Trophic Actions of Bone Marrow-Derived Mesenchymal Stromal Cells for Muscle Repair/Regeneration
Abstract
:1. Introduction
2. Bone Marrow-Derived Mesenchymal Stromal Cells (BM-MSCs)
2.1. Biological Properties
2.2. Contribution of BM-MSCs to Muscle Repair/Regeneration
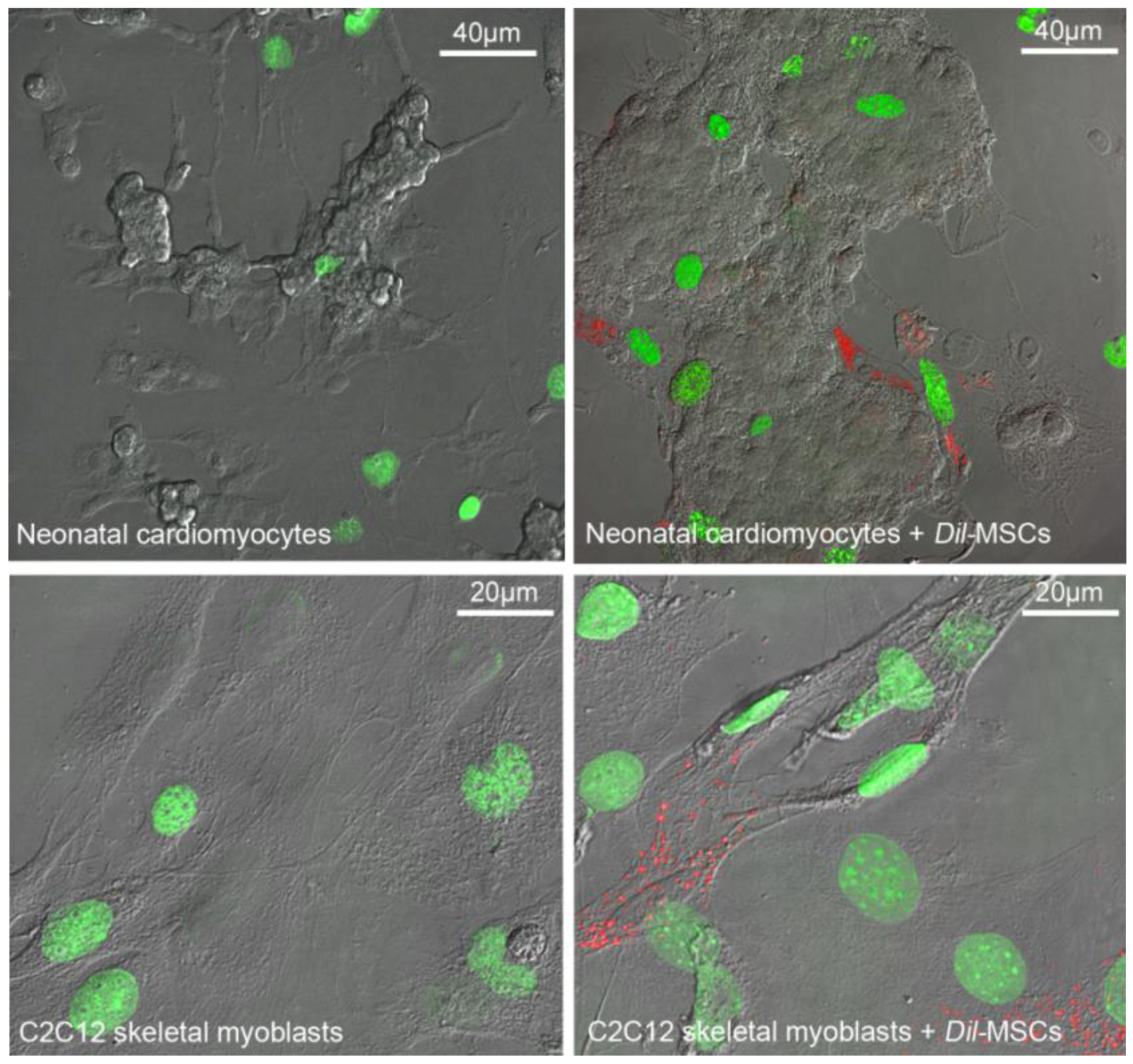
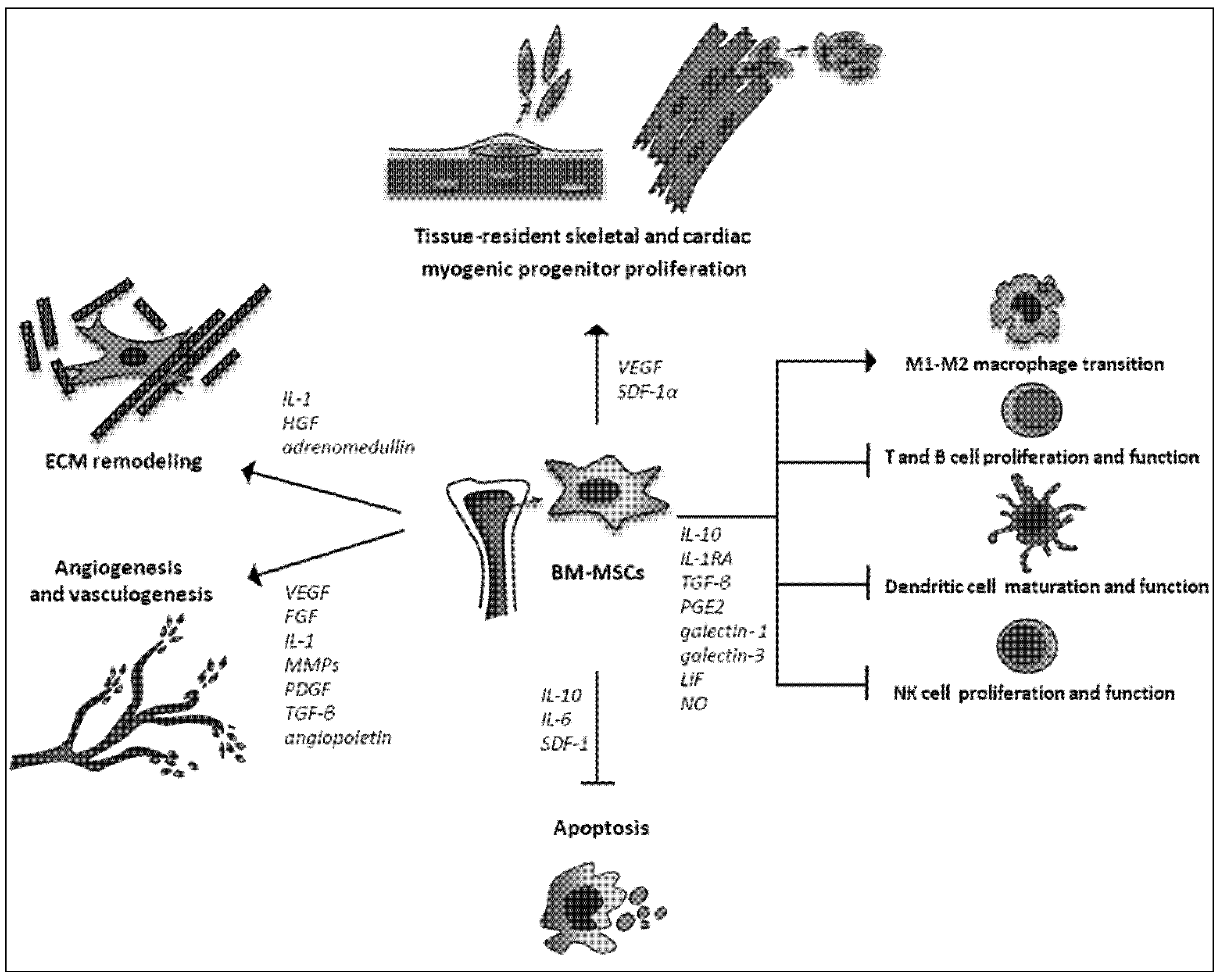
2.3. Trophic Actions
| Trial Name | ClinicalTrials.govIdentifier | Sponsor/ Collaborator | Location | Disease | Source | Route of Delivery | Patients | Status |
|---|---|---|---|---|---|---|---|---|
| Prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) | NCT00587990 | National Heart, Lung, and Blood Institute (NHLBI) | USA | Chronic ischemic left ventricular disfunction | Autologous | Intramyocardial injection | 45 | Completed |
| Johns Hopkins University Specialized Center for Cell Based Therapy | ||||||||
| Stem cell injection to treat heart damage during open heart surgery | NCT01557543 | National Heart, Lung, and Blood Institute (NHLBI) | USA | Heart disease | Autologous | Intramyocardial injection | 24 | Recruiting |
| Ischemic heart disease | ||||||||
| Coronary artery disease | ||||||||
| Safety and efficacy of intracoronary adult human mesenchymal stem cells after acute myocardial infarction | NCT01392105 | Yonsei University | Republic of Korea | Acute myocardial infarction | Autologous | Intracoronary | 80 | Completed |
| FCB | ||||||||
| Pharmicell Co Ltd. | (AMI) | injection | ||||||
| Stem cell therapy for vasculogenesis in patients with severe myocardial ischemia | NCT00260338 | Righospitalet, Copenhagen Denmark | Denmark | Myocardial ischemia | Autologous | Intramyocardial injection | 31 | Completed |
| Jens Kastrup | Coronary heart disease | |||||||
| Autologous mesenchymal stromal cell therapy in heart failure | NCT00644410 | Righospitalet, Copenhagen DenmarkJens Kastrup | Denmark | Heart failure | Autologous | Intramyocardial injection | 60 | Recruiting |
| Prochymal® (human adult stem cells) intravenous infusion following acute myocardial infarction (AMI) | NCT00877903 | Osiris Therapeutics | USA | Acute myocardial infarction | Allogenic | Intravenous injection | 220 | Active, not recruiting |
| Canada | (AMI) | |||||||
| Safety Study of AMI MultiStem® to treat Heart attacks | NCT00677222 | Athersys, Inc | USA | Acute myocardial infarction | Allogenic | Via catheter into peri-vascular space injection | 25 | Completed |
| PPD | ||||||||
| Angiotech Pharmaceuticals | (AMI) | |||||||
| A phase II dose-escalation study to assess the feasibility and safety of transendocardial delivery of three different doses of allogeneic mesenchymal precursor cells (MPCs) in subjects with heart failure | NCT00721045 | Angioblast Systems, U.S. | USA | Heart failure | Allogenic | Trans-endocardial injection | 60 | Unknown (last verified June 2010: active, not recruiting) |
2.4. Strategies to Advance the Therapeutic Properties of BM-MSCs
3. Conclusions and Future Perspectives
Conflict of Interest
References
- Buckingham, M.; Montarras, D. Skeletal muscle stem cells. Curr. Opin. Genet. Dev. 2008, 18, 330–336. [Google Scholar] [CrossRef]
- Chargè, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Dhawan, J.; Rando, T.A. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell. Biol. 2005, 15, 666–673. [Google Scholar] [CrossRef]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef]
- Ten Broek, R.W.; Grefte, S.; von den Hoff, J.W. Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 2010, 224, 7–16. [Google Scholar]
- Turner, N.J.; Badyak, S.F. Regeneration of skeletal muscle. Cell. Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef]
