Shape Control of Strategically Assembled Multishape Memory Vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, A.; Fernández-francos, X.; Serra, À.; De la Flor, S. Phenomenological characterization of sequential dual-curing of off-stoichiometric “thiol-epoxy” systems: Towards applicability. Mater. Des. 2017, 113, 116–127. [Google Scholar] [CrossRef]
- Fernández-Francos, X.; Konuray, A.-O.; Belmonte, A.; De la Flor, S.; Serra, À.; Ramis, X. Sequential curing of off-stoichiometric thiol–epoxy thermosets with a custom-tailored structure. Polym. Chem. 2016, 7, 2280–2290. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, A.; Guzmán, D.; Fernández-francos, X.; De la Flor, S. Effect of the Network Structure and Programming Temperature on the Shape-Memory Response of Thiol-Epoxy “Click” Systems. Polymers 2015, 7, 2146–2164. [Google Scholar] [CrossRef] [Green Version]
- Belmonte, A.; Fernández-Francos, X.; De la Flor, S. New understanding of the shape-memory response in thiol-epoxy click systems: Towards controlling the recovery process. J. Mater. Sci. 2017, 52, 1625–1638. [Google Scholar] [CrossRef]
- Belmonte, A.; Fernández-Francos, X.; De la Flor, S.; Serra, À. Network structure dependence on unconstrained isothermal-recovery processes for shape-memory thiol-epoxy “click” systems. Mech. Time-Depend. Mater. 2016, 1–17. [Google Scholar] [CrossRef]
- Santiago, D.; Fabregat-Sanjuan, A.; Ferrando, F.; De la Flor, S. Recovery stress and work output in hyperbranched poly(ethyleneimine)-modified shape-memory epoxy polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1002–1013. [Google Scholar] [CrossRef]
- Souri, M.; Lu, Y.C.; Erol, A.; Pulla, S.S.; Karaca, H.E. Characterization of unconstraint and constraint shape recoveries of an epoxy based shape memory polymer. Polym. Test. 2015, 41, 231–238. [Google Scholar] [CrossRef]
- Wang, A.; Li, G. Stress memory of a thermoset shape memory polymer. J. Appl. Polym. Sci. 2015, 132, 42112–42123. [Google Scholar] [CrossRef]
- Pascault, J.-P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; CRC Press: Boca Raton, FL, USA, 2002; p. 496. [Google Scholar]
