A Multiple Shape Memory Hydrogel Induced by Reversible Physical Interactions at Ambient Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
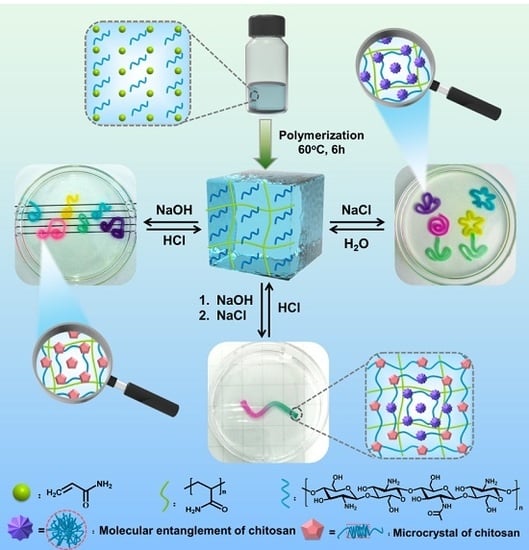
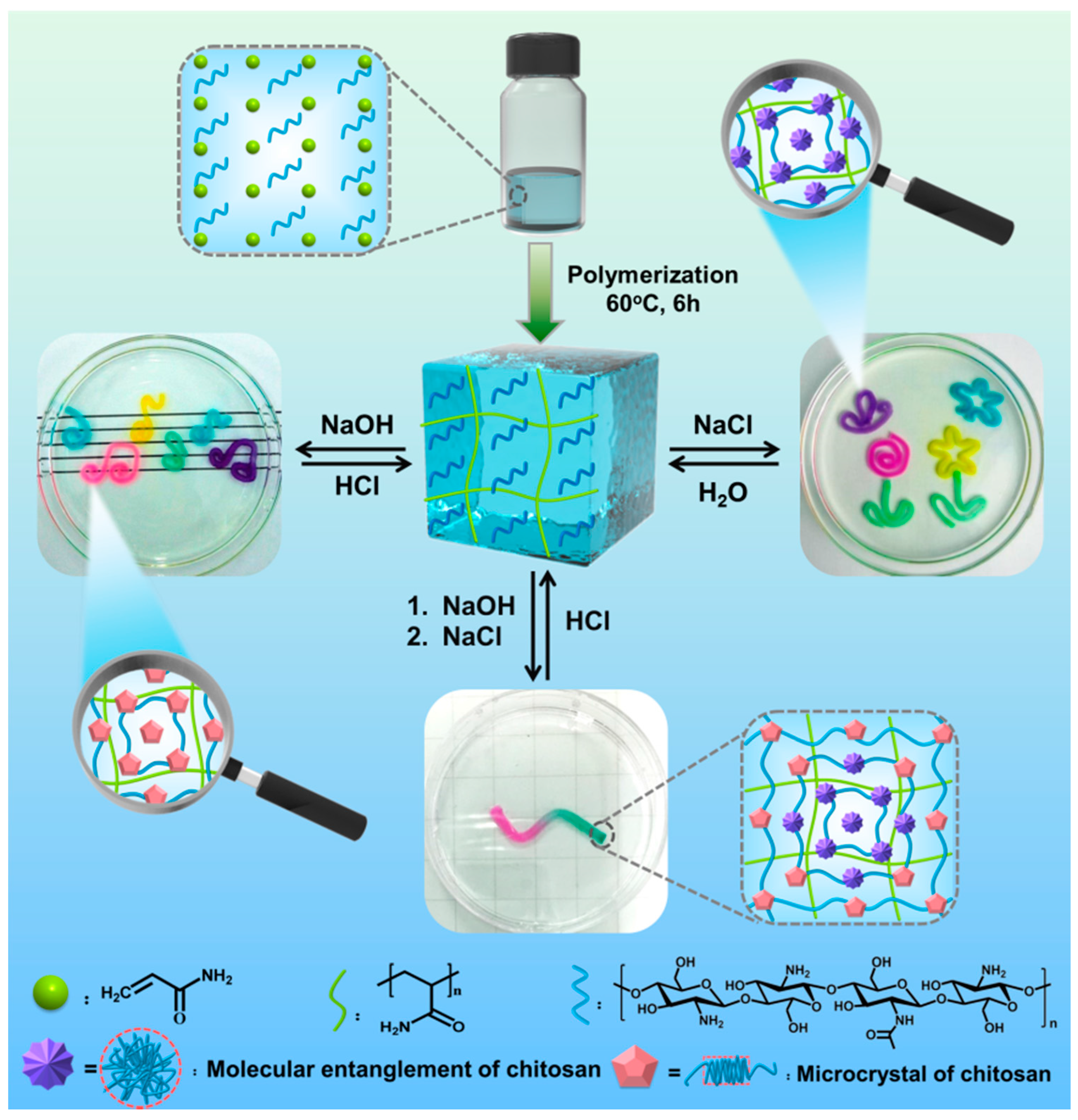
2.3. Preparation of the Hydrogel
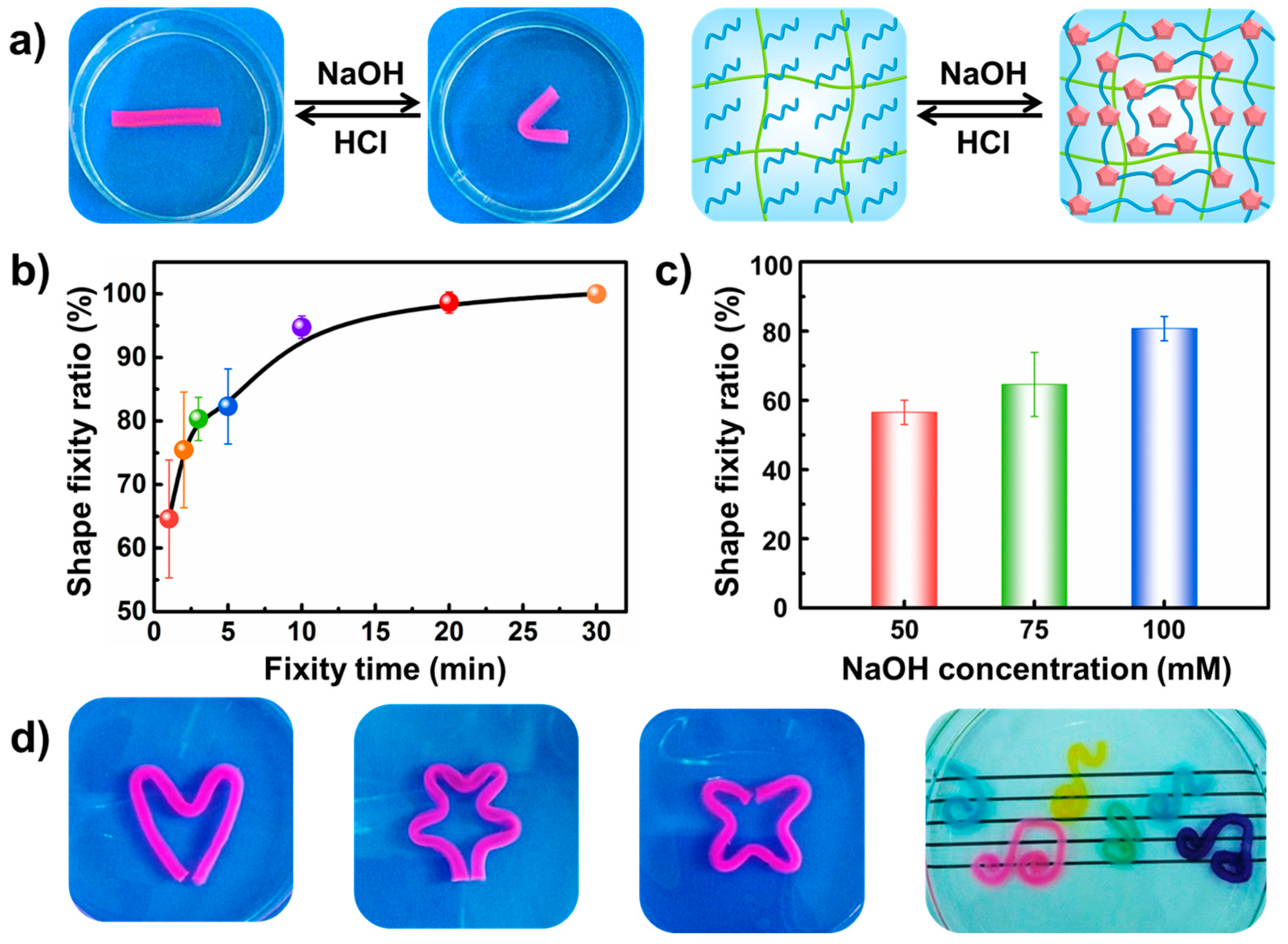
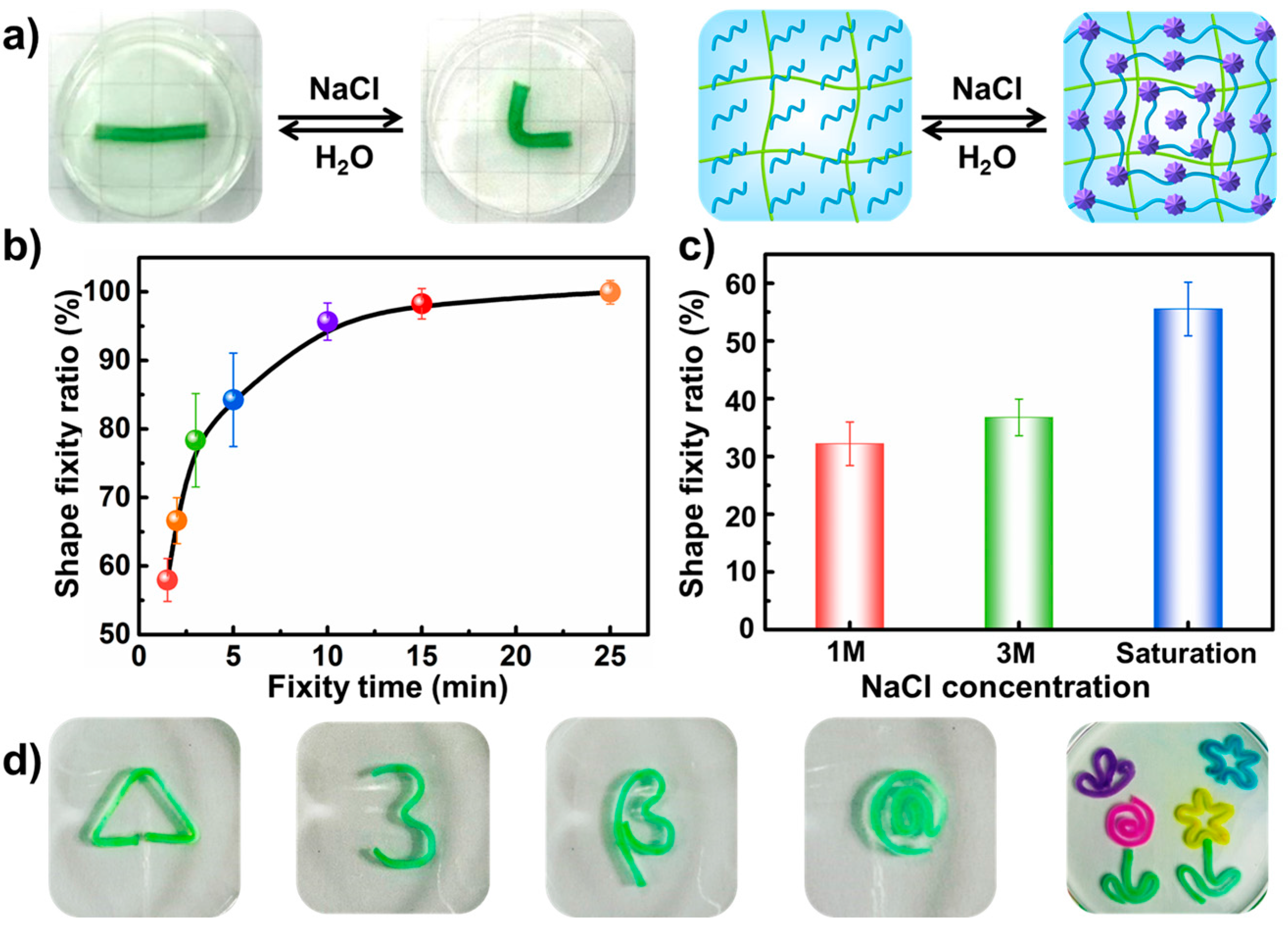
2.4. The Shape Fixity Ratio
3. Result and Discussion
3.1. The Microscopic Changes and Mechanical Properties of PAAm-CS Hydrogel
3.2. Dual Shape Memory Effects of the PAAm-CS Hydrogel
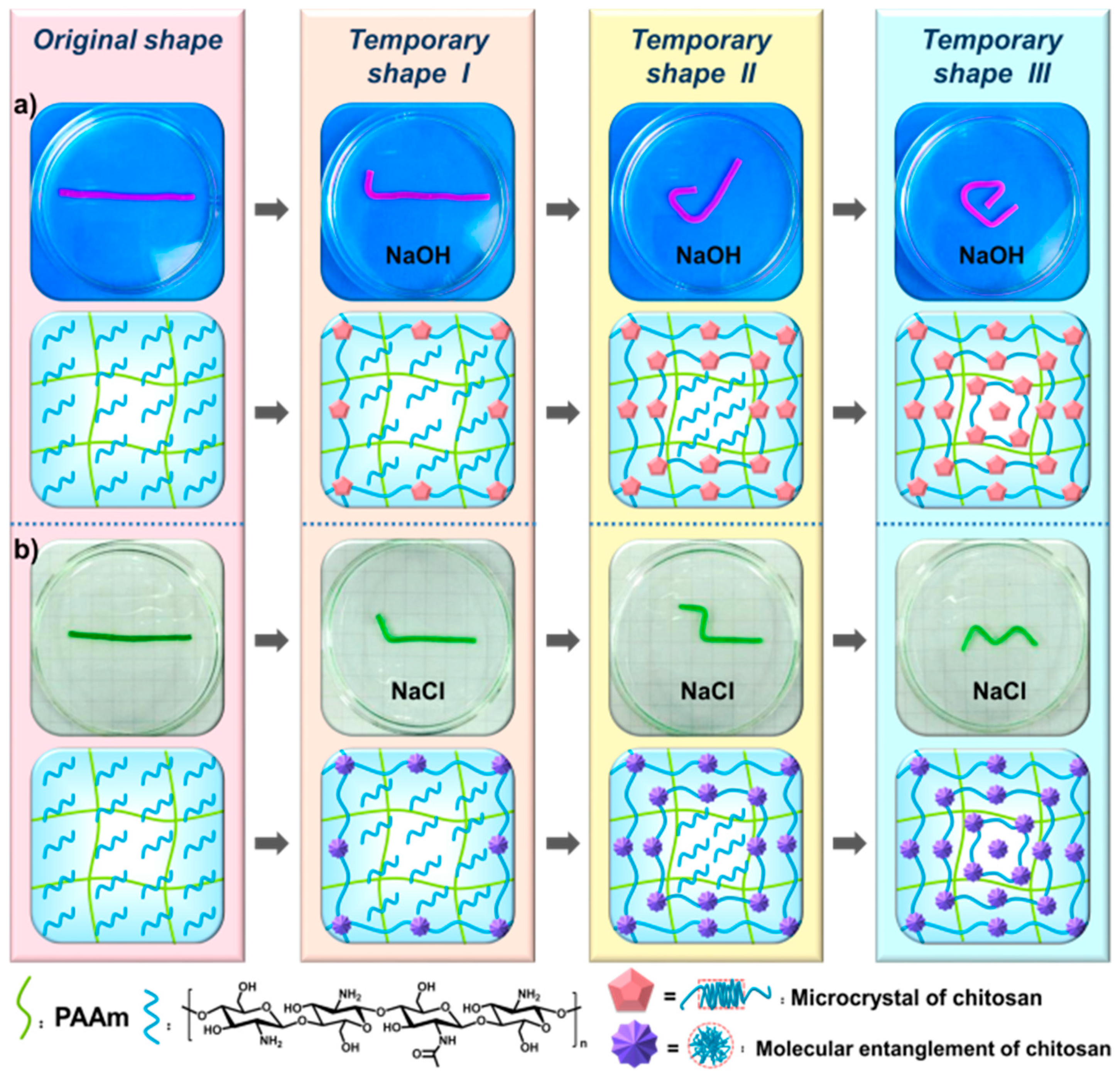
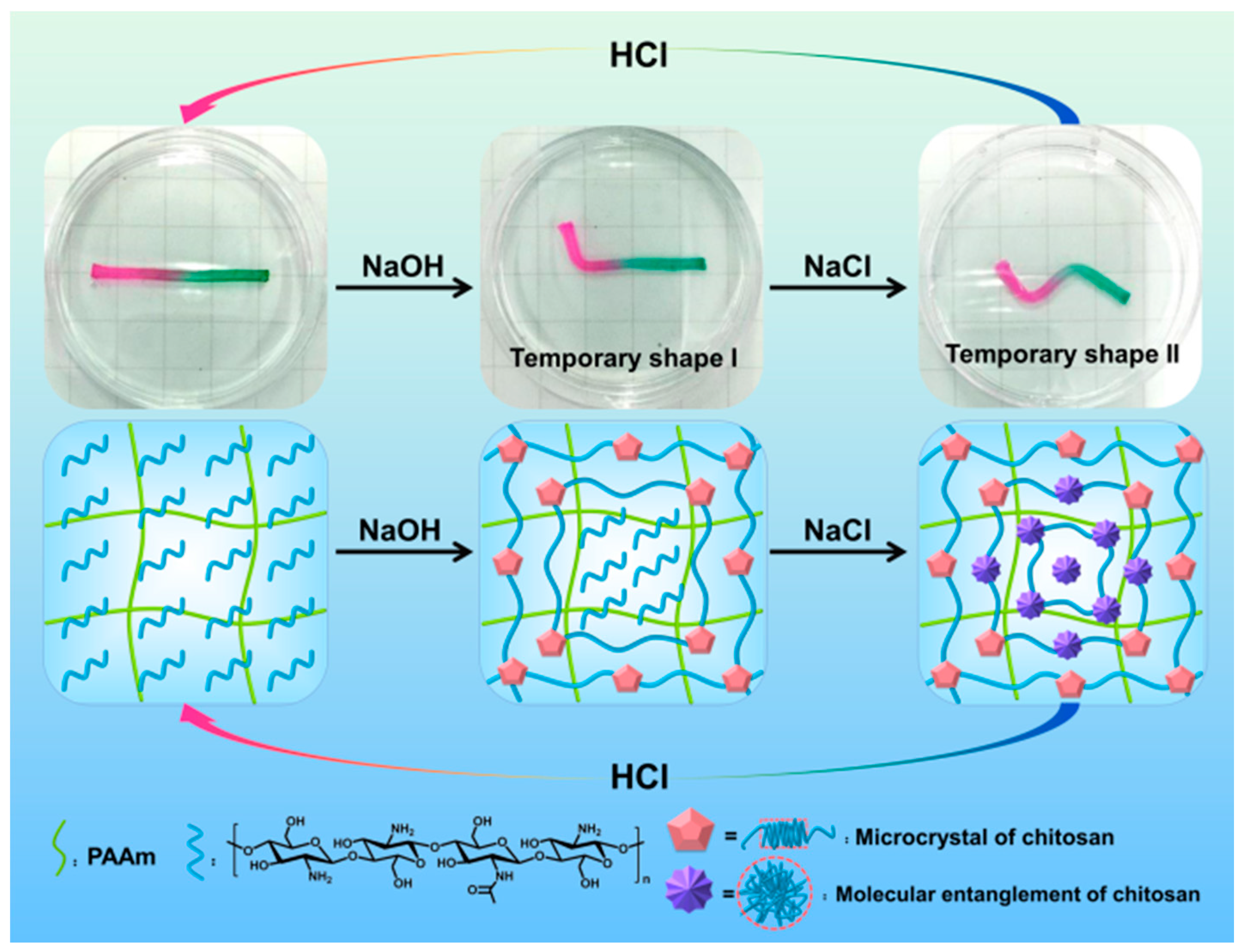
3.3. Multiple Shape Memory Effect of the PAAm-CS Hydrogel
3.4. Triple Shape Memory Effect of the PAAm-CS Hydrogel
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Habault, D.; Zhang, H.J.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.J.; Jia, Y.G.; Zhu, X.X. Biocompound-Based multiple shape memory polymers reinforced by photo-cross-linking. ACS Biomater. Sci. Eng. 2015, 1, 855–863. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.M.; Liu, D.; Wang, W.X.; Liu, Y.; Zhou, S.B. pH-responsive shape memory poly(ethylene glycol)-poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Zhao, L.; Wang, T.; Sun, W.X.; Tong, Z. NIR-Triggered Rapid shape memory PAM–GO–gelatin hydrogels with high mechanical strength. ACS Appl. Mater. Interfaces 2016, 8, 12384–12392. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Lu, C.H.; Orbach, R.; Wang, F.; Qi, X.J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-Stimulated DNA hydrogels exhibiting shape-memory properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Shape-memory polymers: Multifunctional shape-memory polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef] [PubMed]
- Osada, Y.; Matsuda, A. Shape memory in hydrogels. Nature 2002, 376, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49, 3–33. [Google Scholar] [CrossRef]
- Wu, X.L.; Huang, W.M.; Zhao, Y.; Ding, Z.; Tang, C.; Zhang, J.L. Mechanisms of the shape memory effect in polymeric materials. Polymers 2013, 5, 1169–1202. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Serrano, M.C.; Serrano, C.L.; Ameer, G.A. Novel biodegradable shape-memory elastomers with drug-releasing capabilities. Adv. Mater. 2011, 23, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Chen, S.J. A review of actively moving polymers in textile applications. J. Mater. Chem. 2010, 20, 3346–3355. [Google Scholar] [CrossRef]
- Liu, Y.J.; Du, H.Y.; Liu, L.W.; Leng, J.S. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Samuel, C.; Barrau, S.; Lefebvre, J.M.; Raquez, J.M.; Dubois, P. Designing multiple-shape memory polymers with miscible polymer blends: Evidence and origins of a triple-shape memory effect for miscible PLLA/PMMA blends. Macromolecules 2014, 47, 6791–6803. [Google Scholar] [CrossRef]
- Pei, Z.Q.; Yang, Y.; Chen, Q.M.; Wei, Y.; Ji, Y. Regional shape control of strategically assembled multishape memory vitrimers. Adv. Mater. 2016, 28, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Fang, Z.Z.; Zou, W.K.; Zhao, Q.; Xie, T. Thermoset shape-memory polyurethane with intrinsic plasticity enabled by transcarbamoylation. Angew. Chem. Int. Ed. 2016, 55, 11421–11425. [Google Scholar] [CrossRef] [PubMed]