- Filippin, L.I.; Moreira, A.J.; Marroni, N.P.; Xavier, R.M. Nitric oxide and repair of skeletal muscle injury. Nitric. Oxide. 2009, 21, 157–163. [Google Scholar] [CrossRef]
- Wang, W.; Pan, H.; Murray, K.; Jefferson, B.S.; Li, Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am. J. Pathol. 2009, 174, 541–549. [Google Scholar] [CrossRef]
- Carosio, S.; Berardinelli, M.G.; Aucello, M.; Musarò, A. Impact of ageing on muscle cell regeneration. Ageing Res. Rev. 2011, 10, 35–42. [Google Scholar] [CrossRef]
- Laugwitz, K.L.; Moretti, A.; Lam, J.; Gruber, P.; Chen, Y.; Woodard, S.; Lin, L.Z.; Cai, C.L.; Lu, M.M.; Reth, M.; et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005, 433, 647–653. [Google Scholar] [CrossRef]
- Anversa, P.; Kajstura, J.; Leri, A.; Bolli, R. Life and Death of Cardiac Stem Cells A Paradigm Shift in Cardiac Biology. Circulation 2006, 113, 1451–1463. [Google Scholar] [CrossRef]
- Di Nardo, P.; Forte, G.; Ahluwalia, A.; Minieri, M. Cardiac progenitor cells: Potency and control. J. Cell. Physiol. 2010, 224, 590–600. [Google Scholar] [CrossRef]
- Bani, D.; Formigli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J. Cell. Mol. Med. 2010, 14, 2531–2538. [Google Scholar] [CrossRef]
- Balmer, G.M.; Riley, P.R. Harnessing the Potential of Adult Cardiac Stem Cells: Lessons from Haematopoiesis, the Embryo and the Niche. J. Cardiovasc. Transl. Res. 2012, 5, 631–640. [Google Scholar] [CrossRef]
- Torella, D.; Ellison, G.M.; Méndez-Ferrer, S.; Ibanez, B.; Nadal-Ginard, B. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat. Clin. Pract. Cardiovasc Med. 2006, 3, S8–S13. [Google Scholar] [CrossRef]
- van Amerongen, M.J.; Engel, F.B. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J. Cell. Mol. Med. 2008, 12, 2233–2244. [Google Scholar] [CrossRef]
- Noort, W.A.; Sluijter, J.P.; Goumans, M.J.; Chamuleau, S.A.; Doevendans, P.A. Stem cells from in- or outside of the heart: Isolation, characterization, and potential for myocardial tissue regeneration. Pediatr. Cardiol. 2009, 30, 699–709. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Segers, V.F.; Davis, M.E.; MacGillivray, C.; Gannon, J.; Molkentin, J.D.; Robbins, J.; Lee, R.T. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat. Med. 2007, 13, 970–974. [Google Scholar]
- Lyngbaek, S.; Schneider, M.; Hansen, J.L.; Sheikh, S.P. Cardiac regeneration by resident stem and progenitor cells in the adult heart. Basic Res. Cardiol. 2007, 102, 101–114. [Google Scholar] [CrossRef]
- Leri, A.; Kajstura, J.; Anversa, P.; Frishman, W.H. Myocardial regeneration and stem cell repair. Curr. Probl. Cardiol. 2008, 33, 91–153. [Google Scholar] [CrossRef]
- Altarche-Xifró, W.; Curato, C.; Kaschina, E.; Grzesiak, A.; Slavic, S.; Dong, J.; Kappert, K.; Steckelings, M.; Imboden, H.; Unger, T.; et al. Cardiac c-kit+AT2+ cell population is increased in response to ischemic injury and supports cardiomyocyte performance. Stem Cells 2009, 27, 2488–2497. [Google Scholar] [CrossRef]
- Cossu, G.; Sampaolesi, M. New therapies for muscular dystrophy: Cautious optimism. Trends Mol. Med. 2004, 10, 516–520. [Google Scholar] [CrossRef]
- Price, F.D.; Kuroda, K. Rudnicki M.A. Stem cell based therapies to treat muscular dystrophy. Biochim. Biophys. Acta 2007, 1772, 272–283. [Google Scholar] [CrossRef]
- Formigli, L.; Zecchi-Orlandini, S.; Meacci, E.; Bani, D. Skeletal myoblasts for heart regeneration and repair: state of the art and perspectives on the mechanisms for functional cardiac benefits. Curr. Pharm. Des. 2010, 16, 915–928. [Google Scholar] [CrossRef]