- Fernández-Francos, X.; Salla, J.M.; Mantecón, A.; Serra, À.; Ramis, X. Crosslinking of mixtures of DGEBA with 1,6-dioxaspiro[4,4]nonan-2,7-dione initiated by tertiary amines. I. Study of the reaction and kinetic analysis. J. Appl. Polym. Sci. 2008, 109, 2304–2315. [Google Scholar]
- Fernandez-Francos, X.; Cook, W.D.; Serra, A.; Ramis, X.; Liang, G.G.; Salla, J.M. Crosslinking of mixtures of DGEBA with 1,6-dioxaspiro[4,4]nonan-2,7-dione initiated by tertiary amines. Part IV. Effect of hydroxyl groups on initiation and curing kinetics. Polymer 2010, 51, 26–34. [Google Scholar] [CrossRef]
- Anthamatten, M.; Roddecha, S.; Li, J. Energy storage capacity of shape-memory polymers. Macromolecules 2013, 46, 4230–4234. [Google Scholar] [CrossRef]
| System | Critical ratio (rc) 1 | “Thiol–Epoxy” ratio | Thiol (wt %) | DGEBA (wt %) | SU8 (wt %) | 1MI (wt %) |
|---|---|---|---|---|---|---|
| S3-DGEBA | 0.65 | 0.65 | 31.86 | 67.15 | - | 0.99 |
| 0.75 | 35.03 | 63.98 | - | 0.99 | ||
| 0.85 | 37.91 | 61.10 | - | 0.99 | ||
| S4-DGEBA | 0.5 | 0.60 | 28.43 | 70.58 | - | 0.99 |
| 0.70 | 31.65 | 67.36 | - | 0.99 | ||
| 0.80 | 34.59 | 64.42 | - | 0.99 | ||
| S3-SU8-DGEBA | 0.38 | 0.60 | 29.14 | 46.58 | 23.58 | 0.99 |
| 0.70 | 32.42 | 44.42 | 22.49 | 0.99 | ||
| 0.80 | 35.42 | 42.45 | 21.49 | 0.99 |
| Formulation | ∆H1st (J/g) | ∆H2nd (J/g) | ∆H1st (kJ/eq.) 1 | ∆H2nd (kJ/eq.) 1 |
|---|---|---|---|---|
| S3-DGEBA-0.25 | 151 | 291 | 131.6 | 84.4 |
| S3-DGEBA-0.50 | 263 | 197 | 132.0 | 98.7 |
| S3-DGEBA-0.75 | 348 | 82 | 131.9 | 93.2 |
| S3-SU8-DGEBA-0.25 | 147 | 261 | 133.8 | 79.1 |
| S3-SU8-DGEBA-0.50 | 257 | 190 | 134.2 | 99.1 |
| S3-SU8-DGEBA-0.75 | 347 | 83 | 136.3 | 97.8 |
| Formulation | S3-DGEBA | S4-DGEBA | S3-SU8-DGEBA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| “thiol–epoxy” ratio | 0.65 | 0.75 | 0.85 | 0.6 | 0.7 | 0.8 | 0.6 | 0.7 | 0.8 |
| Eg (MPa) | 2150 | 2001 | 2130 | 2200 | 2130 | 2150 | 2310 | 2250 | 2310 |
| Er (MPa) | 10.9 | 9.6 | 8.4 | 18.4 | 15.1 | 14.0 | 19.5 | 17.3 | 14.4 |
| TgDMA (°C) | 80.1 | 70.0 | 61.0 | 98.0 | 89.0 | 81.0 | 96.0 | 81.0 | 73.0 |