- Nochel, U.; Behl, M.; Balk, M.; Lendlein, A. Thermally-induced triple-shape hydrogels: Soft materials enabling complex movements. ACS Appl. Mater. Interfaces 2016, 8, 28068–28076. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.M.; Jiang, R.Q.; Wu, J.J.; Song, J.Z.; Bai, H.; Li, B.G.; Zhao, Q.; Xie, T. Ultrafast digital printing toward 4D shape changing materials. Adv. Mater. 2017, 29, 1605390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zou, W.L.K.; Luo, Y.W.; Xie, T. Shape memory polymer network with thermally distinct elasticity and plasticity. Sci. Adv. 2016, 2, e1501297. [Google Scholar] [CrossRef] [PubMed]
- Zharinova, E.; Heuchel, M.; Weigel, T.; Gerber, D.; Kratz, K.; Lendlein, A. Water-blown polyurethane foams showing a reversible shape-memory effect. Polymers 2016, 8, 412. [Google Scholar] [CrossRef]
- Lu, W.; Le, X.X.; Zhang, J.W.; Huang, Y.J.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.J.; Wang, W.; Gao, H.; Liu, W.G. Fabrication of a shape memory hydrogel based on imidazole-zinc ion coordination for potential cell-encapsulating tubular 2016 scaffold application. Soft Matter 2013, 9, 132–137. [Google Scholar] [CrossRef]