- Negroni, E.; Vallese, D.; Vilquin, J.T.; Butler-Browne, G.; Mouly, V.; Trollet, C. Current advances in cell therapy strategies for muscular dystrophies. Expert Opin. Biol. Ther. 2011, 11, 157–176. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Siminiak, T.; Guarita-Souza, L.C.; Teixeira de Carvalho, K.A.; Gallo, P.; Shim, W.; Condorelli, G. Stem cell therapy in heart diseases: A review of selected new perspectives, practical considerations and clinical applications. Curr. Cardiol. Rev. 2011, 7, 201–212. [Google Scholar] [CrossRef]
- Choi, S.H.; Jung, S.Y.; Kwon, S.M.; Baek, S.H. Perspectives on stem cell therapy for cardiac regeneration. Circ. J. 2012, 76, 1307–1312. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Dellavalle, A.; Diaz-Manera, J.; Messina, G.; Cossu, G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 2010, 120, 11–19. [Google Scholar] [CrossRef]
- Montarras, D.; Morgan, J.; Collins, C.; Relaix, F.; Zaffran, S.; Cumano, A.; Partridge, T.; Buckingham, M. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005, 309, 2064–2067. [Google Scholar]
- Biressi, S.; Rando, T.A. Heterogeneity in the muscle satellite cell population. Semin Cell. Dev. Biol. 2010, 21, 845–854. [Google Scholar]
- Huang, Y.C.; Dennis, R.G.; Baar, K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am. J. Physiol. Cell. Physiol. 2006, 291, C11–C17. [Google Scholar] [CrossRef]
- Harel, I.; Nathan, E.; Tirosh-Finkel, L.; Zigdon, H.; Guimarães-Camboa, N.; Evans, S.M.; Tzahor, E. Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell. 2009, 16, 822–832. [Google Scholar] [CrossRef]
- Sammels, L.M.; Bosio, E.; Fragall, C.T.; Grounds, M.D.; van Rooijen, N.; Beilharz, M.W. Innate inflammatory cells are not responsible for early death of donor myoblasts after myoblast transfer therapy. Transplantation 2004, 77, 1790–1797. [Google Scholar] [CrossRef]
- Chimenti, I.; Gaetani, R.; Barile, L.; Frati, G.; Messina, E.; Giacomello, A. c-kit cardiac progenitor cells: What is their potential? Proc. Natl. Acad. Sci. USA 2009, 106, E78. [Google Scholar]
- Itzhaki-Alfia, A.; Leor, J.; Raanani, E.; Sternik, L.; Spiegelstein, D.; Netser, S.; Holbova, R.; Pevsner-Fischer, M.; Lavee, J.; Barbash, I.M. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation 2009, 120, 2559–2566. [Google Scholar]
- Miyamoto, S.; Kawaguchi, N.; Ellison, G.M.; Matsuoka, R.; Shin'oka, T.; Kurosawa, H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010, 19, 105–116. [Google Scholar] [CrossRef]
- Ellison, G.M.; Galuppo, V.; Vicinanza, C.; Aquila, I.; Waring, C.D.; Leone, A.; Indolfi, C.; Torella, D. Cardiac stem and progenitor cell identification: different markers for the same cell? Front. Biosci. (Schol. Ed.). 2010, 1, 641–652. [Google Scholar]
- Forte, G.; Pietronave, S.; Nardone, G.; Zamperone, A.; Magnani, E.; Pagliari, S.; Pagliari, F.; Giacinti, C.; Nicoletti, C.; Musaró, A.; et al. Human cardiac progenitor cell grafts as unrestricted source of supernumerary cardiac cells in healthy murine hearts. Stem Cells 2011, 29, 2051–2061. [Google Scholar] [CrossRef] [Green Version]
- He, J.Q.; Vu, D.M.; Hunt, G.; Chugh, A.; Bhatnagar, A.; Bolli, R. Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS One 2011, 6, e27719. [Google Scholar]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma'ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Boxall, S.A.; Jones, E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 2012, 975871. [Google Scholar]
- Richardson, J.D.; Nelson, A.J.; Zannettino, A.C.; Gronthos, S.; Worthley, S.G.; Psaltis, P.J. Optimization of the Cardiovascular Therapeutic Properties of Mesenchymal Stromal/Stem Cells-Taking the Next Step. Stem Cell. Rev. 2012, in press. [Google Scholar]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar]
- Zhironkina, O.A.; Shipounova, I.N.; Bigildeev, A.E.; Sats, N.V.; Petinati, N.A.; Drize, N.I. Proliferative potential of multipotent mesenchymal stromal cells from human bone marrow. Bull. Exp. Biol. Med. 2012, 152, 543–547. [Google Scholar] [CrossRef]