| FWHM (°C) | 12.8 | 12.1 | 11.0 | 11.5 | 10.5 | 10.0 | 23.0 | 22.8 | 21.8 |
| tanδ peak | 1.25 | 1.35 | 1.46 | 1.16 | 1.23 | 1.30 | 0.69 | 0.81 | 0.92 |
| Eg/Er | 197 | 208 | 254 | 120 | 141 | 154 | 118 | 130 | 160 |
| Unconstrained | Partially-constrained | Fully-constrained | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Formulation | Rr (%) | ∆Tpeak (°C) | Tpeak (°C) | Vr (%/min) | ∆Tpeak (°C) | Tpeak (°C) | Wrel (mN·mm/mm) | ∆Tpeak (°C) | Tpeak (°C) | FSR (N) |
| S3-DGEBA-0.65 | 99.9 | 2.0 | 69.7 | 73.7 | 4.0 | 75.0 | 57 | 5.0 | 74.0 | 0.74 |
| S3-DGEBA-0.75 | 100.0 | 2.0 | 62.9 | 76.7 | 4.7 | 67.2 | 39 | 6.2 | 64.5 | 0.76 |
| S3-DGEBA-0.85 | 99.5 | 2.3 | 55.0 | 80.3 | 5.2 | 56.7 | 34 | 5.4 | 56.2 | 0.53 |
| S4-DGEBA-0.60 | 99.9 | 1.7 | 92.3 | 79.2 | 3.7 | 93.2 | 187 | 7.6 | 91.0 | 1.71 |
| S4-DGEBA-0.70 | 99.9 | 1.8 | 81.3 | 72.1 | 4.3 | 85.7 | 179 | 6.7 | 81.8 | 1.42 |
| S4-DGEBA-0.80 | 99.8 | 1.8 | 74.9 | 79.0 | 6.2 | 79.6 | 136 | 5.6 | 78.4 | 1.47 |
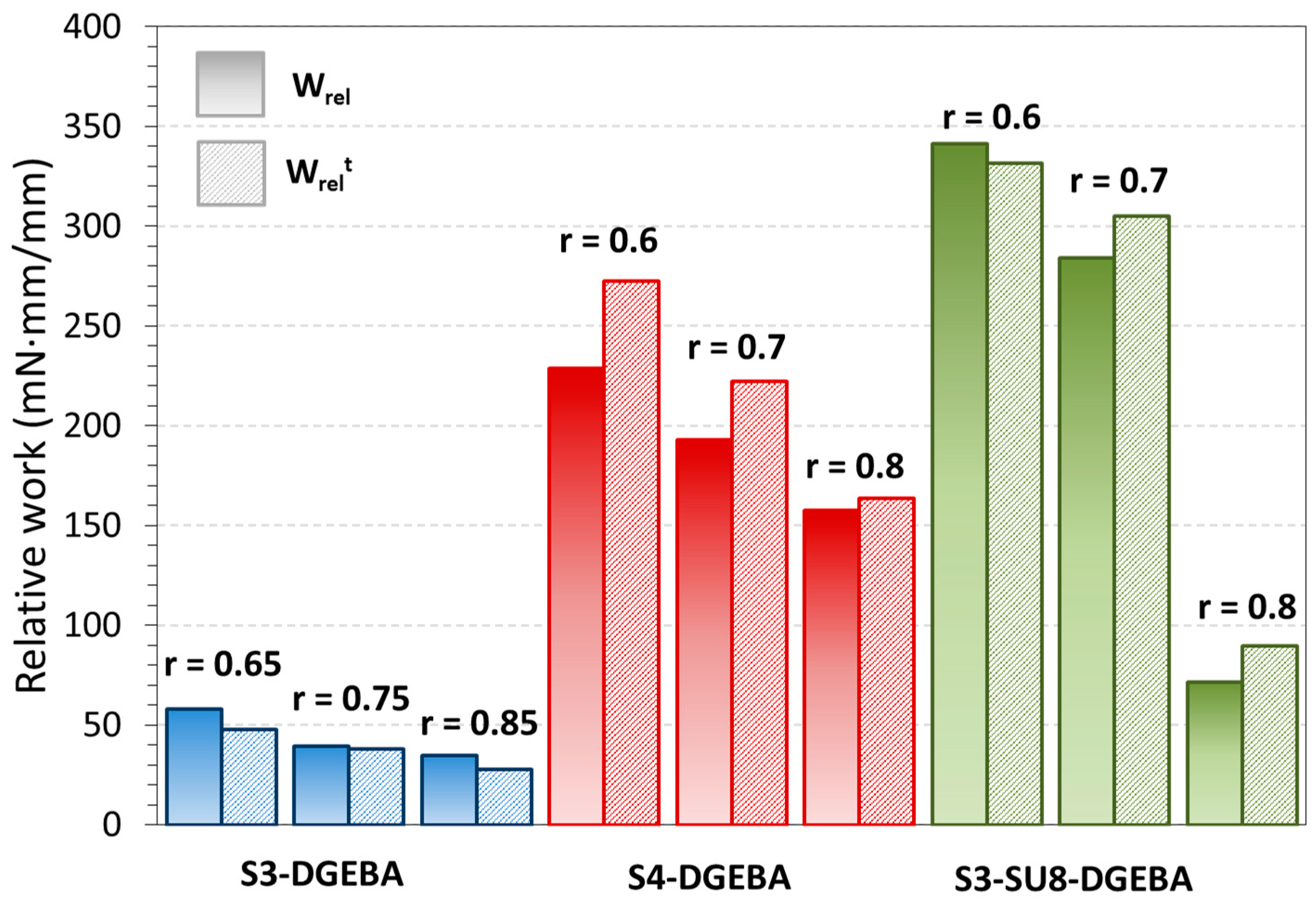
| S3-SU8-DGEBA-0.60 | 99.8 | 4.9 | 84.3 | 34.7 | 10.9 | 87.2 | 314 | 23.9 | 81.9 | 2.92 |
| S3-SU8-DGEBA-0.70 | 99.8 | 4.8 | 74.4 | 37.7 | 12.1 | 77.7 | 247 | 12.4 | 77.8 | 1.79 |
| S3-SU8-DGEBA-0.80 | 99.9 | 4.1 | 66.5 | 45.8 | 7.8 | 67.9 | 49 | 7.0 | 68.3 | 0.89 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmonte, A.; Russo, C.; Ambrogi, V.; Fernández-Francos, X.; De la Flor, S. Epoxy-Based Shape-Memory Actuators Obtained via Dual-Curing of Off-Stoichiometric “Thiol–Epoxy” Mixtures. Polymers 2017, 9, 113. https://doi.org/10.3390/polym9030113
Belmonte A, Russo C, Ambrogi V, Fernández-Francos X, De la Flor S. Epoxy-Based Shape-Memory Actuators Obtained via Dual-Curing of Off-Stoichiometric “Thiol–Epoxy” Mixtures. Polymers. 2017; 9(3):113. https://doi.org/10.3390/polym9030113
Chicago/Turabian StyleBelmonte, Alberto, Claudio Russo, Veronica Ambrogi, Xavier Fernández-Francos, and Silvia De la Flor. 2017. "Epoxy-Based Shape-Memory Actuators Obtained via Dual-Curing of Off-Stoichiometric “Thiol–Epoxy” Mixtures" Polymers 9, no. 3: 113. https://doi.org/10.3390/polym9030113