- Han, Y.J.; Bai, T.; Liu, Y.; Zhai, X.Y.; Liu, W.G. Zinc ion uniquely induced triple shape memory effect of dipole-dipole reinforced ultra-high strength hydrogels. Macromol. Rapid Commun. 2012, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Miyamae, K.; Nakahata, M.; Takashima, Y.; Harada, A. Self-healing, expansion-contraction, and shape-memory properties of a preorganized supramolecular hydrogel through host-guest interactions. Angew. Chem. Int. Ed. 2015, 54, 8984–8987. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Zhou, W.F.; Yang, H.Y.; Li, H.Z.; Chen, Y.; Zhang, X.Y. Shape memory hydrogel based on a hydrophobically-modified polyacrilamide (HMPAM)/α-CD mixture via a host-guest approach. Macromol. Rapid Commun. 2015, 36, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Q.; Cao, Y.; Yuan, Q.J.; Wang, Y.F.; Li, J.H.; Li, B.J.; Zhang, S. Redox- and glucose-induced shape memory polymers. Macromol. Rapid Commun. 2013, 34, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zheng, J.; Wen, X.F.; Cai, Z.Q.; Zhang, J.W.; Chen, T. pH- and sugar-induced shape memory hydrogel based on reversible phenylboronic acid-diol ester bonds. Macromol. Rapid Commun. 