- Haack-Sørensen, M.; Hansen, S.K.; Hansen, L.; Gaster, M.; Hyttel, P.; Ekblond, A.; Kastrup, J. Mesenchymal stromal cell phenotype is not influenced by confluence during culture expansion. Stem Cell. Rev. 2012, in press. [Google Scholar]
- Wakitani, S.; Saito, T.; Caplan, A.I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995, 18, 1417–1426. [Google Scholar] [CrossRef]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999, 103, 697–705. [Google Scholar] [CrossRef]
- He, X.Q.; Chen, M.S.; Li, S.H.; Liu, S.M.; Zhong, Y.; McDonald Kinkaid, H.Y.; Lu, W.Y.; Weisel, R.D.; Li, R.K. Co-culture with cardiomyocytes enhanced the myogenic conversion of mesenchymal stromal cells in a dose-dependent manner. Mol. Cell. Biochem. 2010, 339, 89–98. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.S.; Verfaillie, C.M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Invest. 2002, 109, 1291–1302. [Google Scholar] [Green Version]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Chapel, A.; Bertho, J.M.; Bensidhoum, M.; Fouillard, L.; Young, R.G.; Frick, J.; Demarquay, C.; Cuvelier, F.; Mathieu, E.; Trompier, F.; et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J. Gene Med. 2003, 5, 1028–1038. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef]
- Baiguera, S.; Jungebluth, P.; Mazzanti, B.; Macchiarini, P. Mesenchymal stromal cells for tissue-engineered tissue and organ replacements. Transpl. Int. 2012, 25, 369–382. [Google Scholar] [CrossRef]
- von Bahr, L.; Batsis, I.; Moll, G.; Hägg, M.; Szakos, A.; Sundberg, B.; Uzunel, M.; Ringden, O.; Le Blanc, K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012, 30, 1575–1578. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.B.; Song, Y.P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 2012, 652034. [Google Scholar]
- Cashman, T.J.; Gouon-Evans, V.; Costa, K.D. Mesenchymal Stem Cells for Cardiac Therapy: Practical Challenges and Potential Mechanisms. Stem Cell. Rev. 2012, in press. [Google Scholar]
- Natsu, K.; Ochi, M.; Mochizuki, Y.; Hachisuka, H.; Yanada, S.; Yasunaga, Y. Allogeneic bone marrow-derived mesenchymal stromal cells promote the regeneration of injured skeletal muscle without differentiation into myofibers. Tissue Eng. 2004, 10, 1093–1112. [Google Scholar]
- Matziolis, G.; Winkler, T.; Schaser, K.; Wiemann, M.; Krocker, D.; Tuischer, J.; Perka, C.; Duda, G.N. Autologous bone marrow-derived cells enhance muscle strength following skeletal muscle crush injury in rats. Tissue Eng. 2006, 12, 361–367. [Google Scholar] [CrossRef]
- Winkler, T.; von Roth, P.; Radojewski, P.; Urbanski, A.; Hahn, S.; Preininger, B.; Duda, G.N.; Perka, C. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J. Tissue Eng. Regen. Med. 2012. [Google Scholar] [CrossRef]
- von Roth, P.; Duda, G.N.; Radojewski, P.; Preininger, B.; Perka, C.; Winkler, T. Mesenchymal stem cell therapy following muscle trauma leads to improve muscular regeneration in both male and female rats. Gend. Med. 2012, 9, 129–136. [Google Scholar] [CrossRef]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309, 314–317. [Google Scholar]
- de la Garza-Rodea, A.S.; van der Velde, I.; Boersma, H.; Gonçalves, M.A.; van Bekkum, D.W.; de Vries, A.A.; Knaän-Shanzer, S. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011, 20, 217–231. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar]
- Hoffmann, J.; Glassford, A.J.; Doyle, T.C.; Robbins, R.C.; Schrepfer, S.; Pelletier, M.P. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. Thorac. Cardiovasc. Surg. 2010, 58, 136–142. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, Y.; He, G.; Zhang, F. Augmentation of neovascularization in murine hindlimb ischemia by combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells transplantation. J. Biomed. Sci. 2010, 17, 75. [Google Scholar] [CrossRef]
- Feng, S.W.; Lu, X.L.; Liu, Z.S.; Zhang, Y.N.; Liu, T.Y.; Li, J.L.; Yu, M.J.; Zeng, Y.; Zhang, C. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy 2008, 10, 254–264. [Google Scholar] [CrossRef]
- Nitahara-Kasahara, Y.; Hayashita-Kinoh, H.; Ohshima-Hosoyama, S.; Okada, H.; Wada-Maeda, M.; Nakamura, A.; Okada, T.; Takeda, S. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol. Ther. 2012, 20, 168–177. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.Y.; Lei, Q.F.; Zhang, C.; Li, S.N. Improved motor function in dko mice by intravenous transplantation of bone marrow-derived mesenchymal stromal cells. Cytotherapy 2011, 13, 69–77. [Google Scholar] [CrossRef]
- Shabbir, A.; Zisa, D.; Leiker, M.; Johnston, C.; Lin, H.; Lee, T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation 2009, 87, 1275–1282. [Google Scholar] [CrossRef]
- Amado, L.C.; Saliaris, A.P.; Schuleri, K.H.; St John, M.; Xie, J.S.; Cattaneo, S.; Durand, D.J.; Fitton, T.; Kuang, J.Q.; Stewart, G.; et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA 2005, 102, 11474–11479. [Google Scholar]
- Dai, W.; Hale, S.L.; Martin, B.J.; Kuang, J.Q.; Dow, J.S.; Wold, L.E.; Kloner, R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation 2005, 112, 214–223. [Google Scholar]
- Perin, E.C.; Silva, G.V.; Assad, J.A.; Vela, D.; Buja, L.M.; Sousa, A.L.; Litovsky, S.; Lin, J.; Vaughn, W.K.; Coulter, S.; et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J. Mol. Cell. Cardiol. 2008, 44, 486–495. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, C.H.; Lin, W.W.; Hwang, S.M.; Hsieh, P.C.; Lai, P.H.; Yeh, Y.C.; Chang, Y.; Sung, H.W. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc. Res. 2008, 77, 515–524. [Google Scholar]
- Vela, D.C.; Silva, G.V.; Assad, J.A.; Sousa, A.L.; Coulter, S.; Fernandes, M.R.; Perin, E.C.; Willerson, J.T.; Buja, L.M. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J. Histochem. Cytochem. 2009, 57, 167–176. [Google Scholar]
- Quevedo, H.C.; Hatzistergos, K.E.; Oskouei, B.N.; Feigenbaum, G.S.; Rodriguez, J.E.; Valdes, D.; Pattany, P.M.; Zambrano, J.P.; Hu, Q.; McNiece, I.; et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 14022–14027. [Google Scholar]
- Schuleri, K.H.; Feigenbaum, G.S.; Centola, M.; Weiss, E.S.; Zimmet, J.M.; Turney, J.; Kellner, J.; Zviman, M.M.; Hatzistergos, K.E.; Detrick, B.; et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur. Heart J. 2009, 30, 2722–2732. [Google Scholar]
- Psaltis, P.J.; Carbone, A.; Nelson, A.J.; Lau, D.H.; Jantzen, T.; Manavis, J.; Williams, K.; Itescu, S.; Sanders, P.; Gronthos, S.; et al. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc. Interv. 2010, 3, 974–983. [Google Scholar] [CrossRef]
- Sato, T.; Iso, Y.; Uyama, T.; Kawachi, K.; Wakabayashi, K.; Omori, Y.; Soda, T.; Shoji, M.; Koba, S.; Yokoyama, S.; et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab. Invest. 2011, 91, 553–564. [Google Scholar] [CrossRef]
- Shake, J.G.; Gruber, P.J.; Baumgartner, W.A.; Senechal, G.; Meyers, J.; Redmond, J.M.; Pittenger, M.F.; Martin, B.J. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 2002, 73, 1919–1925. [Google Scholar]
- Makkar, R.R.; Price, M.J.; Lill, M.; Frantzen, M.; Takizawa, K.; Kleisli, T.; Zheng, J.; Kar, S.; McClelan, R.; Miyamota, T.; et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 2005, 10, 225–233. [Google Scholar] [CrossRef]