2015, 36, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xiao, P.; Gu, J.C.; Wen, X.F.; Xu, J.; Zhao, C.Z.; Zhang, J.W.; Chen, T. Self-healable macro-/microscopic shape memory hydrogels based on supramolecular interactions. Chem. Commun. 2014, 50, 12277–12280. [Google Scholar] [CrossRef] [PubMed]
- Le, X.X.; Lu, W.; Zheng, J.; Tong, D.Y.; Zhao, N.; Ma, C.X.; Xiao, H.; Zhang, J.W.; Huang, Y.J.; Chen, T. Stretchable supramolecular hydrogels with triple shape memory effect. Chem. Sci. 2016, 7, 6715–6720. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Le, X.X.; Ma, C.X.; Li, Z.W.; Zheng, J.; Zhang, J.W.; Huang, Y.J.; Chen, T. A multi-responsive hydrogel with triple shape memory effect based on reversible switches. Chem. Commun. 2016, 52, 13292–13295. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D.C. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Lu, H.Z.; Rehman, S.U.; Siddiqb, M.; Yang, H.Y. Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide. Soft Matter 2014, 10, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yan, K.; Bentley, E.W.; Deng, H.B.; Du, Y.M.; Payne, G.F.; Shi, X.W. Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide. ACS Appl. Mater. Interfaces 2014, 6, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.Y.; Lu, W.T.; Ma, J.J.; Yang, L.; Wang, Z.K.; Qin, A.; Hu, Q.L. Orientation in multi-layer chitosan hydrogel: Morphology, mechanism, and design principle. Sci. Rep. 2015, 5, 7635–7642. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Ma, C.; Le, X.; Wang, L.; Lu, W.; Theato, P.; Hu, T.; Zhang, J.; Chen, T. A Multiple Shape Memory Hydrogel Induced by Reversible Physical Interactions at Ambient Condition. Polymers 2017, 9, 138. https://doi.org/10.3390/polym9040138
Xiao H, Ma C, Le X, Wang L, Lu W, Theato P, Hu T, Zhang J, Chen T. A Multiple Shape Memory Hydrogel Induced by Reversible Physical Interactions at Ambient Condition. Polymers. 2017; 9(4):138. https://doi.org/10.3390/polym9040138
Chicago/Turabian StyleXiao, He, Chunxin Ma, Xiaoxia Le, Li Wang, Wei Lu, Patrick Theato, Tuoping Hu, Jiawei Zhang, and Tao Chen. 2017. "A Multiple Shape Memory Hydrogel Induced by Reversible Physical Interactions at Ambient Condition" Polymers 9, no. 4: 138. https://doi.org/10.3390/polym9040138