- Price, M.J.; Chou, C.C.; Frantzen, M.; Miyamoto, T.; Kar, S.; Lee, S.; Shah, P.K.; Martin, B.J.; Lill, M.; Forrester, J.S.; et al. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int. J. Cardiol. 2006, 111, 231–239. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.J.; Qian, H.Y.; Tang, Y.D.; Wang, H.; Zhang, Q. Rosuvastatin treatment activates JAK-STAT Pathway and increases efficacy of allogeneic mesenchymal stem cell transplantation. Circ. J. 2011, 75, 1476–1485. [Google Scholar] [CrossRef]
- Mills, W.R.; Mal, N.; Kiedrowski, M.J.; Unger, R.; Forudi, F.; Popovic, Z.B.; Penn, M.S.; Laurita, K.R. Stem cell therapy enhances electrical viability in myocardial infarction. J. Mol. Cell. Cardiol. 2007, 42, 304–314. [Google Scholar] [CrossRef]
- 90. Costa, A.R.; Panda, N.C.; Yong, S.; Mayorga, M.E.; Pawlowski, G.P.; Fan, K.; Penn, M.S.; Laurita, K.R. Optical mapping of cryoinjured rat myocardium grafted with mesenchymal stem cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H270–277. [Google Scholar] [CrossRef]
- Noiseux, N.; Gnecchi, M.; Lopez-Ilasaca, M.; Zhang, L.; Solomon, S.D.; Deb, A.; Dzau, V.J.; Pratt, R.E. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol. Ther. 2006, 14, 840–850. [Google Scholar] [CrossRef]
- Chong, J.J. Cell Therapy for Left Ventricular Dysfunction: An Overview for Cardiac Clinicians. Heart Lung Circ. 2012, 21, 532–542. [Google Scholar] [CrossRef]
- Chen, S.L.; Fang, W.W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.J.; Zhang, J.J.; Chunhua, R.Z.; Liao, L.M.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar]
- Katritsis, D.G.; Sotiropoulou, P.A.; Karvouni, E.; Karabinos, I.; Korovesis, S.; Perez, S.A.; Voridis, E.M.; Papamichail, M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter. Cardiovasc. Interv. 2005, 65, 321–329. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Tian, N.; Zhang, J.; Yei, F.; Duan, B.; Zhu, Z.; Lin, S.; Kwan, T.W. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J. Invasive Cardiol. 2006, 18, 552–556. [Google Scholar]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; De Maria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef]
- Chin, S.P.; Poey, A.C.; Wong, C.Y.; Chang, S.K.; Teh, W.; Mohr, T.J.; Cheong, S.K. Cryopreserved mesenchymal stromal cell treatment is safe and feasible for severe dilated ischemic cardiomyopathy. Cytotherapy 2010, 12, 31–37. [Google Scholar] [CrossRef]
- Chin, S.P.; Poey, A.C.; Wong, C.Y.; Chang, S.K.; Tan, C.S.; Ng, M.T.; Chew, K.H.; Lam, K.H.; Cheong, S.K. Intramyocardial and intracoronary autologous bone marrow-derived mesenchymal stromal cell treatment in chronic severe dilated cardiomyopathy. Cytotherapy 2011, 13, 814–821. [Google Scholar] [CrossRef]
- Penn, M.S.; Ellis, S.; Gandhi, S.; Greenbaum, A.; Hodes, Z.; Mendelsohn, F.O.; Strasser, D.; Ting, A.E.; Sherman, W. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ. Res. 2012, 110, 304–311. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Shabbir, A.; Zisa, D.; Suzuki, G.; Lee, T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A non invasive therapeutic regimen. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1888–H1897. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Burdon, T.J.; Paul, A.; Noiseux, N.; Prakash, S.; Shum-Tim, D. Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011, 207326. [Google Scholar]
- Lee, R.H.; Oh, J.Y.; Choi, H.; Bazhanov, N. Therapeutic factors secreted by mesenchymal stromal cells and tissue repair. J. Cell. Biochem. 2011, 112, 3073–3078. [Google Scholar] [CrossRef]
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706. [Google Scholar] [CrossRef]
- Prockop, D.J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol. Ther. 2009, 17, 939–946. [Google Scholar] [CrossRef]
- Lee, T. Host tissue response in stem cell therapy. World J. Stem Cells 2010, 2, 61–66. [Google Scholar]
- Sassoli, C.; Pini, A.; Mazzanti, B.; Quercioli, F.; Nistri, S.; Saccardi, R.; Zecchi-Orlandini, S.; Bani, D.; Formigli, L. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: Clues for cardiac regeneration. J. Mol. Cell. Cardiol. 2011, 51, 399–408. [Google Scholar] [CrossRef]
- Sassoli, C.; Pini, A.; Chellini, F.; Mazzanti, B.; Nistri, S.; Nosi, D.; Saccardi, R.; Quercioli, F.; Zecchi-Orlandini, S.; Formigli, L. Bone Marrow Mesenchymal Stromal Cells Stimulate Skeletal Myoblast Proliferation through the Paracrine Release of VEGF. PLoS One 2012, 7, e37512. [Google Scholar]
- Shi, C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology 2012, 136, 133–138. [Google Scholar] [CrossRef]
- Tschöpe, C.; Miteva, K.; Schultheiss, H.P.; Linthout, S.V. Mesenchymal stromal cells: A promising cell source for the treatment of inflammatory cardiomyopathy. Curr. Pharm. Des. 2011, 17, 3295–3307. [Google Scholar]
- Zanin, M.; Germinario, E.; Dalla Libera, L.; Sandonà, D.; Sabbadini, R.A.; Betto, R.; Danieli-Betto, D. Trophic action of sphingosine 1-phosphate in denervated rat soleus muscle. Am. J. Physiol. Cell. Physiol. 2008, 294, C36–C46. [Google Scholar] [CrossRef]
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef]
- Frey, S.P.; Jansen, H.; Raschke, M.J.; Meffert, R.H.; Ochman, S. VEGF Improves Skeletal Muscle Regeneration After Acute Trauma and Reconstruction of the Limb in a Rabbit Model. Clin. Orthop. Relat. Res. 2012, in press. [Google Scholar]
- Gehrig, S.M.; van der Poel, C.; Hoeflich, A.; Naim, T.; Lynch, G.S.; Metzger, F. Therapeutic potential of PEGylated insulin-like growth factor I for skeletal muscle disease evaluated in two murine models of muscular dystrophy. Growth Horm. IGF Res. 2012, 22, 69–75. [Google Scholar] [CrossRef]
- Urbanek, K.; Rota, M.; Cascapera, S.; Bearzi, C.; Nascimbene, A.; De Angelis, A.; Hosoda, T.; Chimenti, S.; Baker, M.; Limana, F.; et al. Cardiac stem cells possess growth factor-receptor systems that following activation regenerate the infarcted myocardium improving ventricular function and long-term survival. Circ. Res. 2005, 97, 663–667. [Google Scholar] [CrossRef]
- Bani, D.; Bigazzi, M. Relaxin as a cardiovascular drug: a promise kept. Curr. Drug Saf. 2011, 6, 324–328. [Google Scholar] [CrossRef]
- Ellison, G.M.; Torella, D.; Dellegrottaglie, S.; Perez-Martinez, C.; Perez de Prado, A.; Vicinanza, C.; Purushothaman, S.; Galuppo, V.; Iaconetti, C.; Waring, C.D.; et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J. Am. Coll. Cardiol. 2011, 58, 977–986. [Google Scholar]
- Nistri, S.; Pini, A.; Sassoli, C.; Squecco, R.; Francini, F.; Formigli, L.; Bani, D. Relaxin promotes growth and maturation of mouse neonatal cardiomyocytes in vitro: clues for cardiac regeneration. J. Cell. Mol. Med. 2012, 16, 507–519. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008, 3, e1886. [Google Scholar]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007, 110, 3499–3506. [Google Scholar] [CrossRef]
- Kortesidis, A.; Zannettino, A.; Isenmann, S.; Shi, S.; Lapidot, T.; Gronthos, S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 2005, 105, 3793–3801. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Zhang, M.; Mal, N.; Kiedrowski, M.; Chacko, M.; Askari, A.T.; Popovic, Z.B. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007, 21, 3197–3207. [Google Scholar] [CrossRef]
- Yin, Q.; Jin, P.; Liu, X.; Wei, H.; Lin, X.; Chi, C.; Liu, Y.; Sun, C.; Wei, Y. SDF-1α inhibits hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells through PI3K/Akt and ERK1/2 signaling pathways. Mol. Biol. Rep. 2011, 38, 9–16. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Epstein, S.E. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ. Res. 2004, 95, 354–363. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004, 94, 678–685. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.J.; Cao, D.Y.; Li, X.; Zhang, L.Y.; He, Y.; Yue, S.Q.; Wang, D.S.; Dou, K.F. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J. Gastroenterol. 2012, 18, 1048–1058. [Google Scholar] [CrossRef]
- Ohnishi, S.; Sumiyoshi, H.; Kitamura, S.; Nagaya, N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007, 581, 3961–3966. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Guo, L.; Kong, X.; Yang, J.; Zheng, F.; Zhang, L.; Huang, Y. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol. Cells 2010, 29, 9–19. [Google Scholar] [CrossRef]
- Mazhari, R.; Hare, J.M. Mechanisms of action of mesenchymal stem cells in cardiac repair: Potential influences on the cardiac stem cell niche. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, S21–S26. [Google Scholar] [CrossRef]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Steinhauser, M.L.; Gannon, J.; Lee, R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell 2011, 8, 389–398. [Google Scholar] [CrossRef]
- Tang, J.M.; Wang, J.N.; Zhang, L.; Zheng, F.; Yang, J.Y.; Kong, X.; Guo, L.Y.; Chen, L.; Huang, Y.Z.; Wan, Y.; et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc. Res. 2011, 91, 402–411. [Google Scholar] [CrossRef]
- Formigli, L.; Benvenuti, S.; Mercatelli, R.; Quercioli, F.; Tani, A.; Mirabella, C.; Dama, A.; Saccardi, R.; Mazzanti, B.; Cellai, I.; et al. Dermal matrix scaffold engineered with adult mesenchymal stem cells and platelet-rich plasma as a potential tool for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2012, 6, 125–134. [Google Scholar] [CrossRef]
- Giannelli, M.; Chellini, F.; Sassoli, C.; Francini, F.; Pini, A.; Squecco, R.; Nosi, D.; Bani, D.; Zecchi-Orlandini, S.; Formigli, L. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: Effects and mechanisms of action. J. Cell. Physiol. 2012, in press. [Google Scholar]
- Guo, Y.H.; He, J.G.; Wu, J.L.; Yang, L.; Zhang, D.S.; Tan, X.Y.; Qi, R.D. Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy 2008, 10, 857–867. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Zheng, F.; Kong, X.; Guo, L.; Yang, J.; Zhang, L.; Huang, Y. Combination of chemokine and angiogenic factor genes and mesenchymal stem cells could enhance angiogenesis and improve cardiac function after acute myocardial infarction in rats. Mol. Cell. Biochem. 2010, 339, 107–118. [Google Scholar]
- Chen, C.H.; Wei, H.J.; Lin, W.W.; Chiu, I.; Hwang, S.M.; Wang, C.C.; Lee, W.Y.; Chang, Y.; Sung, H.W. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc. Res. 2008, 80, 88–95. [Google Scholar]
- Khait, L.; Hecker, L.; Blan, N.R.; Coyan, G.; Migneco, F.; Huang, Y.C.; Birla, R.K. Getting to the heart of tissue engineering. J. Cardiovasc. Transl. Res. 2008, 1, 71–84. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sassoli, C.; Zecchi-Orlandini, S.; Formigli, L. Trophic Actions of Bone Marrow-Derived Mesenchymal Stromal Cells for Muscle Repair/Regeneration. Cells 2012, 1, 832-850. https://doi.org/10.3390/cells1040832
Sassoli C, Zecchi-Orlandini S, Formigli L. Trophic Actions of Bone Marrow-Derived Mesenchymal Stromal Cells for Muscle Repair/Regeneration. Cells. 2012; 1(4):832-850. https://doi.org/10.3390/cells1040832
Chicago/Turabian StyleSassoli, Chiara, Sandra Zecchi-Orlandini, and Lucia Formigli. 2012. "Trophic Actions of Bone Marrow-Derived Mesenchymal Stromal Cells for Muscle Repair/Regeneration" Cells 1, no. 4: 832-850. https://doi.org/10.3390/cells1040832
APA StyleSassoli, C., Zecchi-Orlandini, S., & Formigli, L. (2012). Trophic Actions of Bone Marrow-Derived Mesenchymal Stromal Cells for Muscle Repair/Regeneration. Cells, 1(4), 832-850. https://doi.org/10.3390/cells1040832




